A Review on the Challenges of Using Zeolite 13X as Heat Storage Systems for the Residential Sector
Abstract
:1. Introduction
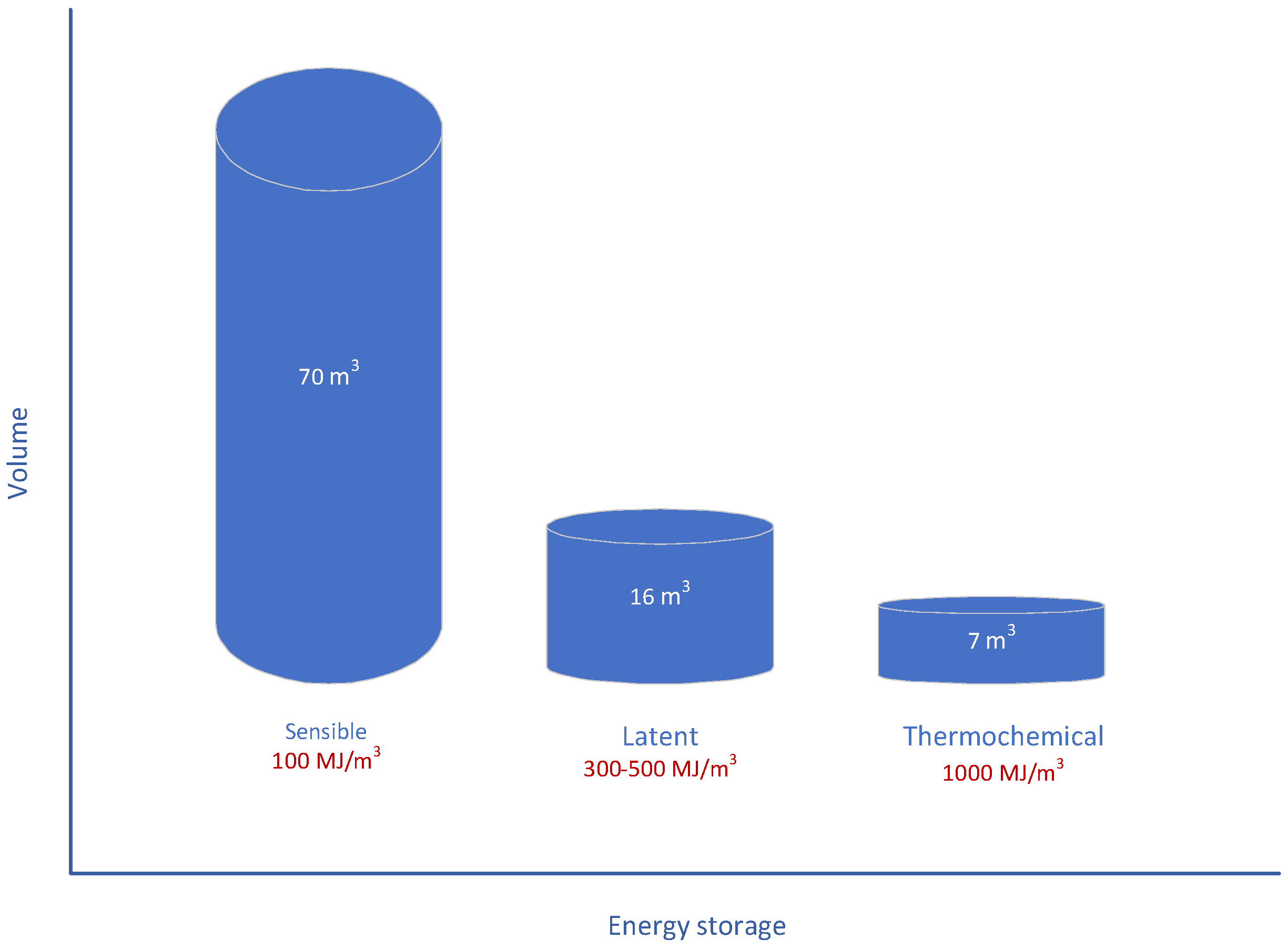
2. Thermal Energy Storage Systems
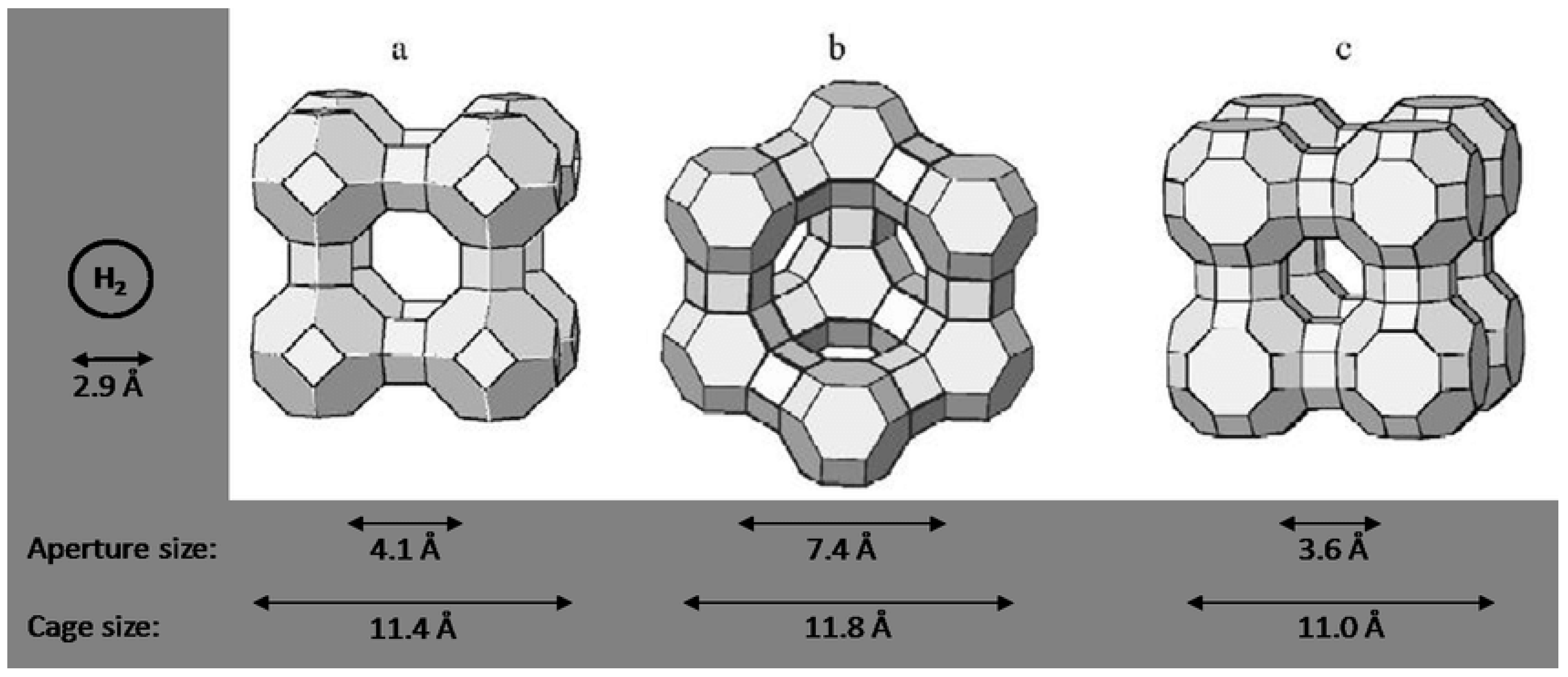
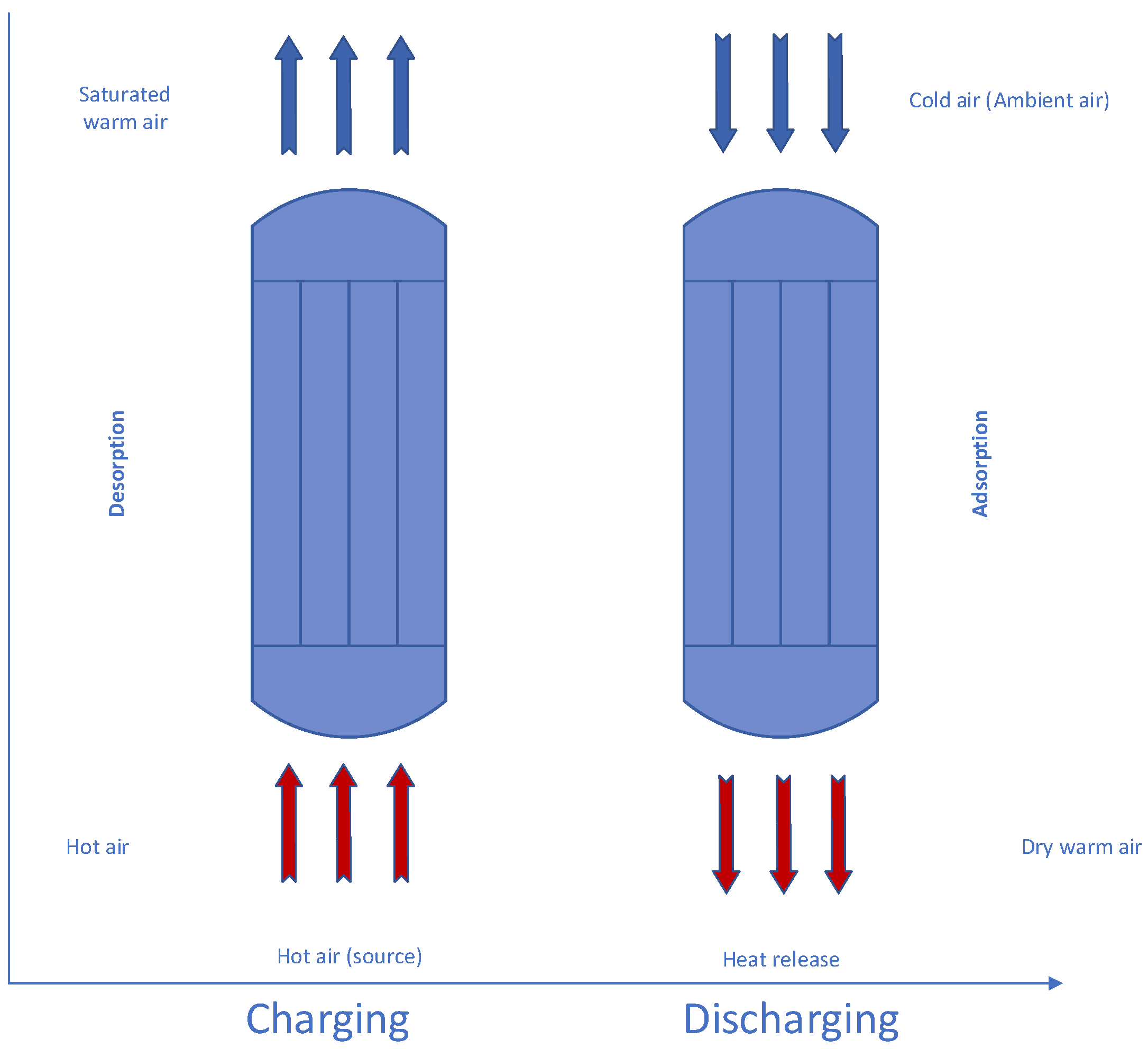
3. Adsorption Heat Materials
4. Technical Challenge
4.1. Required Temperature Supply
4.2. Relative Humidity Control
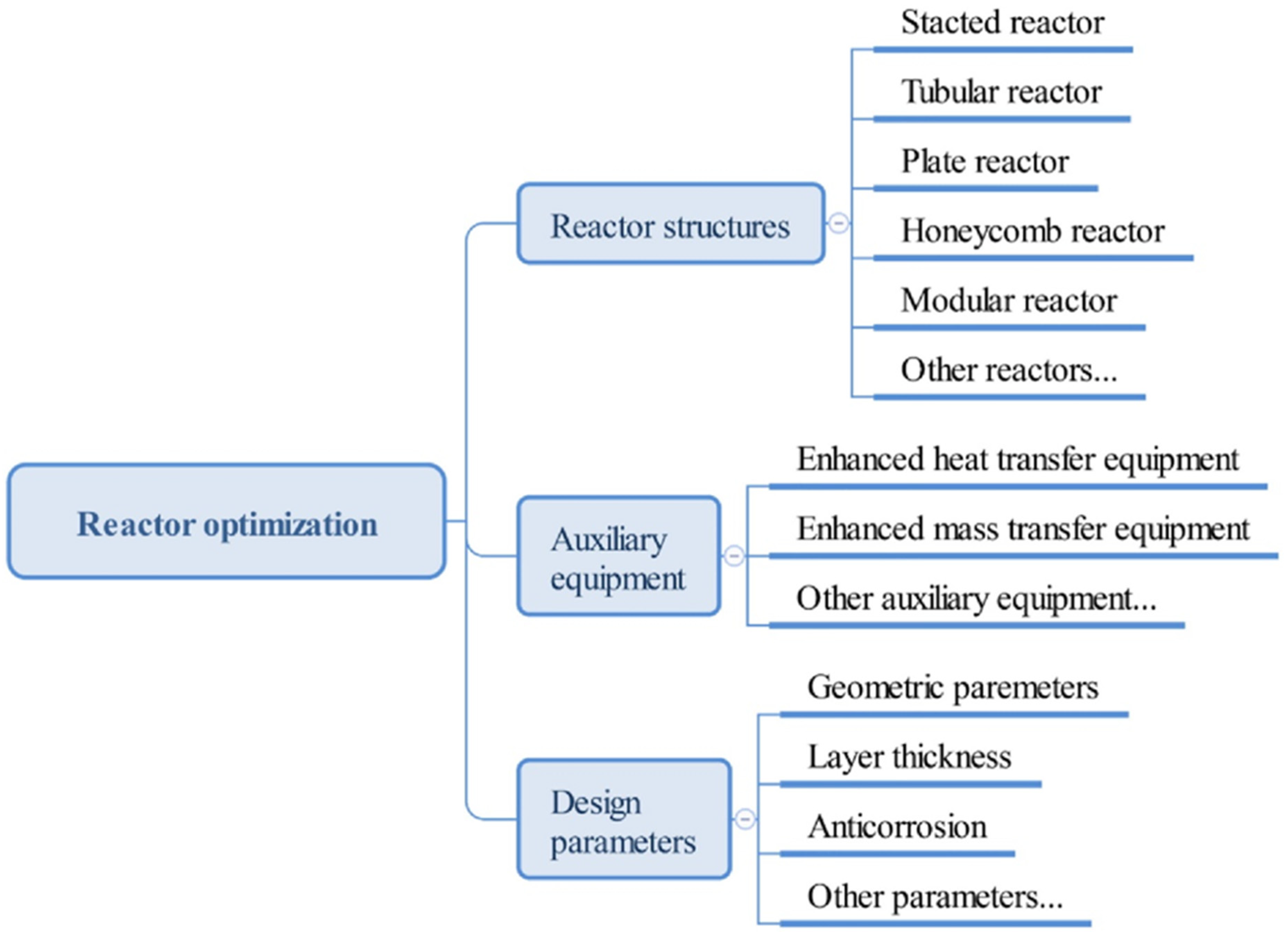
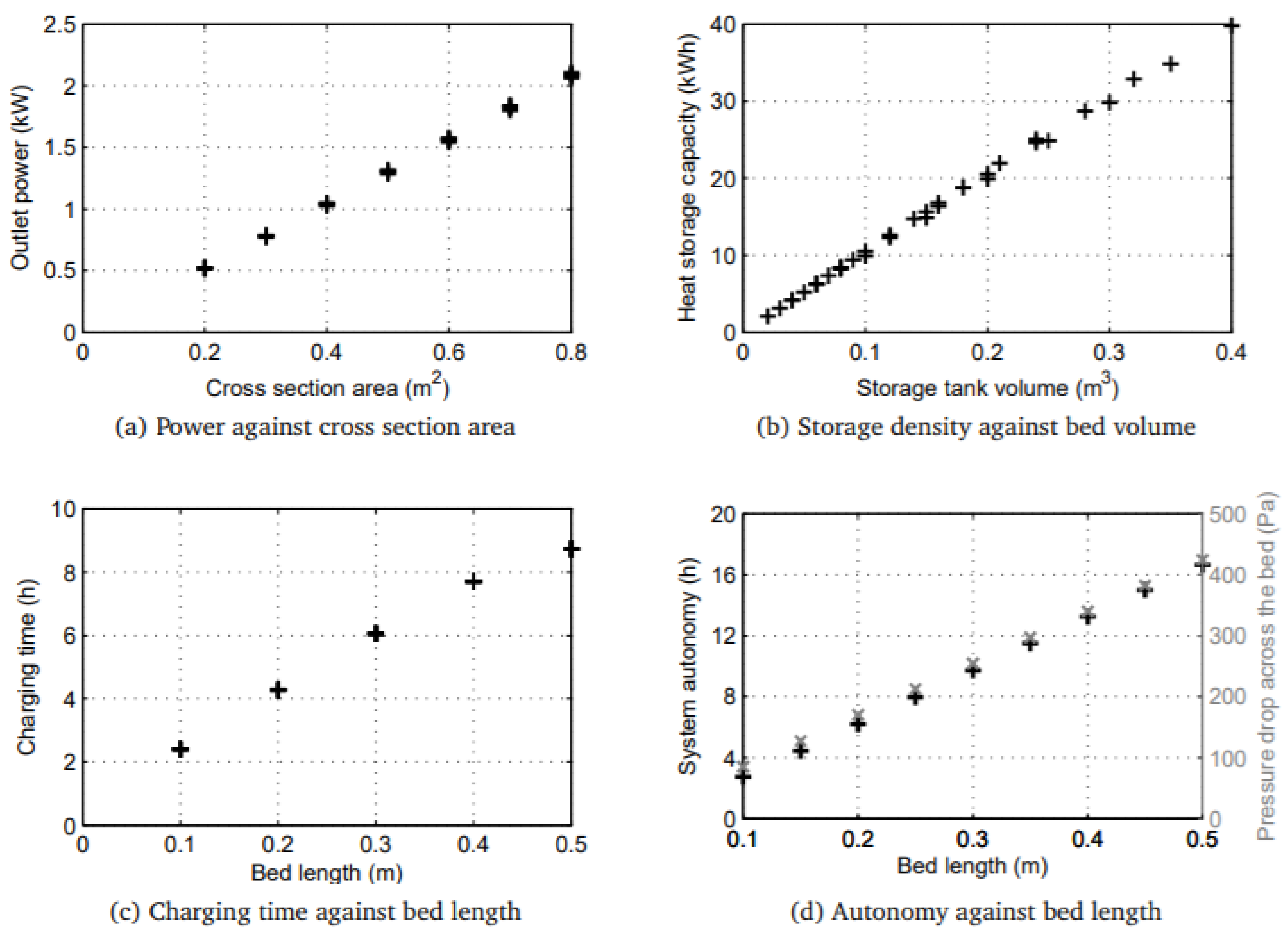
4.3. Reduce Reactor Size
- outlet power is directly proportional to cross section area (a)
- heat storage capacity is directly equivalent to storage tank volume (b)
- charging time and discharging time is directly proportional to bed length (c,d)
4.4. System Output Power
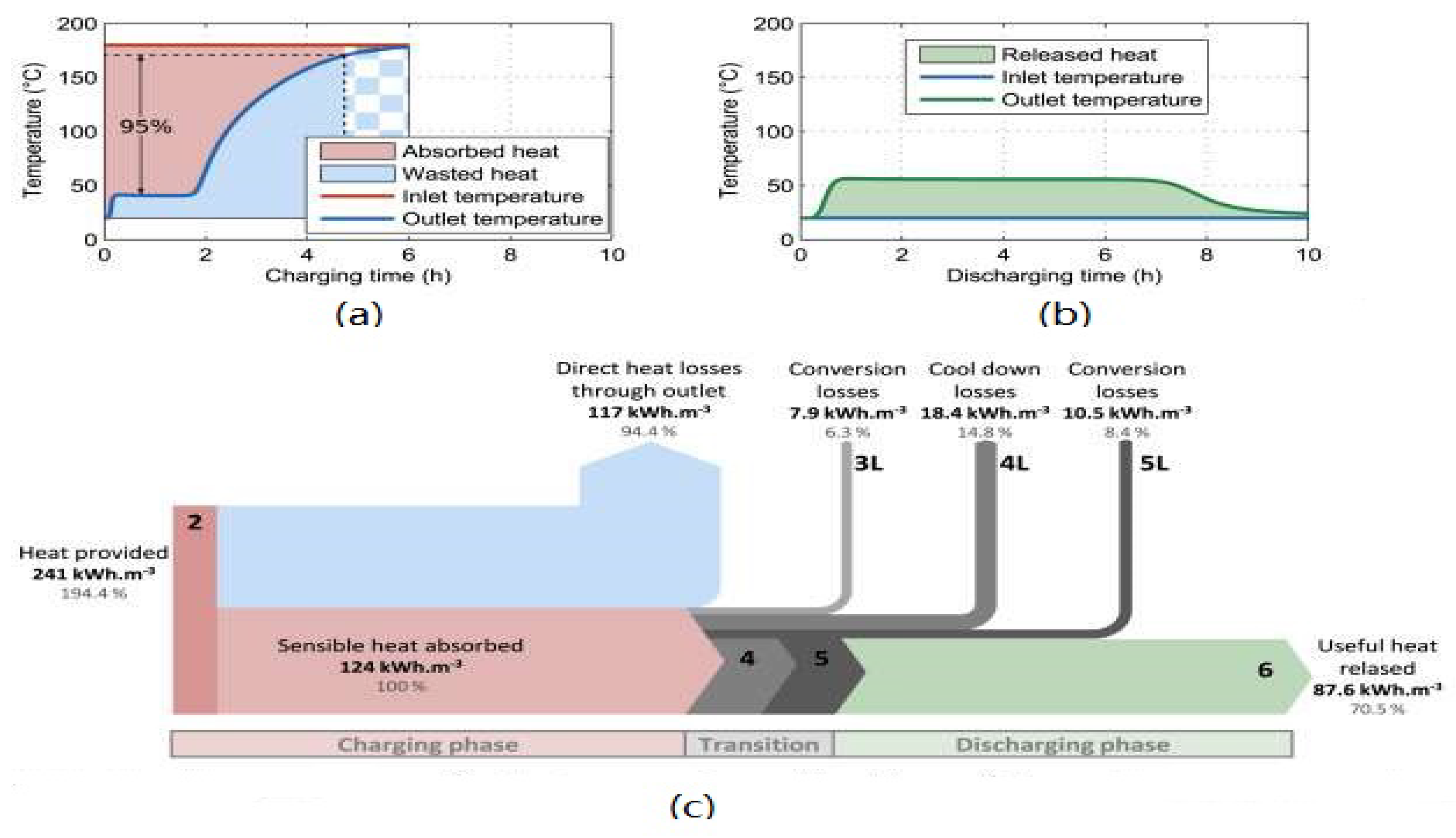
4.5. Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, R. Sorption Thermal Energy Storage: Concept, Process, Applications and Perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- Dincer, I.; Ezan, M.A. Thermal Energy Storage Methods BT—Heat Storage: A Unique Solution for Energy Systems; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–84. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A Review of Potential Materials for Thermal Energy Storage in Building Applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Q.; Huang, H.; Wu, Q.; Xiao, Y. Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: A review. Sol. Energy 2020, 214, 149–178. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Edge, J. Hydorgen Adsorption and Dynamics in Clay Minerals; UCL (University College London): London, UK, 2014; p. 269. [Google Scholar]
- Rocky, K.A.; Pal, A.; Rupam, T.H.; Palash, M.L.; Saha, B.B. Recent Advances of Composite Adsorbents for Heat Transformation Applications. Therm. Sci. Eng. Prog. 2021, 23, 100900. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Hauer, A.; Jaehnig, D.; Kerskes, H.; Henning, T.N.; Visscher, K.; Laevemann, E.; Peltzer, M.; Henning, H.-M. Thermal Energy Storage for Solar and Low Energy Buildings State of the Art; International Energy Agency: Bern, Switzerland, 2005. [Google Scholar]
- Zhao, Q.; Lin, J.; Huang, H.; Wu, Q.; Shen, Y.; Xiao, Y. Optimization of Thermochemical Energy Storage Systems Based on Hydrated Salts: A Review. Energy Build. 2021, 244, 111035. [Google Scholar] [CrossRef]
- Anderson, R.; Shiri, S.; Bindra, H.; Morris, J.F. Experimental Results and Modeling of Energy Storage and Recovery in a Packed Bed of Alumina Particles. Appl. Energy 2014, 119, 521–529. [Google Scholar] [CrossRef]
- Lahmidi, H.; Mauran, S.; Goetz, V. Definition, Test and Simulation of a Thermochemical Storage Process Adapted to Solar Thermal Systems. Sol. Energy 2006, 80, 883–893. [Google Scholar] [CrossRef]
- Stitou, D.; Mazet, N.; Mauran, S. Experimental Investigation of a Solid/Gas Thermochemical Storage Process for Solar Air-Conditioning. Energy 2012, 41, 261–270. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Jaenig, D.; Kerskes, H.; Summer, K.; van Essen, M.; Weber, R. Laboratory Tests of Chemical Reactions and Prototype Sorption Storage Units. A Report of IEA Solar Heating and Cooling Programme—Task 32: Advanced Storage Concepts for Solar and Low Energy Buildings; Solar Energy Research Center SERC Högskolan Dalarna: Borlänge, Sweden, 2008; p. 55. [Google Scholar]
- Oktariani, E.; Tahara, K.; Nakashima, K.; Noda, A.; Xue, B.; Wijayanta, A.T.; Nakaso, K.; Fukai, J. Experimental Investigation on the Adsorption Process for Steam Generation Using a Zeolite-Water System. J. Chem. Eng. Jpn. 2012, 45, 355–362. [Google Scholar] [CrossRef]
- Tolich, K. Lecture 11 Second Law of Thermodynamics; Canvas Learning Management System. University of Washington; Available online: https://canvas.uw.edu/files/40309275/download?download_frd=1&verifier=wfvQGeN0R7J6PC33VKp7O1cbquwVpa6FELtQAxl0 (accessed on 1 December 2021).
- van Alebeek, R.; Scapino, L.; Beving, M.A.J.M.; Gaeini, M.; Rindt, C.C.M.; Zondag, H.A. Investigation of a Household-Scale Open Sorption Energy Storage System Based on the Zeolite 13X/Water Reacting Pair. Appl. Therm. Eng. 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Gondre, D. Numerical Modeling and Analysis of Heat and Mass Transfers in an Adsorption Heat Storage Tank: Influences of Material Properties, Operating Conditions and System Design on Storage Performances. Ph.D. Thesis, University of Lyon, Lyon, France, 21 March 2016. [Google Scholar]
- Johannes, K.; Kuznik, F.; Hubert, J.L.; Durier, F.; Obrecht, C. Design and Characterisation of a High Powered Energy Dense Zeolite Thermal Energy Storage System for Buildings. Appl. Energy 2015, 159, 80–86. [Google Scholar] [CrossRef]
- Zettl, B.; Englmair, G.; Steinmaurer, G. Development of a Revolving Drum Reactor for Open-Sorption Heat Storage Processes. Appl. Therm. Eng. 2014, 70, 42–49. [Google Scholar] [CrossRef]
- Zondag, H.; Kikkert, B.; Smeding, S.; de Boer, R.; Bakker, M. Prototype Thermochemical Heat Storage with Open Reactor System. Appl. Energy 2013, 109, 360–365. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Jaenig, D.; Kerskes, H.; Summer, K.; van Essen, M.; Weber, R. Laboratory Tests of Chemical Reactions and Prototype Sorption Storage Units. Report B4 of Subtask B; IEA-SHC: Paris, France, 2008. [Google Scholar]
- Kuznik, F.; Gondre, D.; Johannes, K.; Obrecht, C.; David, D. Numerical Modelling and Investigations on a Full-Scale Zeolite 13X Open Heat Storage for Buildings. Renew. Energy 2019, 132, 761–772. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Pel, L.; Adan, O.C.G. Experimental Studies for the Cyclability of Salt Hydrates for Thermochemical Heat Storage. J. Energy Storage 2016, 5, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Michel, B.; Mazet, N.; Neveu, P. Experimental Investigation of an Innovative Thermochemical Process Operating with a Hydrate Salt and Moist Air for Thermal Storage of Solar Energy: Global Performance. Appl. Energy 2014, 129, 177–186. [Google Scholar] [CrossRef] [Green Version]
- de Boer, R.; Smeding, S.F.; Zondag, H.A.; Krol, G. Development of a Prototype System for Seasonal Solar Heat Storage Using an Open Sorption Process. In Proceedings of the Eurotherm Seminar, Lleida, Spain, 28–30 May 2014; pp. 1–9. [Google Scholar]







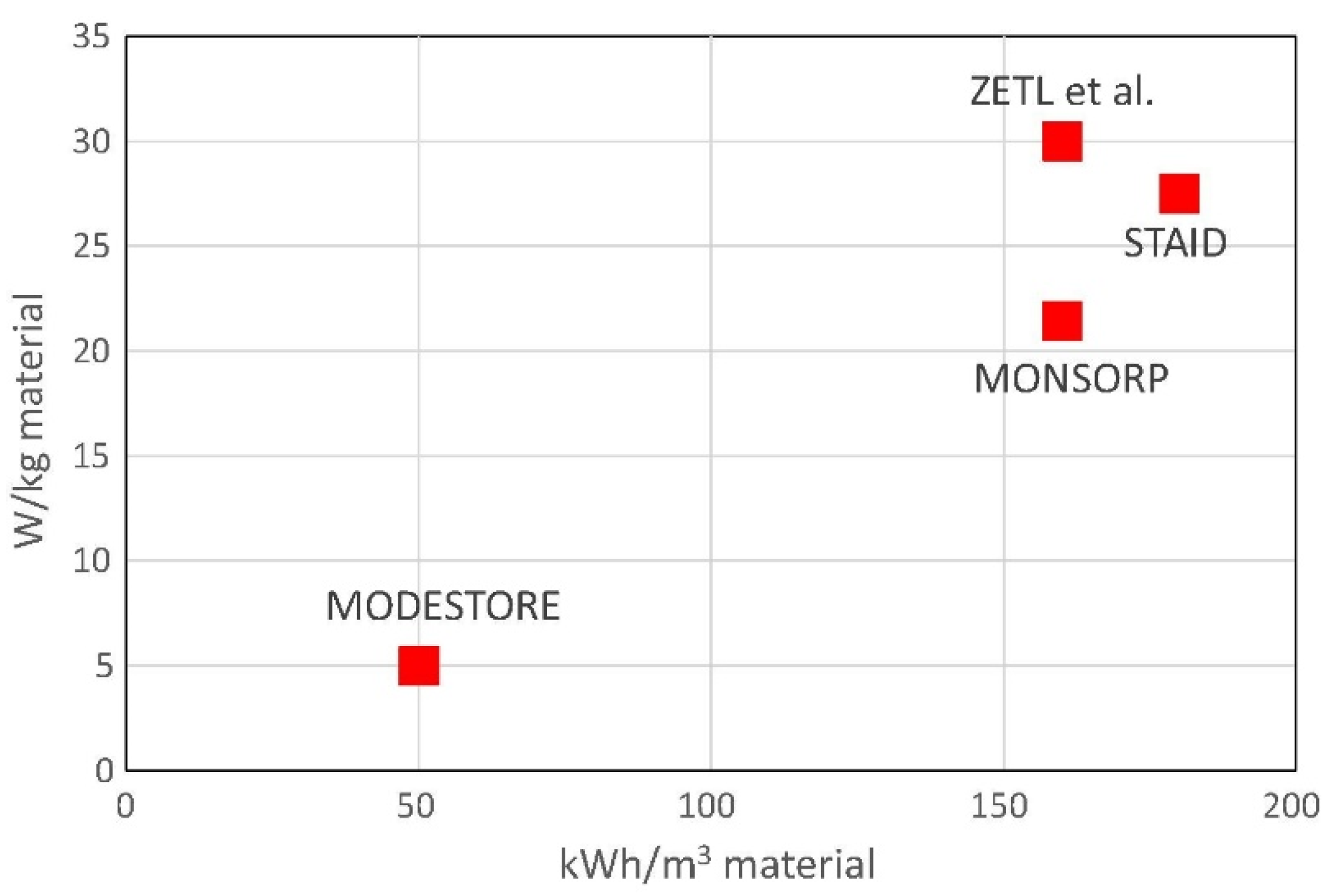
| Setup Name | Year | Inlet Temperature [°C] | Heat Source | Outlet Temperature [°C] | Energy Density [kWh/m3] | Max Power [kW] |
|---|---|---|---|---|---|---|
| Alebeek [16] | 2018 | 180 | Electrical heater | 13 | 54 | 4.4 |
| STAID [18] | 2015 | 120–180 | Electrical heater | 20 | 114 | 2.25 |
| ASIC [19] | 2014 | 230–180 | Electrical heater | 25 | 148 | 1.5 |
| E-HUB/ECN [20] | 2013 | 185 | Oil to air exchanger (Electrical heater) | 25–60 | 58 | 0.4 |
| MONDESTORE [21] | 2008 | 180 | Electrical heater | 25 | 57 | |
| MONOSORP [11] | 2006 | 170 | Electrical heater | 20 | 120 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaei, A.; Zanj, A. A Review on the Challenges of Using Zeolite 13X as Heat Storage Systems for the Residential Sector. Energies 2021, 14, 8062. https://doi.org/10.3390/en14238062
Banaei A, Zanj A. A Review on the Challenges of Using Zeolite 13X as Heat Storage Systems for the Residential Sector. Energies. 2021; 14(23):8062. https://doi.org/10.3390/en14238062
Chicago/Turabian StyleBanaei, Amirhossein, and Amir Zanj. 2021. "A Review on the Challenges of Using Zeolite 13X as Heat Storage Systems for the Residential Sector" Energies 14, no. 23: 8062. https://doi.org/10.3390/en14238062






