Investigating the Effects of Ultrasonic Frequency and Membrane Technology on Biodiesel Production from Chicken Waste
Abstract
:1. Introduction
- 1-
- It has a renewable energy source.
- 2-
- It is nontoxic and decomposable.
- 3-
- It contains less pollutants (no sulfur).
- 4-
- It has lower toxic gas emissions.
- 5-
- It is relatively nonflammable due to its high ignition point [5].
2. Materials, Methods, and Analysis
2.1. Materials and Methods
Materials
2.2. Experimental Setup
- Carrier gas: He, 99/999%
- Constant column flow: 1.0 mL/min
- Injector temperature: 280 °C, split ratio: 1:100
- GC column: Varian, VF-1MS
- Column length: 30 m
- Inside diameter: 0.25 mm
- Film thickness: 0.25 micrometer
2.3. Experimental Protocol
2.3.1. Esterification
2.3.2. Transesterification Reaction
3. Results and Discussions
3.1. Experimental Design and Statistical Analysis
3.2. Effect of Process Variables on Biodiesel Production
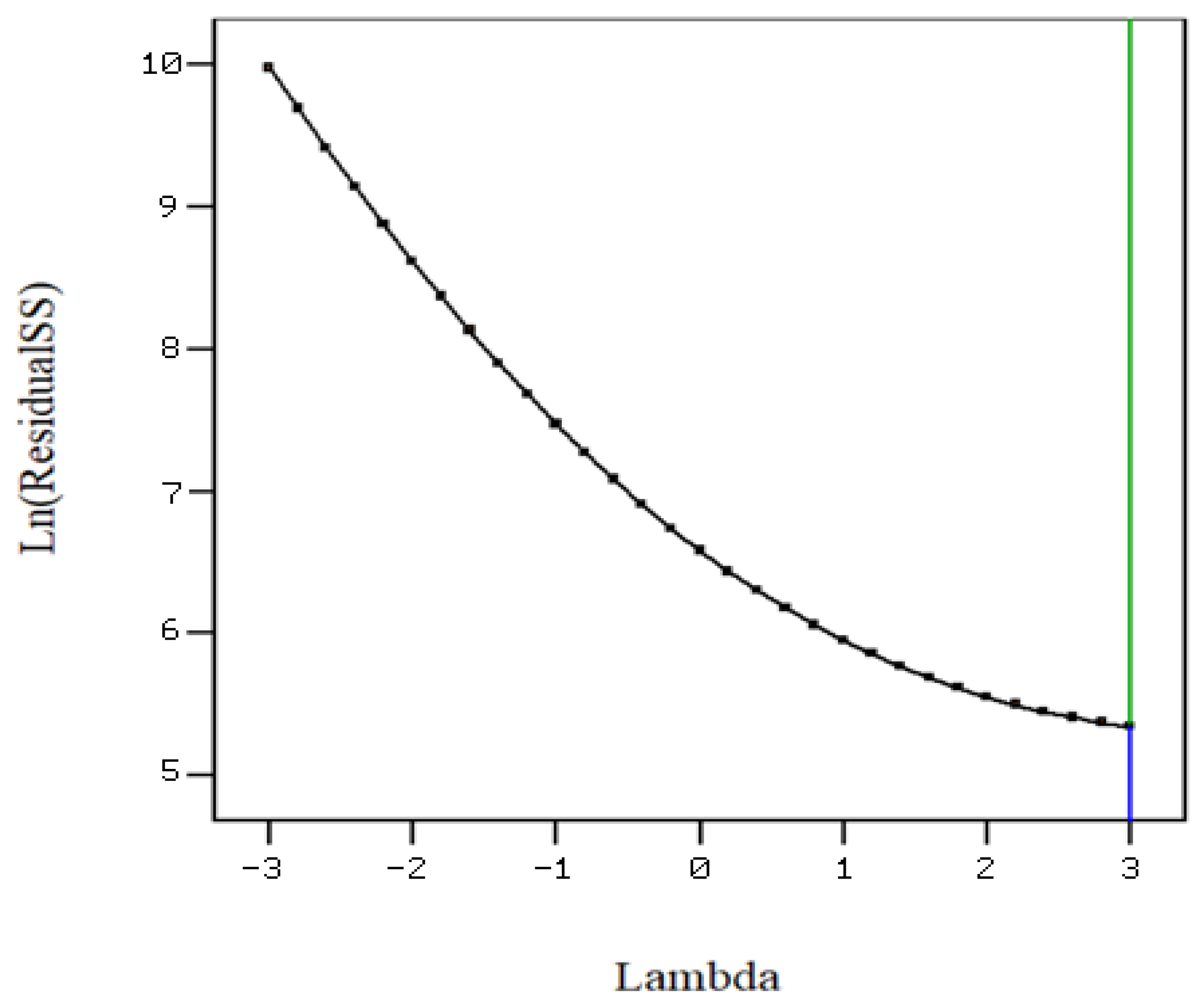
3.3. Optimization
4. Analysis
4.1. FA Contents
4.2. Physicochemical Characterization
5. Modeling of Chicken Feet Biodiesel Production in a Membrane Reactor System
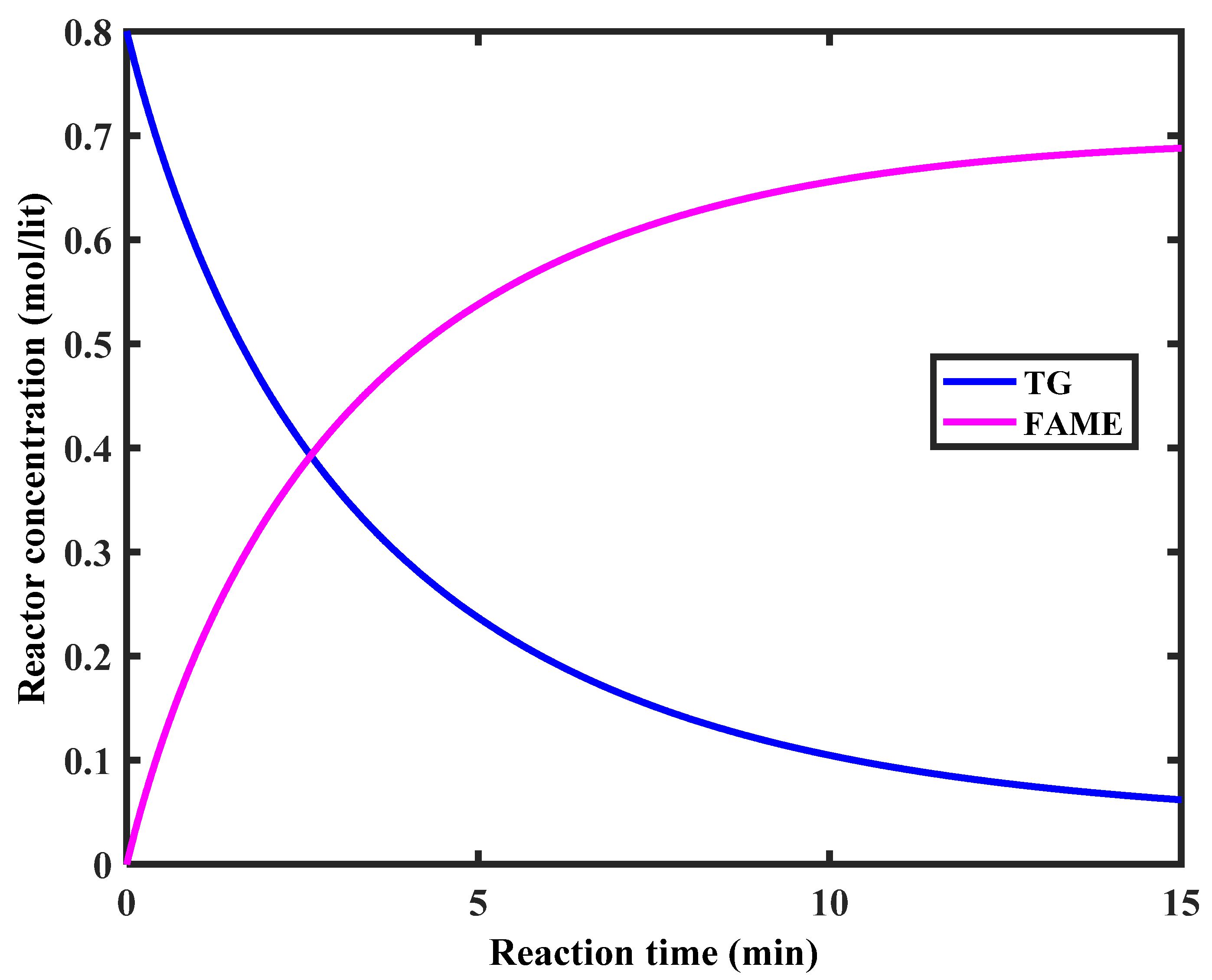
5.1. Determination of the Reaction Kinetics
5.2. Mathematical Modeling of the Membrane Reactor System
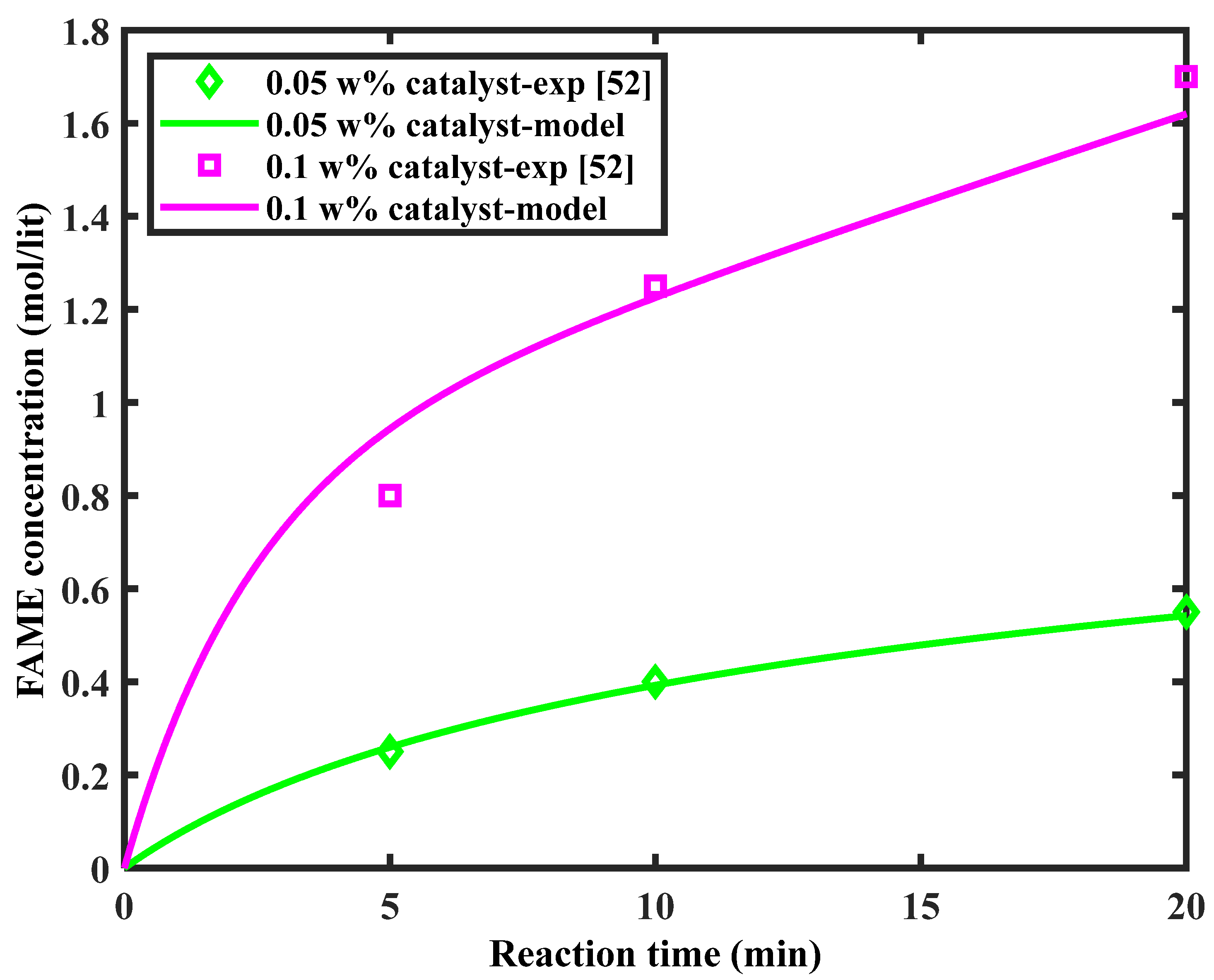
5.2.1. Model Validation
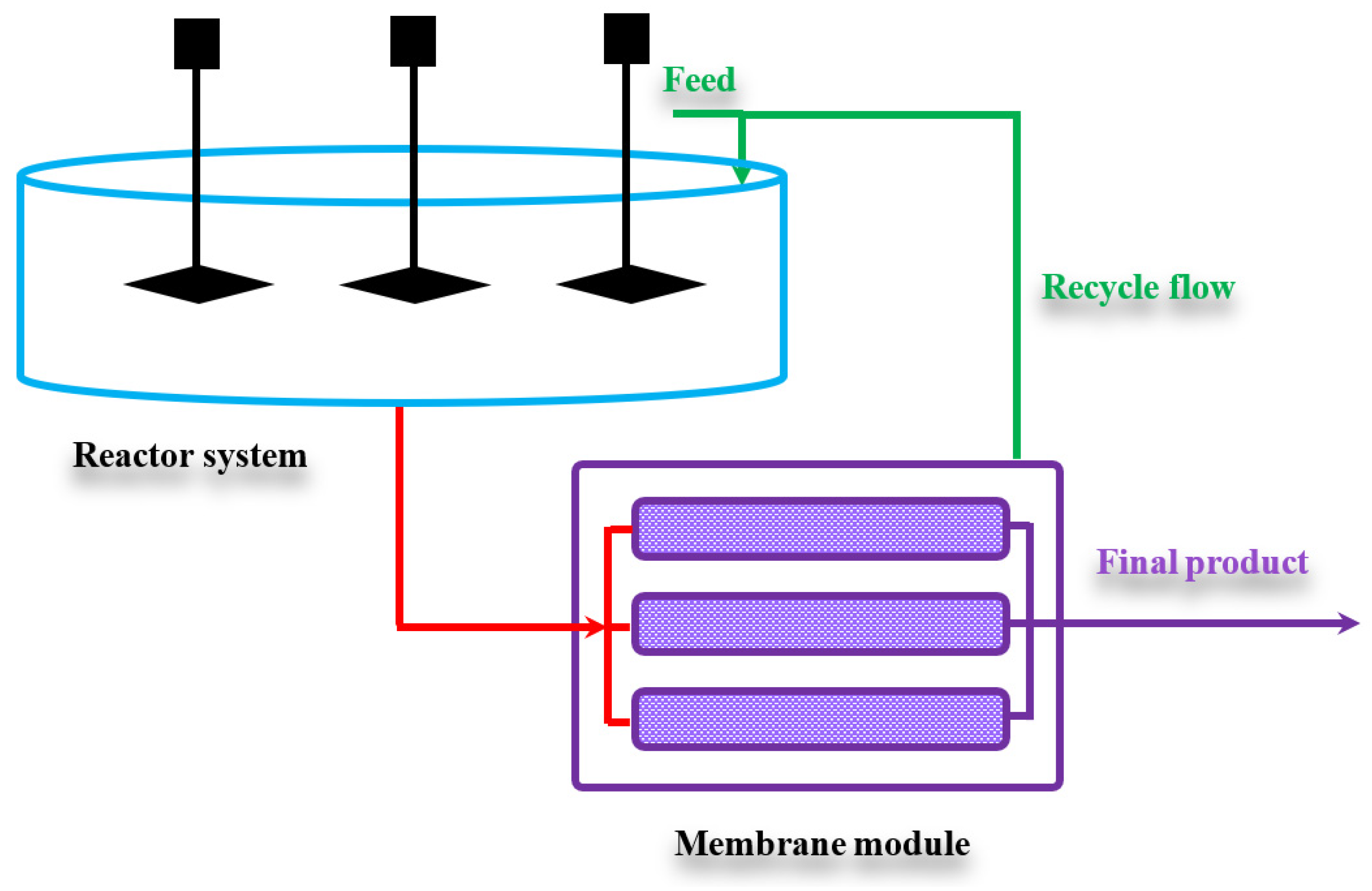
5.2.2. The Proposed Membrane System for Chicken Feet Biodiesel
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ardebili, M.S.; Ghobadian, B.; Najafi, G.; Chegeni, A. Biodiesel production potential from edible oil seeds in Iran. Renew. Sustain. Energy Rev. 2011, 15, 3041–3044. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rahimpour, M.R.; Ghasemi, Y.; Raeissi, S. The Biodiesel of Microalgae as a Solution for Diesel Demand in Iran. Energies 2018, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Bazooyar, B.; Darabkhani, H.G. Design and numerical analysis of a 3 kWe flameless microturbine combustor for hydrogen fuel. Int. J. Hydrogen Energy 2019, 44, 11134–11144. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A. Process and Techno-Economic Analysis for Fuel and Chemical Production by Hydrodeoxygenation of Bio-Oil. Catalysts 2019, 9, 1021. [Google Scholar] [CrossRef] [Green Version]
- Jian-Xun, W.; Huang, Q.D.; Huang, F.H.; Huang, Q.J. Lipase-catalyzed production of biodiesel from high acid value waste oil using ultrasonic assistant. Chin. J. Biotechnol. 2007, 23, 1121–1128. [Google Scholar] [CrossRef]
- Roosta, A.; Bardool, R. A Predictive Correlation for Dynamic Viscosity of Fatty Acid Methyl Esters and Biodiesel. J. Am. Oil Chem. Soc. 2019, 96, 741–750. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Hanif, M.A.; Qasim, M. Biodiesel production from waste tallow. Fuel 2008, 87, 2961–2966. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Optimization of pretreatment reaction for methyl ester production from chicken fat. Fuel 2010, 89, 4035–4039. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Capote, F.P.; de Castro, M.L. Analytical Applications of Ultrasound; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Suslick, K.S. The chemical effects of ultrasound. Sci. Am. 1989, 260, 80–87. Available online: https://www.jstor.org/stable/24987145 (accessed on 1 February 1989). [CrossRef]
- Ghavami, S.; Gregory, A.; Webb, J.; Bayat, M.; Denis, M.; Kumar, V.; Milbrand, T.A.; Larson, A.N.; Fatemi, M.; Alizad, A. Ultrasound Radiation Force for the Assessment of Bone Fracture Healing in Children: An In Vivo Pilot Study. Sensors 2019, 19, 955. [Google Scholar] [CrossRef] [Green Version]
- Tagliapietra, S.; Gaudino, E.C.; Cravotto, G. The use of power ultrasound for organic synthesis in green chemistry. In Power Ultrasonics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 997–1022. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B. Intensified synthesis of biodiesel using hydrodynamic cavitation reactors based on the interesterification of waste cooking oil. Fuel 2014, 137, 285–292. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Conversion of vegetable oil to biodiesel using ultrasonic irradiation. Chem. Lett. 2003, 32, 716–717. [Google Scholar] [CrossRef]
- Bastante, J.S.; Ortega-Román, C.; Pinzi, S.; Lara-Raya, F.R.; Leiva-Candia, D.E.; Dorado, M.P. Ultrasound-Assisted Biodiesel Production from Camelina sativa oil. Bioresour. Technol. 2015, 185, 116–124. [Google Scholar] [CrossRef]
- Van Gerpen, J.; Shanks, B.; Pruzsko, R.; Clements, D.; Knothe, G. Biodiesel Production Technology; National Renewable Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil an economical source for biodiesel a review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Kelkar, M.A.; Gogate, P.R.; Pandit, A.B. Intensification of esterification of acids for synthesis of biodiesel using acoustic and hydrodynamic cavitation. Ultrason. Sonochem. 2008, 15, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.; Assis, J.C.; Mendonça, D.R.; Santos, I.T.; Guimaraes, P.R.; Pontes, L.A.; Teixeira, J.S. Comparison between conventional and ultrasonic preparation of beef tallow biodiesel. Fuel Process. Technol. 2009, 90, 1164–1166. [Google Scholar] [CrossRef]
- Santos, F.F.; Matos, L.J.; Rodrigues, S.; Fernandes, F.A. Optimization of the production of methyl esters from soybean waste oil applying ultrasound technology. Energy Fuels 2009, 23, 4116–4120. [Google Scholar] [CrossRef]
- Santos, F.F.; Malveira, J.Q.; Cruz, M.G.; Fernandes, F.A. Production of biodiesel by ultrasound assisted esterification of Oreochromis niloticus oil. Fuel 2010, 89, 275–279. [Google Scholar] [CrossRef]
- Yin, X.; Duan, X.; You, Q.; Dai, C.; Tan, Z.; Zhu, X. Biodiesel production from soybean oil deodorizer distillate using calcined duck eggshell as catalyst. Energy Convers. Manag. 2016, 112, 199–207. [Google Scholar] [CrossRef]
- Singh, A.; Pal, A.; Maji, S. Biodiesel production from microalgae oil through conventional and ultrasonic methods, Energy Sources, Part A: Recovery. Util. Environ. Eff. 2017, 39, 806–810. [Google Scholar] [CrossRef]
- Suryanto, A.; Sabara, H.Z.; Ismail, H.; Artiningsih, A.; Zainuddin, U.; Almukmin, A.; Nurichsan, U.; Niswah, F. Production Biodiesel from Kapok Seed Oil Using Ultrasonic. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Saint Petersburg, Russia, 2018; pp. 12–23. [Google Scholar]
- Rabu, R.A.; Janajreh, I.; Honnery, D. Transesterification of waste cooking oil: Process optimization and conversion rate evaluation. Energy Convers. Manag. 2013, 65, 764–769. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sindhanaiselvan, S.; Gandhi, N.N.; Devi, S.S.; Renganathan, S. Optimization and kinetic studies on biodiesel production from underutilized ceiba pentandra oil. Fuel 2013, 103, 693–698. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from non-edible oil using sequential combination of microwave and ultrasound. Fuel Process. Technol. 2013, 106, 62–69. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Goswami, P.P.; Malani, R.S.; Moholkar, V.S. Ultrasonic biodiesel synthesis from crude Jatropha curcas oil with heterogeneous base catalyst: Mechanistic insight and statistical optimization. Ultrason. Sonochem. 2014, 21, 1050–1064. [Google Scholar] [CrossRef]
- Canoira, L.; Gamero, M.R.; Querol, E.; Alcantara, R.; Lapuerta, M.; Oliva, F. Biodiesel from low-grade animal fat: Production process assessment and biodiesel properties characterization. Ind. Eng. Chem. Res. 2008, 47, 7997–8004. [Google Scholar] [CrossRef]
- Abid, M.; Touzani, A.; Benhima, R. Synthesis of biodiesel from chicken’s skin waste by homogeneous transesterification. Int. J. Sustain. Eng. 2019, 12, 272–280. [Google Scholar] [CrossRef]
- Fangrui, M.; Milford, A.H. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel; Springer: London, UK, 2008; pp. 111–119. [Google Scholar]
- Othmana, R.; Mohammad, A.W.; Ismail, M.; Salimon, J. Application of polymeric solvent resistant nanofiltration membranes for biodiesel production. J. Membr. Sci. 2010, 348, 287–297. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T.; Subhash, B.; Tan, S.H. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef]
- Sokac, T.; Gojun, M.; Tusek, A.J.; Salic, A.; Zelic, B. Purification of biodiesel produced by lipase catalysed transesterification by ultrafiltration: Selection of membranes and analysis of membrane blocking mechanisms. Renew. Energy 2020, 159, 642–651. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Amiri, Y.Y.; Zeynali, R. Basile. In Current Trends and Future Developments on (Bio-) Membranes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 4; p. 92. [Google Scholar]
- Naderi, A.; Chung, T.S.; Weber, M.; Maletzko, C. High performance dual-layer hollow fiber membrane of sulfonated polyphenylsulfone/Polybenzimidazole for hydrogen purification. Membr. Sci. 2019, 591, 117292. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Barbieri, G. Selective Mass Transport of CO2 Containing Mixtures through Zeolite Membranes. Membr. Sci. Res. 2020, 6, 333–343. [Google Scholar] [CrossRef]
- Gilani, N.; Towfighi, J.; Rashidi, A.; Mohammadi, T.; Omidkhah, M.R.; Sadeghian, A. Investigation of H2S separation from H2S/CH4 mixtures using functionalized and non-functionalized vertically aligned carbon nanotube membranes. Appl. Surf. Sci. 2013, 270, 115–123. [Google Scholar] [CrossRef]
- Chatterjee, G.; Houde, A.A.; Stern, S.A. Poly(ether urethane) and poly(ether urethane urea) membranes with high H2S/CH4 selectivity. Membr. Sci. 1997, 135, 99–106. [Google Scholar] [CrossRef]
- Alamsyaha, R.; Loebisa, E.H. Design and technical testing for crude biodiesel reactor using dry methods: Comparison of energy analysis. Energy Procedia 2014, 47, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Atadashi, I.M. Purification of crude biodiesel using dry washing and membrane technologies. Alex. Eng. J. 2015, 14, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Nascimento, S.M.; Pereira, I.G.; Martins, M.I.; Cardoso, V.L.; Reis, M. Biodiesel purification using micro and ultrafiltration membranes. Renew. Energy 2013, 58, 15–20. [Google Scholar] [CrossRef]
- Noriega, M.A.; Narváez, P.C.; Habert, A.C. Biodiesel separation using ultrafiltration poly(ether sulfone) hollow fiber membranes: Improving biodiesel and glycerol rich phases settling. Chem. Eng. Res. Des. 2018, 138, 32–42. [Google Scholar] [CrossRef]
- Noriega, M.A.; Narváez, P.C.; Habert, A.C. Simulation and validation of biodiesel production in Liquid-Liquid Film Reactors integrated with PES hollow fibers membranes. Fuel 2018, 227, 367–378. [Google Scholar] [CrossRef]
- Talaghat, M.R.; Jokar, S.M.; Modarres, E. Mathematical modeling of methyl ester concentration distribution in a continuous membrane tubular reactor and comparison with conventional tubular reactor. Heat Mass Transf. 2017, 53, 3099–3108. [Google Scholar] [CrossRef]
- Cao, P.; Dube, M.A.; Tremblay, A.Y. Methanol recycling in the production of biodiesel in a membrane reactor. Fuel 2008, 87, 825–833. [Google Scholar] [CrossRef]
- Cao, P.; Dube´, M.A.; Tremblay, A.Y. High-purity fatty acid methyl ester production from canola, soybean, palm, and yellow grease lipids by means of a membrane reactor. Biomass Bioenergy 2008, 32, 1028–1036. [Google Scholar] [CrossRef]
- Cao, P.; Tremblay, A.Y.; Dube, M.A. Kinetics of canola oil transesterification in a membrane reactor. Ind. Eng. Chem. Res. 2009, 48, 2533–2541. [Google Scholar] [CrossRef]
- Arteaga, H.; Siche, R.; Pagador, S.; Cáceres, H. Effect of transesterification temperature and time on yield and calorific value of biodiesel from refined fat of chicken. Sci. Agropecu. 2010, 1, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Getahun, E.; Getahune, N. Experimental investigation and characterization of biodiesel production from leather industry fleshing Wastes. Renew. Sustain. Energy 2013, 2, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Zauzi, N.S.A. Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew. Energy 2017, 114, 437–447. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 52, pp. 218–286. [Google Scholar]
- Rana-Madaria, P.; Nagarajan, M.; Rajapagol, C.; Garg, B.S. Removal of chromium from aqueous solutions by treatment with carbon aerogel electrodes using response surface methodology. Ind. Eng. Chem. Res. 2005, 44, 6549–6559. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Deng, S.; Cooke, P.; Munson-McGee, S.; Rhodes, I.; Lammers, P.; Nirmalakhandan, N. Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour. Technol. 2011, 102, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.E.; Gude, V.G. Kinetics of ultrasonic transesterification of waste cooking oil. Sustain. Energy 2014, 33, 1051–1058. [Google Scholar] [CrossRef]









| Chicken Feet Oil | |
|---|---|
| Acid Value | 3 mg KOH/g |
| FFA | 1.5% |
| Boiling Point | >220 °C |
| Variable | Symbol | Coded Factor Level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Methanol-to-oil molar ratio | A | 3 | 6 | 9 | 12 | 15 |
| Catalyst amount (wt%) | B | 0.5 | 1 | 1.5 | 2 | 2.5 |
| Frequency (kHz) | C | 25 | 45 | |||
| Factor 1 | Factor 2 | Factor 3 | Biodiesel Yield | Biodiesel Yield |
|---|---|---|---|---|
| A: Methanol: Oil | B: Catalyst (wt%) | C: Frequency (kHz) | Actual % | Predicted % |
| 9:01 | 0.5 | 25 | 24.99 | 33.22 |
| 6:01 | 2 | 25 | 69.99 | 72.5 |
| 6:01 | 2 | 45 | 71.72 | 69.16 |
| 9:01 | 2.5 | 25 | 63.77 | 62.14 |
| 15:01 | 1.5 | 45 | 64.27 | 59.13 |
| 6:01 | 1 | 25 | 83.92 | 83.33 |
| 3:01 | 1.5 | 45 | 46.43 | 55.3 |
| 15:01 | 1.5 | 25 | 59.56 | 64.64 |
| 3:01 | 1.5 | 25 | 67.06 | 61.49 |
| 9:01 | 2.5 | 45 | 70.86 | 72.13 |
| 9:01 | 0.5 | 45 | 78.6 | 77.45 |
| 12:01 | 1 | 45 | 88.52 | 89.84 |
| 12:01 | 2 | 25 | 74.33 | 74.28 |
| 9:01 | 1.5 | 25 | 80.16 | 80.49 |
| 12:01 | 1 | 25 | 87.09 | 85.69 |
| 9:01 | 1.5 | 45 | 71.63 | 77.08 |
| 6:01 | 1 | 45 | 87.17 | 87.71 |
| 9:01 | 1.5 | 45 | 82.6 | 77.08 |
| 12:01 | 2 | 45 | 71.06 | 71.11 |
| 9:01 | 1.5 | 25 | 80.03 | 80.49 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 6.065 × 1011 | 11 | 5.514 × 1010 | 10.32 | 0.0014 | Significant |
| A: M: O | 1.41 × 109 | 1 | 1.41 × 109 | 0.26 | 0.6213 | |
| B: Catalyst.C | 3.245 × 109 | 1 | 3.245 × 109 | 0.61 | 0.4582 | |
| C: Frequency | 1.205 × 1010 | 1 | 1.205 × 1010 | 2.26 | 0.1715 | |
| AB | 231,800,000 | 1 | 231,800,000 | 0.043 | 0.8402 | |
| BC | 3.207 × 1010 | 1 | 3.207 × 1010 | 6 | 0.0399 | |
| A2 | 1.459 × 1011 | 1 | 1.459 × 1011 | 27.31 | 0.0008 | |
| B2 | 8.871 × 1010 | 1 | 8.871 × 1010 | 16.61 | 0.0036 | |
| A2B | 1.282 × 1011 | 1 | 1.282 × 1011 | 24 | 0.0012 | |
| AB2 | 5.799 × 108 | 1 | 5.799 × 108 | 0.11 | 0.7502 | |
| B2C | 8.034 × 1010 | 1 | 8.034 × 1010 | 15.04 | 0.0047 | |
| A2B2 | 9.164 × 1010 | 1 | 9.164 × 1010 | 17.16 | 0.0032 | |
| Residual | 4.273 × 1010 | 8 | 5.342 × 109 | |||
| Lack of Fit | 2.351 × 1010 | 6 | 3.919 × 109 | 0.41 | 0.8334 | Not Significant |
| Pure Error | 1.922 × 1010 | 2 | 9.609 × 109 | |||
| Cor Total | 6.493 × 1011 | 19 | ||||
| Std.Dev | 73,086.43 | R2 | 0.9342 | |||
| Mean | 402,100 | Adjusted R2 | 0.8437 |
| Retention Time (min) | Identified Compounds (Methyl + Acid) | % | Type of FA |
|---|---|---|---|
| 14.11 | Tetradecanoic acid | 1.13 | Saturated |
| 15.16 | Pentadecanoic acid | 0.13 | Saturated |
| 15.83 | 7,10-Hexadecadienoic acid | 0.32 | Unsaturated |
| 15.99 | 9-Hexadecenoic acid (Palmitoleic acid) | 16.45 | Unsaturated |
| 16.21 | Hexadecanoic acid (Palmitic acid) | 19.18 | Saturated |
| 16.9 | 9-Octadecenoic acid (z) (Oleic acid) | 0.36 | Unsaturated |
| 17.14 | Heptadecanoic acid (Margaric acid) | 0.2 | Saturated |
| 17.6 | 6,9,12-Octadecatrienoic acid (Linolenic acid) | 0.65 | Unsaturated |
| 17.86 | 9-Octadecenoic acid (Elaidic acid) | 47.98 | Unsaturated |
| 18.09 | Octadecanoic acid (Stearic acid) | 8.14 | Saturated |
| 18.17 | 9,12-Octadecadienoic acid (Linolelaidic acid) | 0.28 | Unsaturated |
| 19.19 | 5,8,11,14-Eicosatetraenoic acid | 1.28 | Unsaturated |
| 19.25 | 5,8,11-Eicosatrienoic acid | 0.3 | Unsaturated |
| 19.34 | 7,10,13-Eicosatrienoic acid | 1.04 | Unsaturated |
| 19.55 | 11-Eicosenoic acid | 1.89 | Unsaturated |
| 19.77 | Eicosanoic acid (Arachidic acid) | 0.13 | Saturated |
| 20.8 | Methyl Arachidonate | 0.33 | Unsaturated |
| 20.85 | Cyclooctene,3-ethenyl | 0.22 | Saturated |
| Property | Unit | Method Used (ASTM…) | EN 14214 [44] | ASTM D6751-10 [43] | ASTM 6751-02 [4,45] | Diesel Fuel ASTM D975 | Biodiesel (This Study) |
|---|---|---|---|---|---|---|---|
| Density (@ 15 °C) | g/cm3 | D4052-91 | 0.86–0.90 | - | 0.87–0.9 | 0.85 | 0.872 |
| Acid value | mg KOH/g | - | 0.5 max | 0.5 max | - | 0.5 | 0.56 |
| Saponification value | mg/kg | - | - | - | - | - | 190.74 |
| Iodine value | gI2/100 g | - | 120 max | - | - | 38.3 | 40.6 |
| Cetane number | - | D613 | 51 min | 46–70 | 47 min | 40–55 | 65.78 |
| Flash point | °C | D92-85 | 120 min | 130 min | 130 min | 52 min | 164 |
| Fire point | °C | D92-85 | - | - | - | - | 184 |
| Cloud point | °C | D2500-02 | - | - | −3 to 12 | - | 5 |
| Kinematic viscosity (@ 40 °C) | mm2/s | D92-85 | 3.5–5.0 | 1.9–6.0 | 1.9–6 | - | 4.5 |
| Parameter | Value |
|---|---|
| α (L/mol·min·kHz) | 9.500 × 10−5 |
| β (L/mol·min) | 3.263 × 10−2 |
| γ | 1 |
| δ | 1 |
| Parameter | Value |
|---|---|
| Initial concentration of TG (mole/L) | 0.8 |
| Initial concentration of methanol (mole/L) | 9.84 |
| Reactor volume (L) | 80 |
| Reaction temperature (°C) | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, S.F.M.; Parvasi, P.; Jokar, S.M.; Basile, A. Investigating the Effects of Ultrasonic Frequency and Membrane Technology on Biodiesel Production from Chicken Waste. Energies 2021, 14, 2133. https://doi.org/10.3390/en14082133
Haghighi SFM, Parvasi P, Jokar SM, Basile A. Investigating the Effects of Ultrasonic Frequency and Membrane Technology on Biodiesel Production from Chicken Waste. Energies. 2021; 14(8):2133. https://doi.org/10.3390/en14082133
Chicago/Turabian StyleHaghighi, Seyyedeh Faezeh Mirab, Payam Parvasi, Seyyed Mohammad Jokar, and Angelo Basile. 2021. "Investigating the Effects of Ultrasonic Frequency and Membrane Technology on Biodiesel Production from Chicken Waste" Energies 14, no. 8: 2133. https://doi.org/10.3390/en14082133





