Characterization and Impact of Waste Plastic Oil in a Variable Compression Ratio Diesel Engine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fuels
2.2. Gas Chromatography Analysis
2.3. Experimental Setup and Procedure
3. Results and Discussion
3.1. Test Fuels
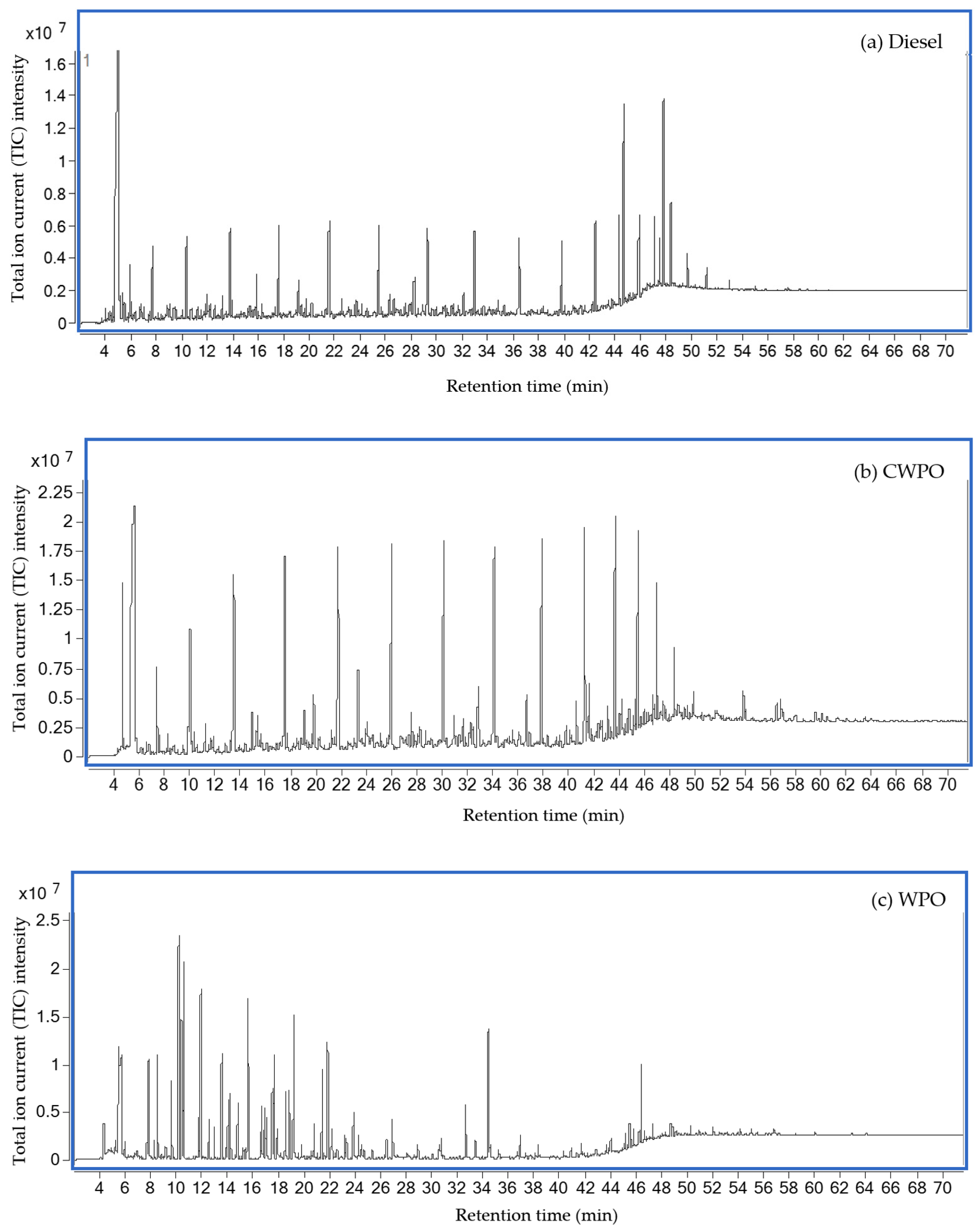
3.1.1. Chemical Compositions
3.1.2. Chemical and Physical Properties of the Test Fuels
3.2. Engine Tests
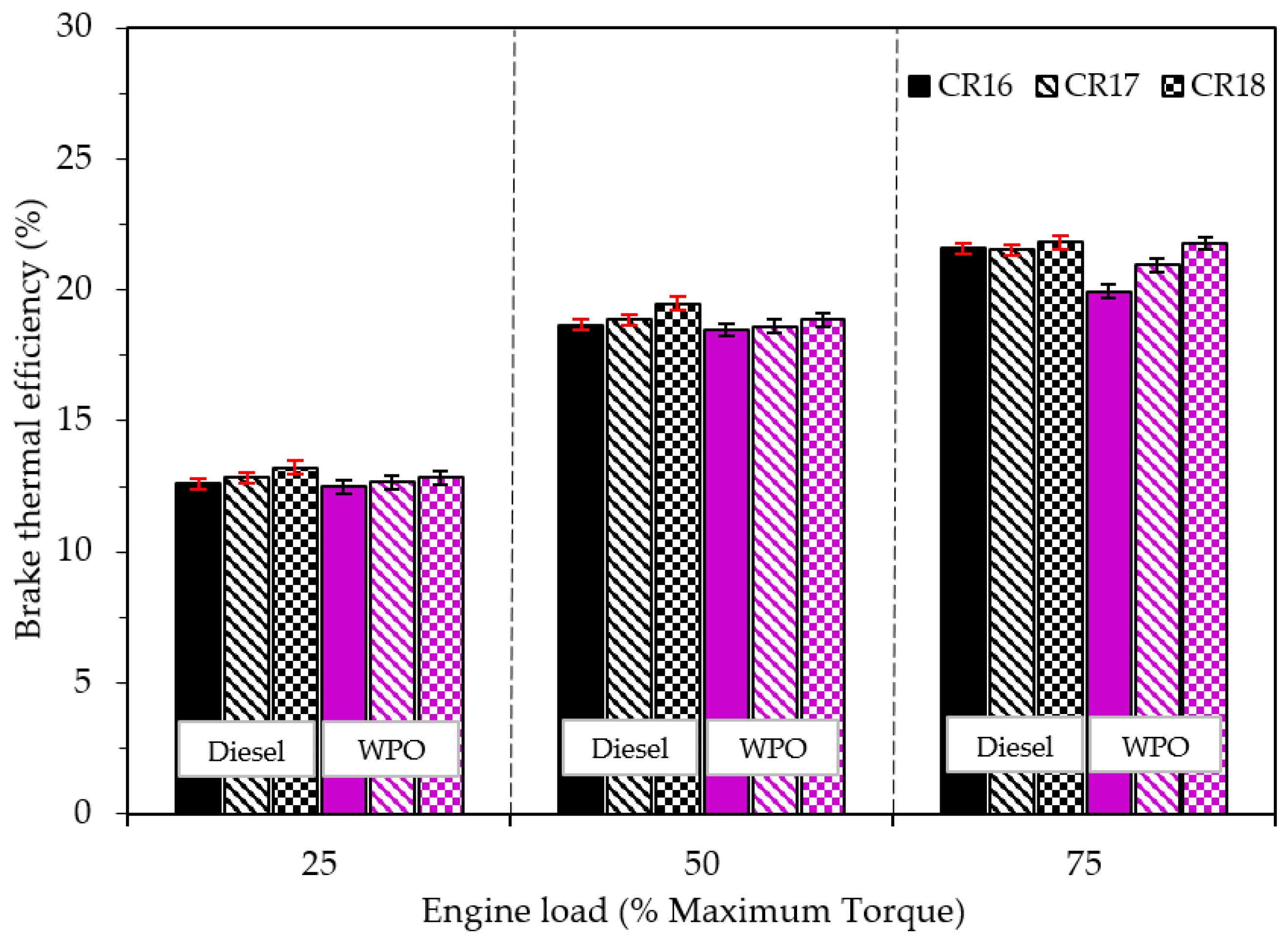
3.2.1. Engine Performance
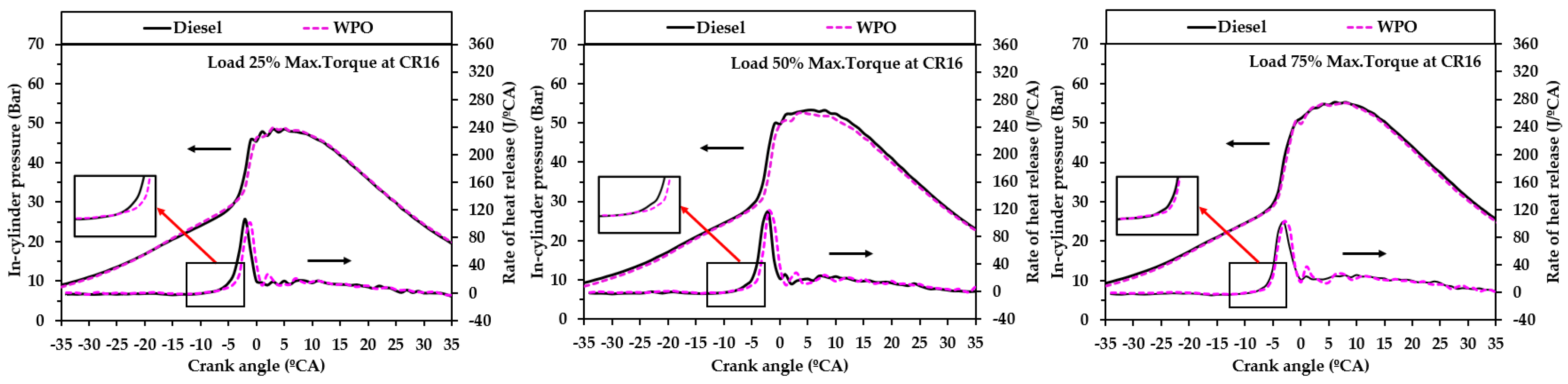
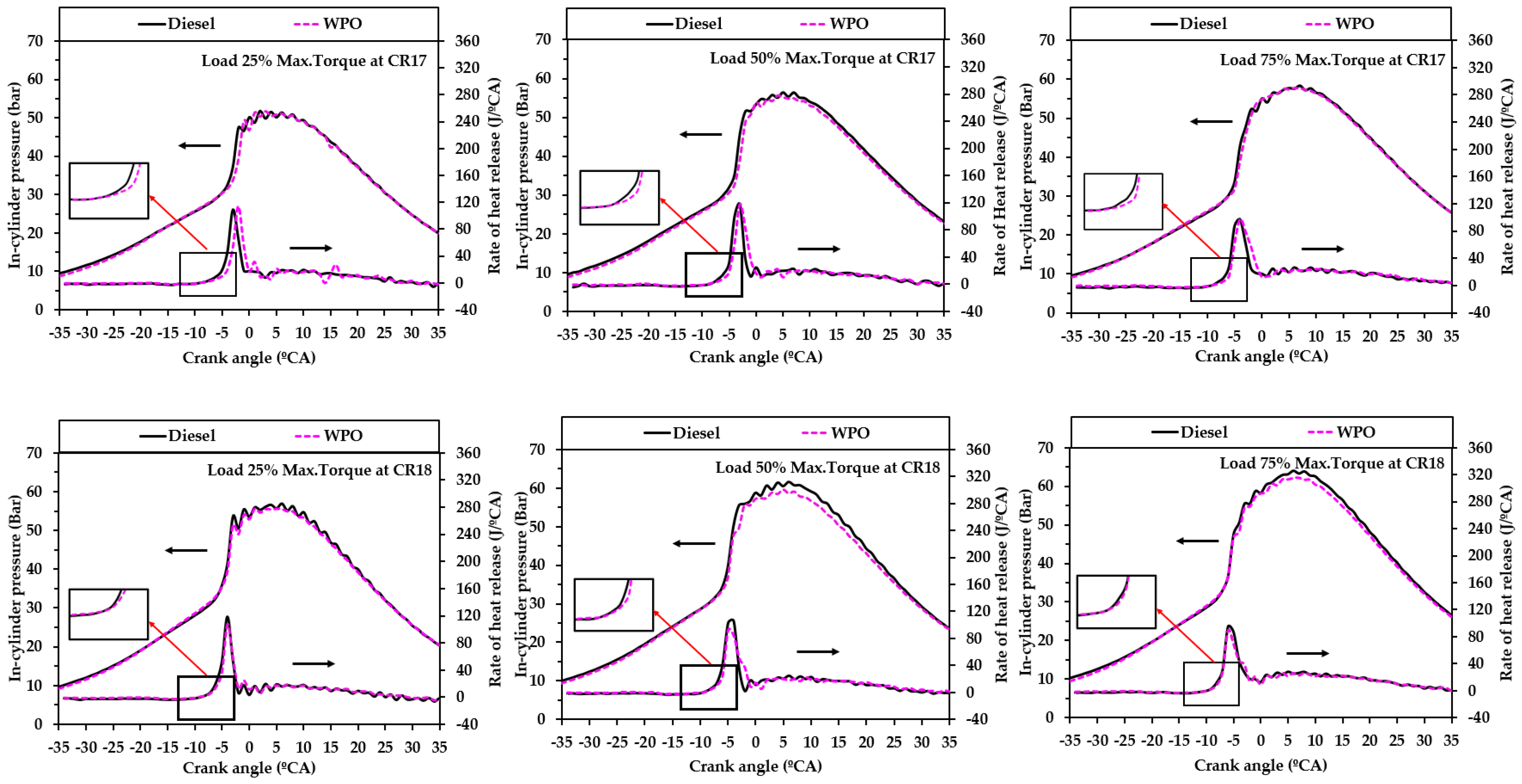
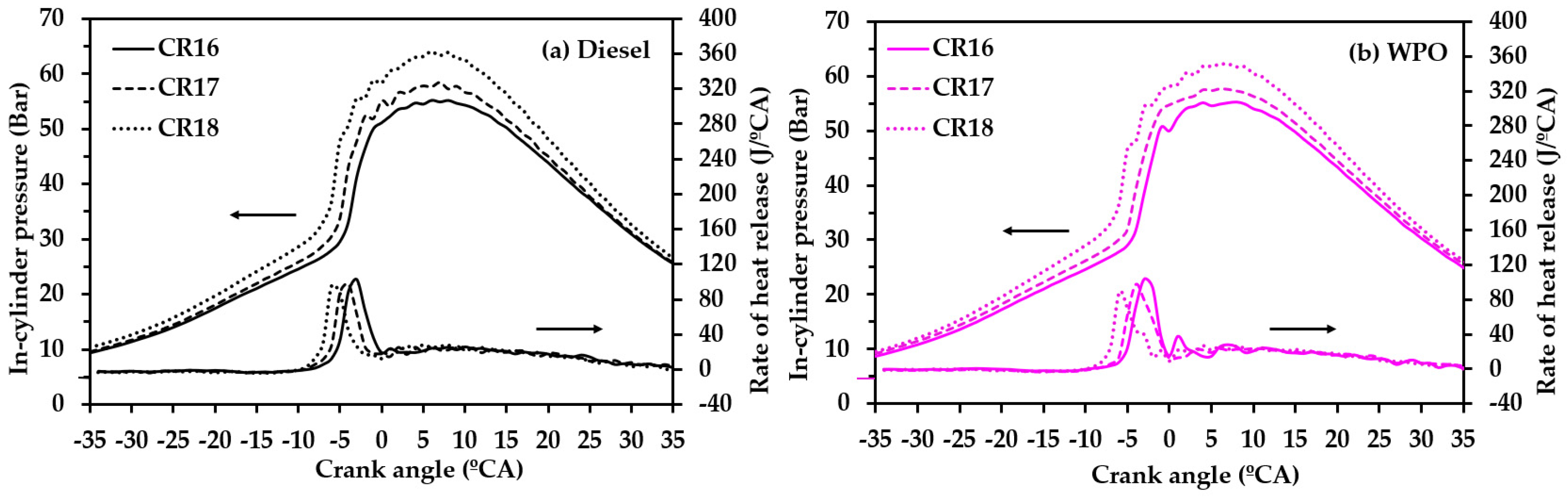
3.2.2. Combustion Characteristics
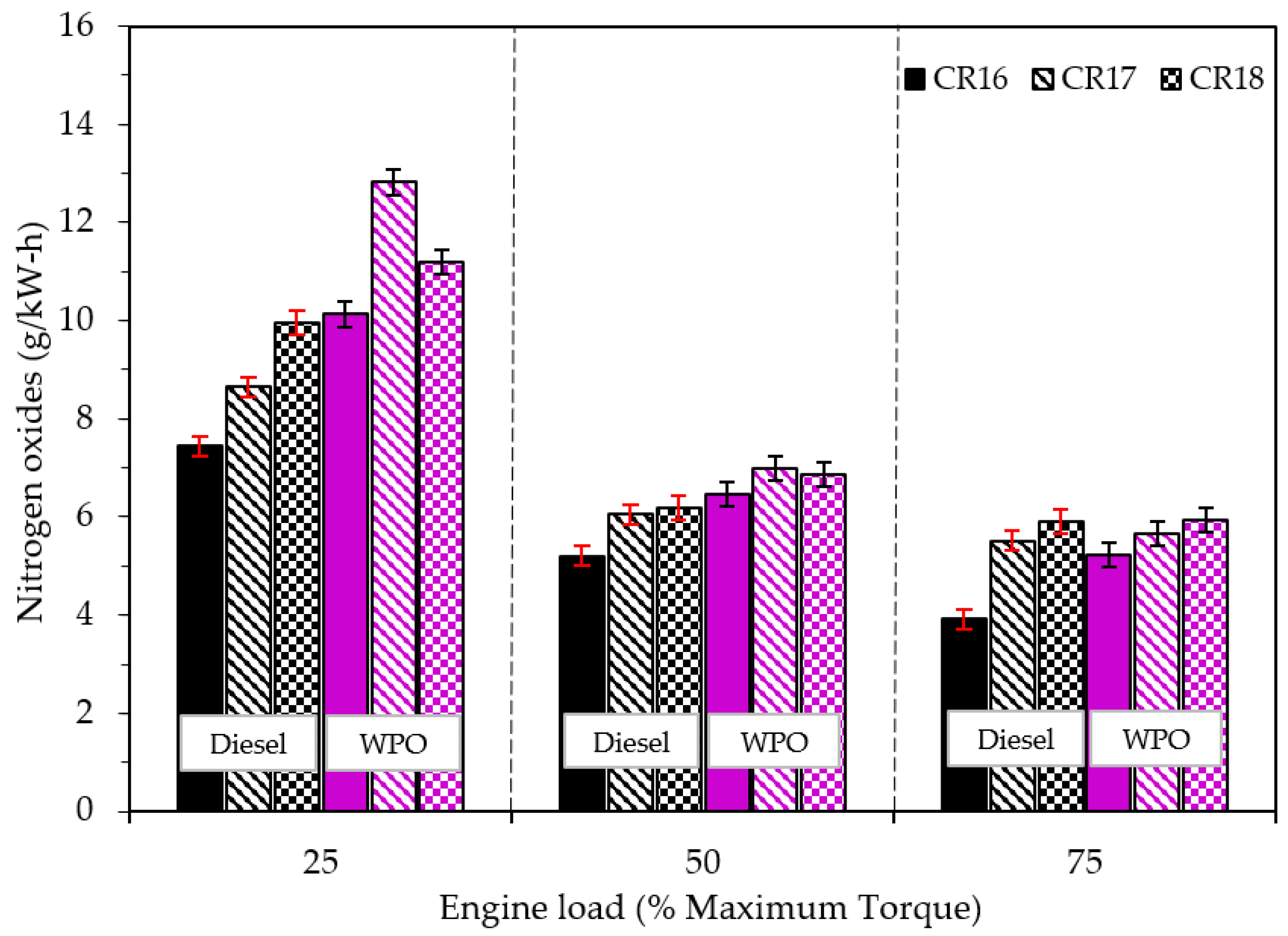
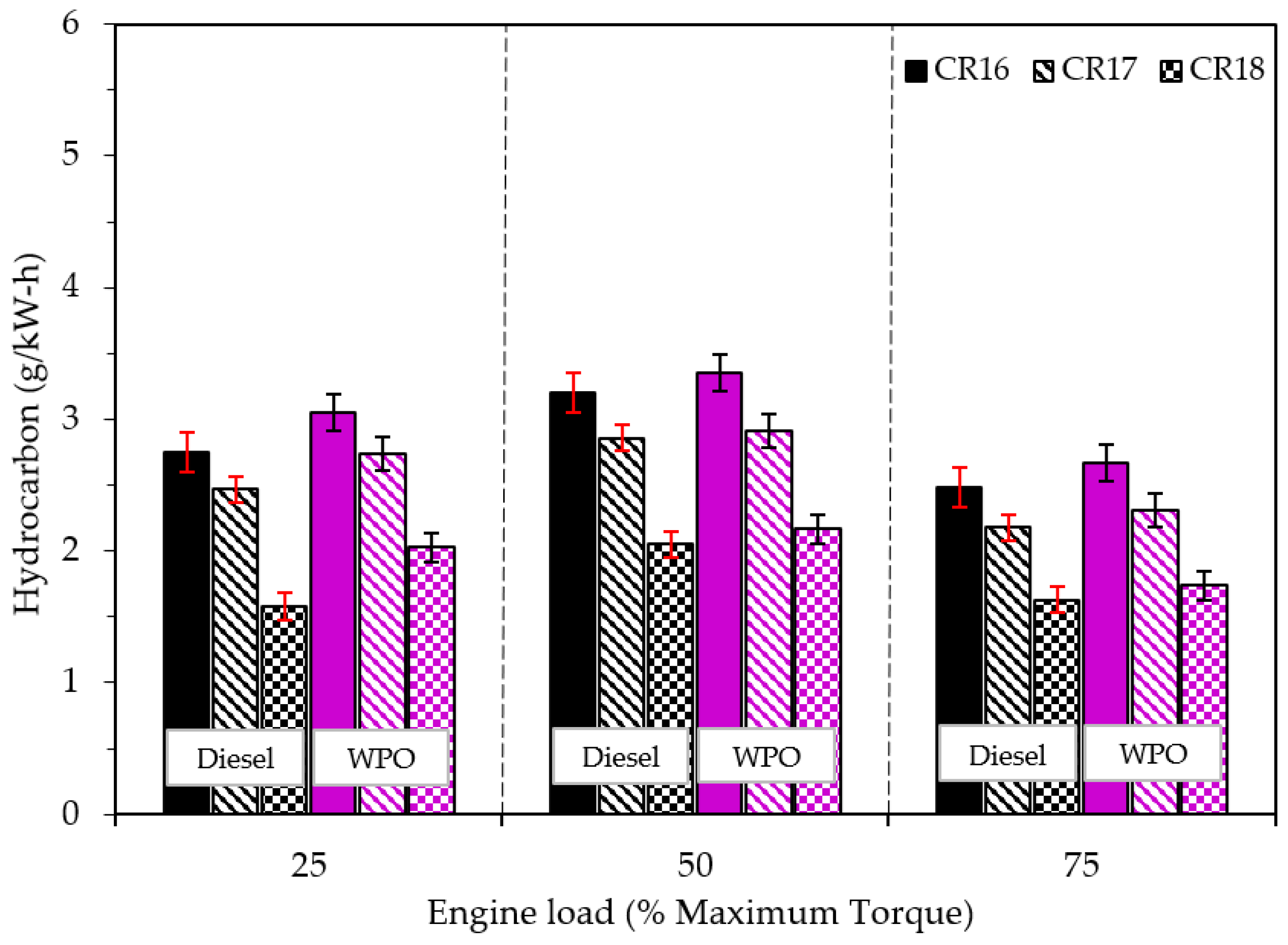
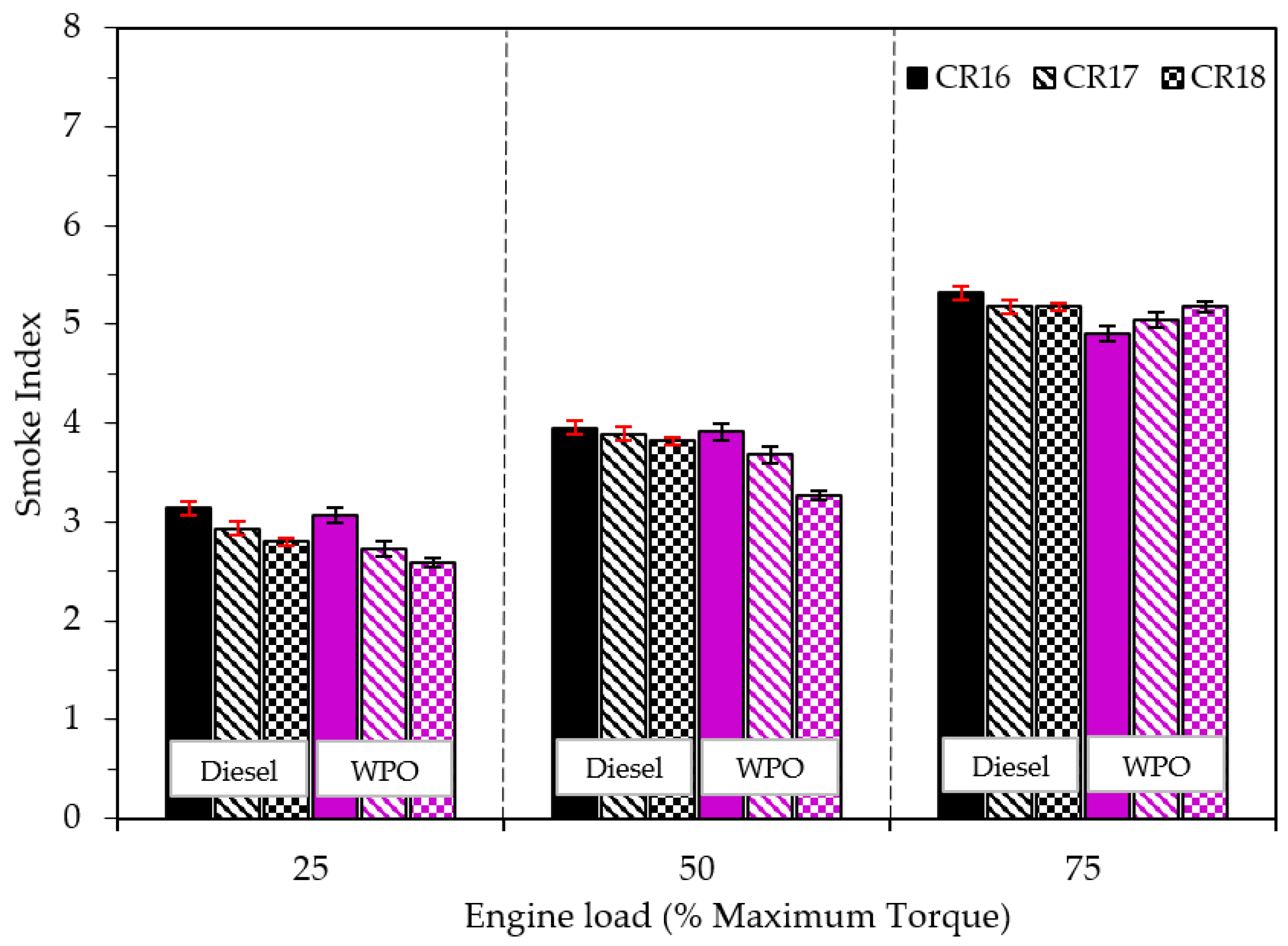
3.2.3. Emission Characteristics
4. Conclusions
- A lower cetane index and a higher percentage of long chain carbon compounds (C12–C20) resulted in higher NOx, CO, and HC emissions caused by the combustion of WPO.
- The disadvantage of emissions by the use of WPO can be alleviated when the engine operates at a high engine operating load and a high compression ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BSFC | brake-specific fuel consumption |
| BTE | brake thermal efficiency |
| CO | carbon monoxide |
| CWPO | crude waste plastic oil |
| CR | compression ratio |
| GC–MS | gas chromatography–mass spectrometry |
| HC | hydrocarbon |
| ICP | in-cylinder pressure |
| NO | nitric oxide |
| NO2 | nitrogen dioxide |
| NOX | nitrogen oxides |
| RoHR | rate of heat release |
| TDC | top dead center |
| VCR | variable compression ratio |
| WPO | distilled waste plastic oil |
References
- Suthiwong, R. Renewable Energy policy. In NDC Security Review; Thailand National Defence College: Bangkok, Thailand, 2017; Volume 1, p. 12. [Google Scholar]
- Alternative Energy and Efficiency Information Center, Department of Alternative Energy Development and Efficiency, Ministry of energy. Thailand Alternative Energy Situation 2017; Department of Alternative Energy Development and Efficiency, Ministry of energy: Bangkok, Thailand, 2017; Volume 15, ISSN 1686-5170.
- Energy Policy and Planning Office, Ministry of Energy, Thailand. Thailand Integrated Energy Blueprint 2015–2036. 2015. Available online: http://www.eppo.go/index.php/th/plan-policy/tieb (accessed on 10 December 2020).
- Pollution Control Department. Municipal Solid Waste Situation of Thailand Report 2018; PCD—Pollution Control Department, Ministry of Natural Resources and Environment: Bangkok, Thailand, 2018. Available online: http://infofile.pcd.go.th/Waste/Wst2018.pdf (accessed on 9 November 2020).
- Pollution Control Department. Draft Integrated Plastic Waste Management Plan (2017–2021). 2017. Available online: http://infofile.pcd.go.th/law/DraftWastePlan60-64.pdf?CFID=1835558&CFTOKEN=98563117 (accessed on 23 November 2020).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Al-Salem, S.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Traivivatana, S.; Wangjiraniran, W. Thailand Integrated Energy Blueprint (TIEB): One Step towards Sustainable Energy Sector. Energy Procedia 2019, 157, 492–497. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P.; Tigga, V.P. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- Phetyim, N.; Pivsa-Art, S. Prototype Co-Pyrolysis of Used Lubricant Oil and Mixed Plastic Waste to Produce a Diesel-Like Fuel. Energies 2018, 11, 2973. [Google Scholar] [CrossRef] [Green Version]
- Khairil; Riayatsyah, T.M.I.; Bahri, S.; Sofyan, S.E.; Jalaluddin, J.; Kusumo, F.; Silitonga, A.S.; Padli, Y.; Jihad, M.; Shamsuddin, A.H. Experimental Study on the Performance of an SI Engine Fueled by Waste Plastic Pyrolysis Oil–Gasoline Blends. Energies 2020, 13, 4196. [Google Scholar] [CrossRef]
- Pratama, N.N.; Saptoadi, H. Characteristics of Waste Plastics Pyrolytic Oil and Its Applications as Alternative Fuel on Four Cylinder Diesel Engines. Int. J. Renew. Energy Dev. 2014, 3, 13–20. [Google Scholar]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Venkatesan, H.; Sivamani, S.; Bhutoria, K.; Vora, H.H. Experimental study on combustion and performance characteristics in a DI CI engine fuelled with blends of waste plastic oil. Alex. Eng. J. 2018, 57, 2257–2263. [Google Scholar] [CrossRef]
- Devaraj, J.; Robinson, Y.; Ganapathi, P. Experimental investigation of performance, emission and combustion characteristics of waste plastic pyrolysis oil blended with diethyl ether used as fuel for diesel engine. Energy 2015, 85, 304–309. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Experimental evaluation of a diesel engine fuelled by pyrolysis oils produced from low-density polyethylene and ethylene–vinyl acetate plastics. Fuel Process. Technol. 2017, 161, 125–131. [Google Scholar] [CrossRef]
- Sukjit, E.; Liplap, P.; Maithomklang, S.; Arjharn, W. Experimental Investigation on a DI Diesel Engine Using Waste Plastic Oil Blended with Oxygenated Fuels. SAE Tech. Pap. 2017, 1. [Google Scholar] [CrossRef]
- Mani, M.; Nagarajan, G.; Sampath, S. Characterisation and effect of using waste plastic oil and diesel fuel blends in compression ignition engine. Energy 2011, 36, 212–219. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Narayanan, S.; Ramesh, A. Combustion and emission analysis of hydrogenated waste polypropylene pyrolysis oil blended with diesel. J. Hazard. Mater. 2020, 386, 121453. [Google Scholar] [CrossRef]
- Das, A.K.; Hansdah, D.; Mohapatra, A.K.; Panda, A.K. Energy, Exergy and Emission Analysis on a DI Single Cylinder Diesel engine using pyrolytic waste plastic oil diesel blend. J. Energy Inst. 2020, 93, 1624–1633. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Ramesh, A. Experimental investigation to identify the type of waste plastic pyrolysis oil suitable for conversion to diesel engine fuel. J. Clean. Prod. 2020, 246, 119066. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Convers. Manag. 2015, 105, 951–956. [Google Scholar] [CrossRef]
- Rinaldini, C.A.; Mattarelli, E.; Savioli, T.; Cantore, G.; Garbero, M.; Bologna, A. Performance, emission and combustion characteristics of a IDI engine running on waste plastic oil. Fuel 2016, 183, 292–303. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sankaranarayanan, G. Investigation on environmental factors of waste plastics into oil and its emulsion to control the emission in DI diesel engine. Ecotoxicol. Environ. Saf. 2016, 134, 440–444. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Kaimal, V.K.; Vijayabalan, P. A detailed investigation of the combustion characteristics of a DI diesel engine fuelled with plastic oil and rice bran methyl ester. J. Energy Inst. 2017, 90, 324–330. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of waste plastic oil-biodiesel blends as alternative fuels for diesel engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Damodharan, D.; Sathiyagnanam, A.P.; Rana, D.; Kumar, B.R.; Saravanan, S. Extraction and characterization of waste plastic oil (WPO) with the effect of n-butanol addition on the performance and emissions of a DI diesel engine fueled with WPO/diesel blends. Energy Convers. Manag. 2017, 131, 117–126. [Google Scholar] [CrossRef]
- Lobato, J.; Rodríguez, J.F.; Jiménez, C.; Llanos, J.; Nieto-Márquez, A.; Inarejos, A.M. Consequence analysis of an explosion by simple models: Texas refinery gasoline explosion case. Afinidad 2009, 66, 372–379. [Google Scholar]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Jayabal, S.; Thirumal, P. Investigation on performance, emission and combustion characteristics of variable compression engine fuelled with diesel, waste plastics oil blends. J. Braz. Soc. Mech. Sci. Eng. 2016, 39, 19–28. [Google Scholar] [CrossRef]
- Verma, A.; Raghuvansi, A.; Quraishi, M.A.; Tirkey, J.V.; Verma, C. Engine Fuel Production from Waste Plastic Pyrolysis (WPO) and Performance Evaluation in a CI engine with Diesel Blend. J. Mater. Environ. Sci. 2018, 9, 1712–1721. [Google Scholar]
- Bridjesh, P.; Geetha, N.K. Effect of Diethyl Carbonate as Additive to Waste Plastic Oil on Performance and Emission of a Diesel Engine. Orient. J. Chem. 2020, 36, 189–194. [Google Scholar] [CrossRef]
- Rao, B.G.; Bharadwaz, Y.D.; Virajitha, C.; Rao, V.D. Effect of injection parameters on the performance and emission characteristics of a variable compression ratio diesel engine with plastic oil blends–An experimental study. Energy Environ. 2018, 29, 492–510. [Google Scholar]
- Syamsiro, M.; Saptoadi, H.; Kismurtono, M.; Mufrodi, Z.; Yoshikawa, K. Utilization of waste polyethylene pyrolysis oil as partial substitute for diesel fuel in a DI diesel engine. Int. J. Smart Grid Clean Energy 2019, 8, 38–47. [Google Scholar] [CrossRef]
- Hirkude, J.; Padalkar, A.S. Experimental investigation of the effect of compression ratio on performance and emissions of CI engine operated with waste fried oil methyl ester blend. Fuel Process. Technol. 2014, 128, 367–375. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988; p. 508. [Google Scholar]
- Uyumaz, A.; Aydoğan, B.; Solmaz, H.; Yılmaz, E.; Hopa, D.Y.; Bahtli, T.A.; Solmaz, Ö.; Aksoy, F. Production of waste tyre oil and experimental investigation on combustion, engine performance and exhaust emissions. J. Energy Inst. 2019, 92, 1406–1418. [Google Scholar] [CrossRef]
- Kobori, S.; Kamimoto, T.; Aradi, A.A. A study of ignition delay of diesel fuel sprays. Int. J. Engine Res. 2000, 1, 29–39. [Google Scholar] [CrossRef]
- Vasudeva, M.; Sharma, S.; Mohapatra, S.K.; Kundu, K. Performance and exhaust emission characteristics of variable compression ratio diesel engine fuelled with esters of crude rice bran oil. SpringerPlus 2016, 5, 293. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, S.; Rajendran, S. Assessment of performance, combustion, and emission behavior of novel annona biodiesel-operated diesel engine. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Cambridge, UK, 2019; pp. 391–405. [Google Scholar]
- Saravanan, S.; Nagarajan, G.; Sampath, S. A Correlation for the Ignition Delay of a CI Engine Fuelled with Diesel and Biodiesel. Int. J. Green Energy 2014, 11, 542–557. [Google Scholar] [CrossRef]
- Majewski, W.A.; Khair, M.K. Diesel Emissions and Their Control; Society of Automotive Engineers: Wallendale, PA, USA, 2006; pp. 109–110. [Google Scholar]
- Panda, A.K.; Murugan, S.; Singh, R.K. Performance and emission characteristics of diesel fuel produced from waste plastic oil obtained by catalytic pyrolysis of waste polypropylene. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 568–576. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.D.; García-Contreras, R.; Tsolakis, A. The effect of the addition of individual methyl esters on the combustion and emissions of ethanol and butanol -diesel blends. Energy 2012, 42, 364–374. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K. Eco friendly biofuels for CI engine applications. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Cambridge, UK, 2019; pp. 407–440. [Google Scholar]



| Diesel Engine Specifications | Types of Fuel | Performance | Combustion | Emissions | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BSFC | BTE | ICP | RoHR | NOX | CO | HC | Smoke | |||
| AKSA-A4CRX46TI, 4-cylinder, 4-stroke, 68 kW at 1500 rpm | WPO | - |  |  |  |  |  |  | - | [14] |
| Kirloskar AV1, DI, 1-cylinder, 4-stroke, 3.7 kW at 1500 rpm | WPO |  |  |  |  |  |  |  |  | [16] |
| AKSA1-A4CRX46TI, 4-cylinder, 4-stroke, 68 kW at 1500 rpm | WPO |  |  |  |  |  |  |  |  | [17] |
| 4JA1, DI, 4-cylinder, 4-stroke, 68 kW at 1500 rpm | WPO |  |  |  |  |  |  |  |  | [18] |
| Kirloskar TAF1, DI, 1-cylinder, 4-stroke, 4.4 kW at 1500 rpm | WPO |  |  | - | - |  |  |  | - | [19] |
| Eicher E483, DI, 4-cylinder, 4-stroke, Max. Power 70 kW | WPO |  |  |  |  |  |  |  |  | [20] |
| Kirloskar TV1, DI, 1-cylinder, 4-stroke, 5.2 kW at 1500 rpm | WPO |  |  | - | - |  |  |  |  | [21] |
| DI, Turbocharger, 4-cylinder in line T/C, 4-stroke, 70 kW | WPO |  |  |  |  |  |  |  | - | [22] |
| DI, 1-cylinder, 4-stroke, 3.7 kW at 1500 rpm | WPO | - |  |  |  | - | - | - | - | [23] |
| Lombardini-Kohler FOCS 1.4, IDI, 1-cylinder, 4-stroke, | WPO |  |  |  |  | - | - | - |  | [24] |
| Kirloskar TAF1, DI, 1-cylinder, 4-stroke, 4.4 kW at 1500 rpm | WPO | - |  |  |  |  | - |  |  | [25] |
| AKSA-A4CRX46TI, 4-cylinder, 4-stroke, 68 kW at 1500 rpm | WPO |  |  |  |  |  |  |  | - | [26] |
| DI, 1-cylinder, 4-stroke, 3.7 kW at 1500 rpm | WPO-RME |  |  |  |  | - | - | - | - | [27] |
| Kirloskar TV1, DI, 1-cylinder, 4-stroke, 3.5 kW at 1500 rpm | WPO-POME WPO-COME |  |  |  |  |  |  |  |  | [28] |
| Kirloskar TAF1, DI, 1-cylinder, 4-stroke, 4.4 kW at 1500 rpm | WPO-BU |  |  | - | - |  |  |  |  | [29] |
 —increases,
—increases,  —decreases, WPO—waste plastic oil, RME—rice bran methyl ester, POME—palm methyl ester, COME—castor methyl ester, BU—Butanol, DI—Direct injection.
—decreases, WPO—waste plastic oil, RME—rice bran methyl ester, POME—palm methyl ester, COME—castor methyl ester, BU—Butanol, DI—Direct injection.| Parameter | Measuring Techniques | Measuring Range | Resolution | Accuracy |
|---|---|---|---|---|
| TESTO 350 | ||||
| NO | Chemiluminescence | 0–4000 ppm | 1 ppm | ±5 <100 ppm |
| NO2 | Chemiluminescence | 0–500 ppm | 0.1 ppm | ±5 <100 ppm |
| CO | Nondispersive Infrared | 0–10,000 ppm | 1 ppm | ±10 <200 ppm |
| HC | Flame Ionization Detector | 0–40,000 ppm | 10 ppm | ±400 ppm |
| TESTO 308 | ||||
| Smoke index | Photodiode (filter paper) | 0–6 | 0.1 | ±0.2 |
| Carbon Content | Diesel | CWPO | WPO |
|---|---|---|---|
| C4 | ND | 2.77 | ND |
| C5 | ND | 0.84 | ND |
| C6 | ND | 3.76 | ND |
| C7 | ND | 5.38 | ND |
| C8 | 2.54 | 34.02 | 1.03 |
| C9 | 5.61 | 19.94 | 0.73 |
| C10 | 5.23 | 28.49 | 2.28 |
| C11 | 4.18 | 0.53 | 5.71 |
| C12 | 7.08 | 0.75 | 9.78 |
| C13 | 6.39 | ND | 10.08 |
| C14 | 8.09 | ND | 11.03 |
| C15 | 5.66 | 0.67 | 10.48 |
| C16 | 5.95 | 0.19 | 8.71 |
| C17 | 15.80 | 0.26 | 7.90 |
| C18 | 4.30 | 0.32 | 8.88 |
| C19 | 16.11 | 0.36 | 7.86 |
| C20 | 3.90 | 0.24 | 8.10 |
| C21 | 3.19 | 0.36 | 3.63 |
| C22 | ND | 0.27 | 2.29 |
| C23 | 2.36 | 0.20 | 1.08 |
| C24 | ND | 0.19 | 0.43 |
| C25 | 1.19 | 0.20 | ND |
| C26 | 1.13 | 0.18 | ND |
| C27 | 0.62 | ND | ND |
| C28 | 0.34 | 0.08 | ND |
| C29 | 0.33 | 0.08 | ND |
| Carbon Content | Area Percentage | ||
|---|---|---|---|
| Diesel | CWPO | WPO | |
| C4–C11 | 13.38 | 95.72 | 9.74 |
| C12–C20 | 75.00 | 2.79 | 82.83 |
| >C20 | 11.62 | 1.49 | 7.43 |
| Properties | Test Method | Diesel | CWPO | WPO |
|---|---|---|---|---|
| Kinematic viscosity at 40 °C (cSt) | ASTM D445 | 3.44 | 1.66 | 3.11 |
| Surface tension (mN/m) | ASTM D971 | - | 27.77 | 27.85 |
| Specific gravity at 15.6 °C | ASTM D1298 | 0.835 | 0.900 | 0.824 |
| Density at 15.6 °C (kg/m3) | ASTM D1298 | 834 | 899 | 823 |
| Flash point (°C) | ASTM D93 | 66 | 35 | 54 |
| Gross calorific value (MJ/kg) | ASTM D240 | 45.56 | 37.72 | 45.24 |
| Cetane index | ASTM D976 | 56.57 | - | 46.7 |
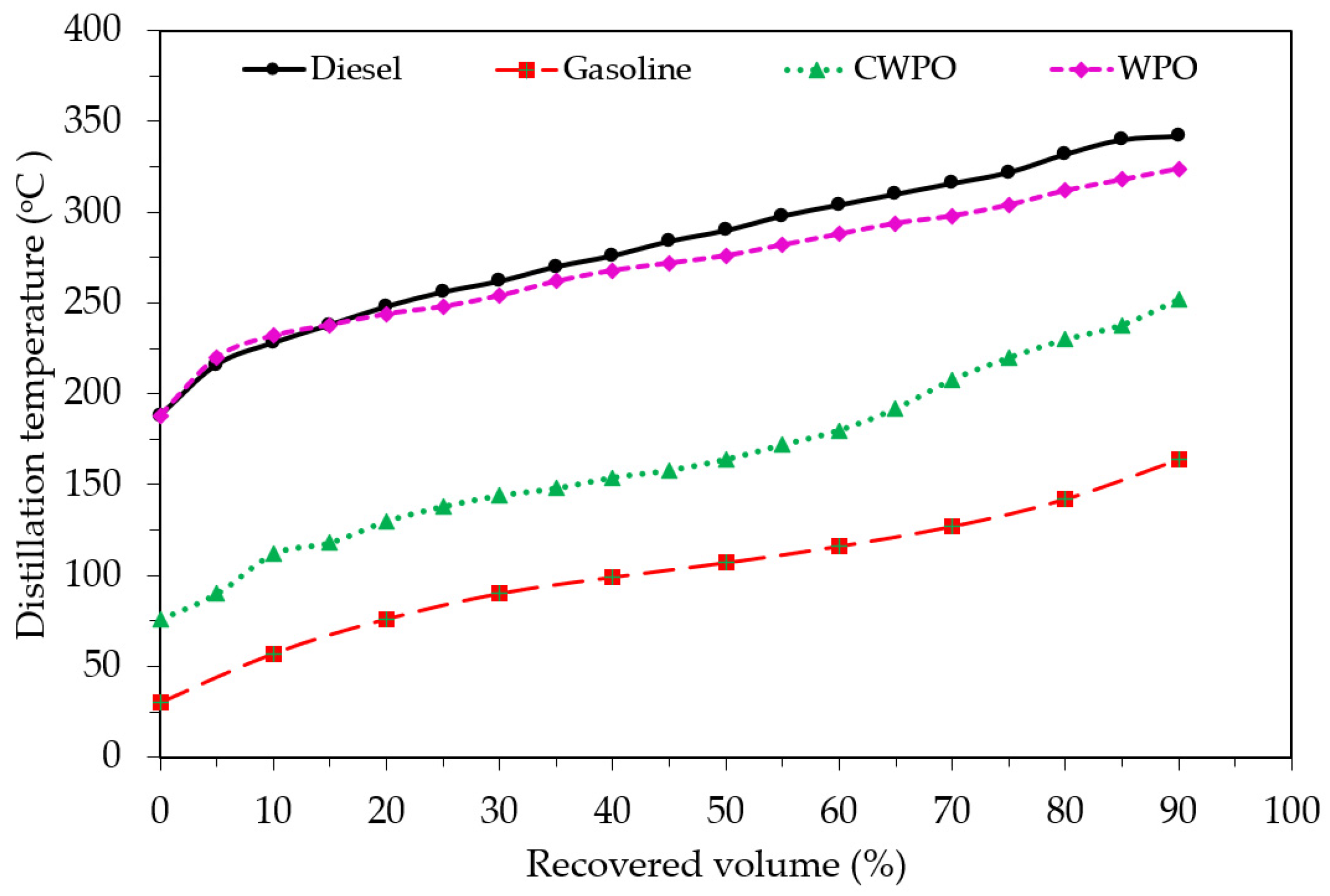
| Distillation temperature (°C) | ASTM D86 | |||
| 10% Recovered (°C) | ASTM D86 | 228 | 112 | 232 |
| 50% Recovered (°C) | ASTM D86 | 290 | 164 | 276 |
| 90% Recovered (°C) | ASTM D86 | 348 | 252 | 324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wathakit, K.; Sukjit, E.; Kaewbuddee, C.; Maithomklang, S.; Klinkaew, N.; Liplap, P.; Arjharn, W.; Srisertpol, J. Characterization and Impact of Waste Plastic Oil in a Variable Compression Ratio Diesel Engine. Energies 2021, 14, 2230. https://doi.org/10.3390/en14082230
Wathakit K, Sukjit E, Kaewbuddee C, Maithomklang S, Klinkaew N, Liplap P, Arjharn W, Srisertpol J. Characterization and Impact of Waste Plastic Oil in a Variable Compression Ratio Diesel Engine. Energies. 2021; 14(8):2230. https://doi.org/10.3390/en14082230
Chicago/Turabian StyleWathakit, Khatha, Ekarong Sukjit, Chalita Kaewbuddee, Somkiat Maithomklang, Niti Klinkaew, Pansa Liplap, Weerachai Arjharn, and Jiraphon Srisertpol. 2021. "Characterization and Impact of Waste Plastic Oil in a Variable Compression Ratio Diesel Engine" Energies 14, no. 8: 2230. https://doi.org/10.3390/en14082230






