Magnetic Steel Slag Biochar for Ammonium Nitrogen Removal from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
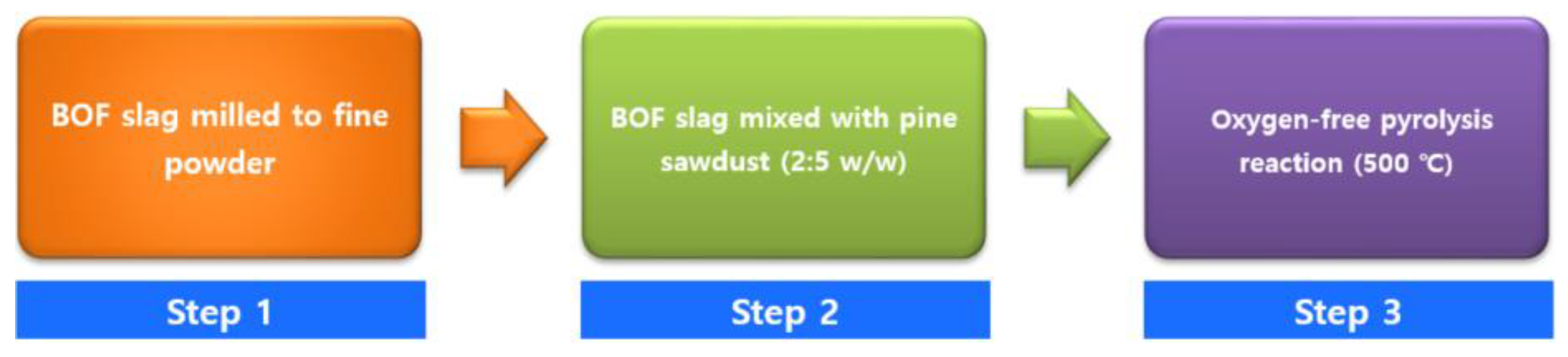
2.2. Preparation of Biochar
2.3. Physicochemical Property Analysis of Slag Biochar Complex
2.4. Batch Adsorption Experiments
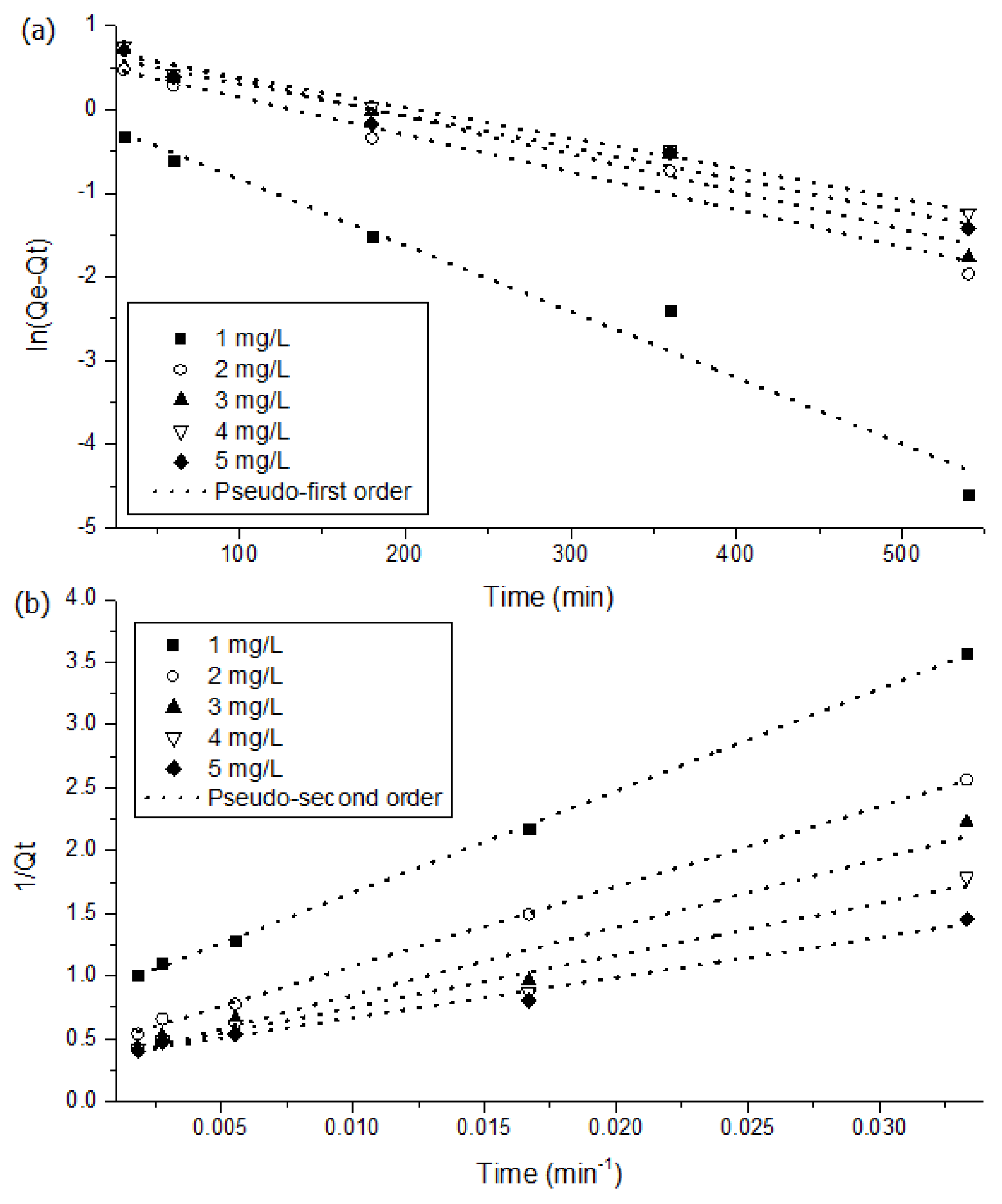
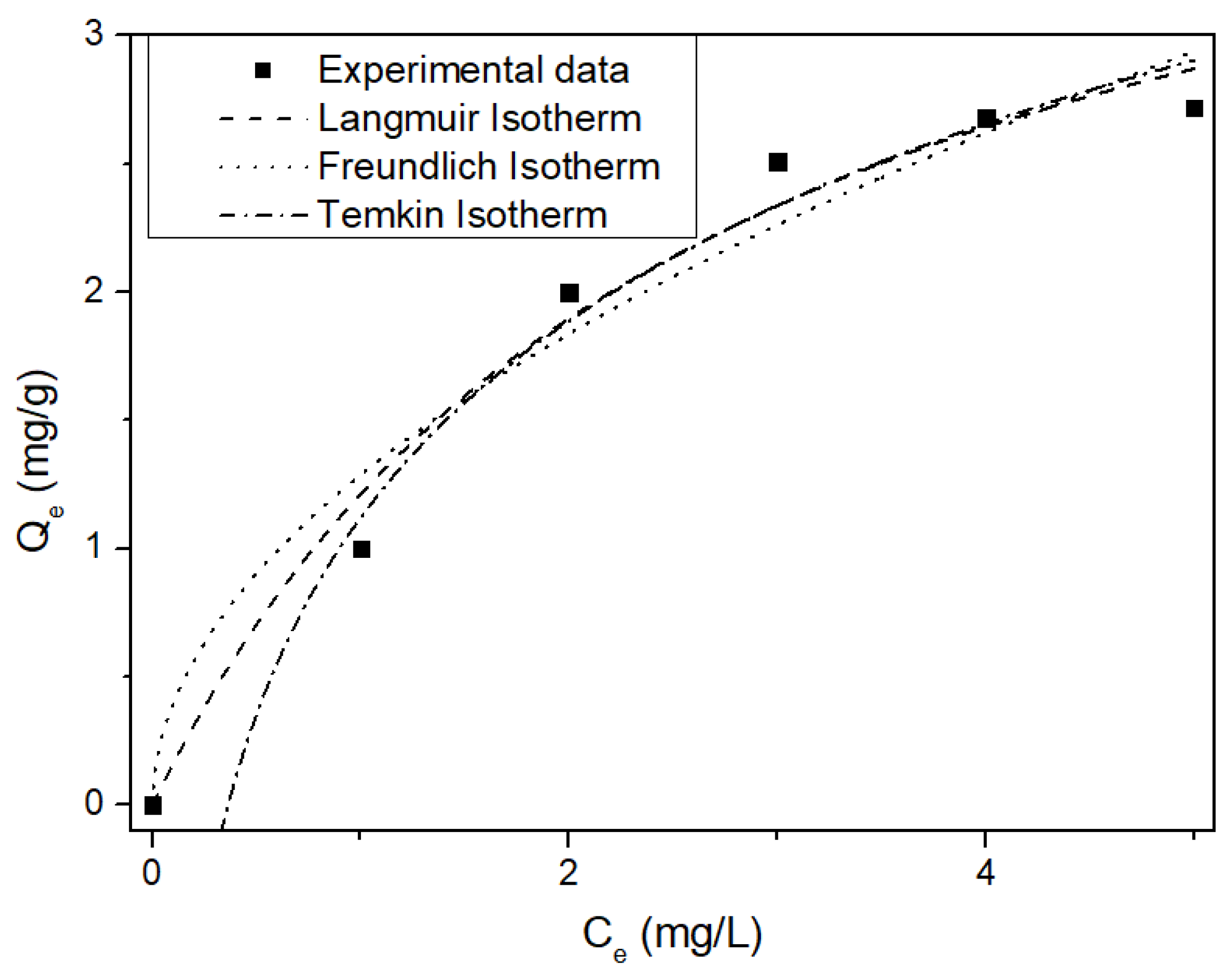
2.5. NH4-N Adsorption Kinetics and Isotherms
3. Results and Discussion
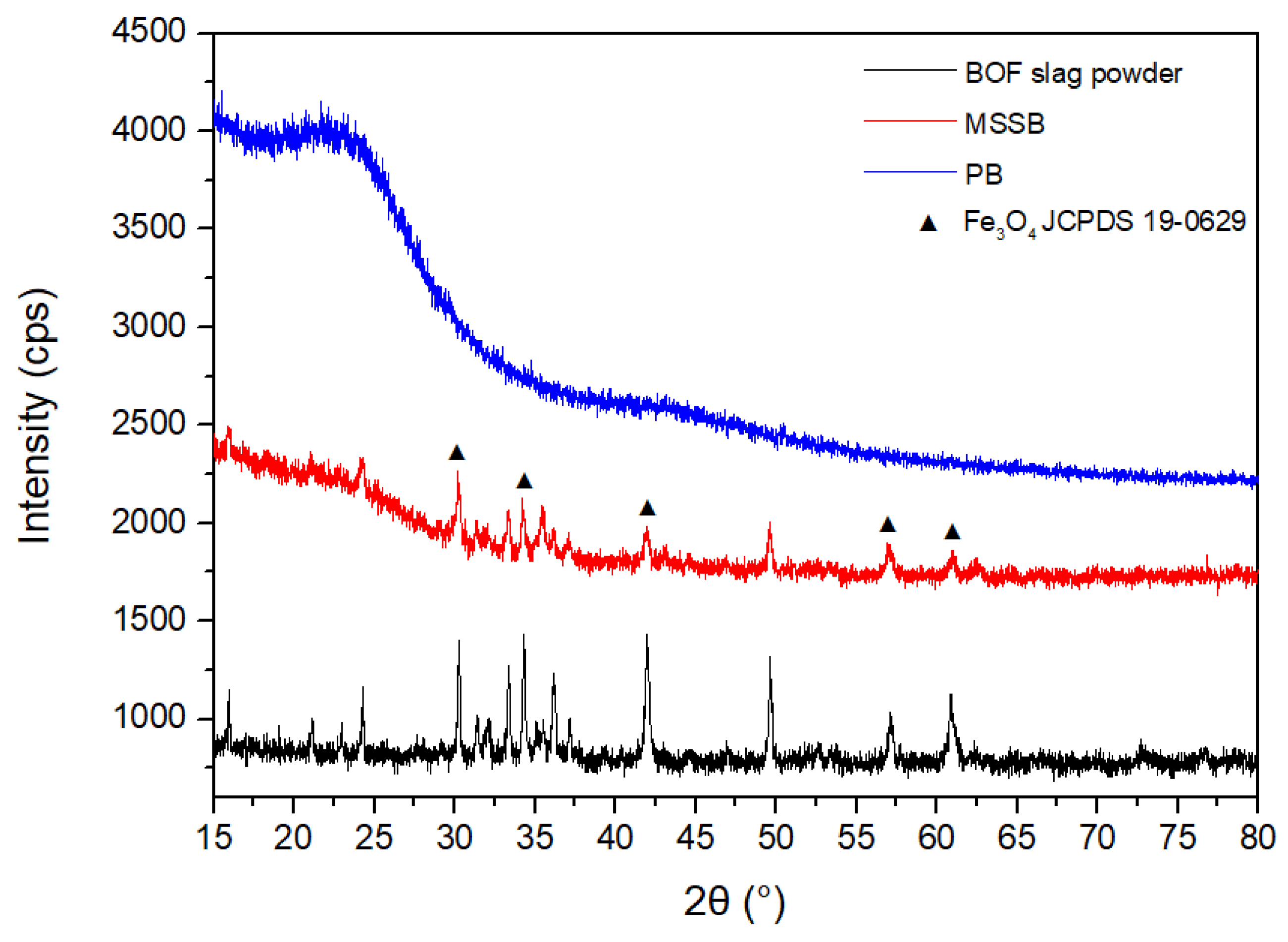
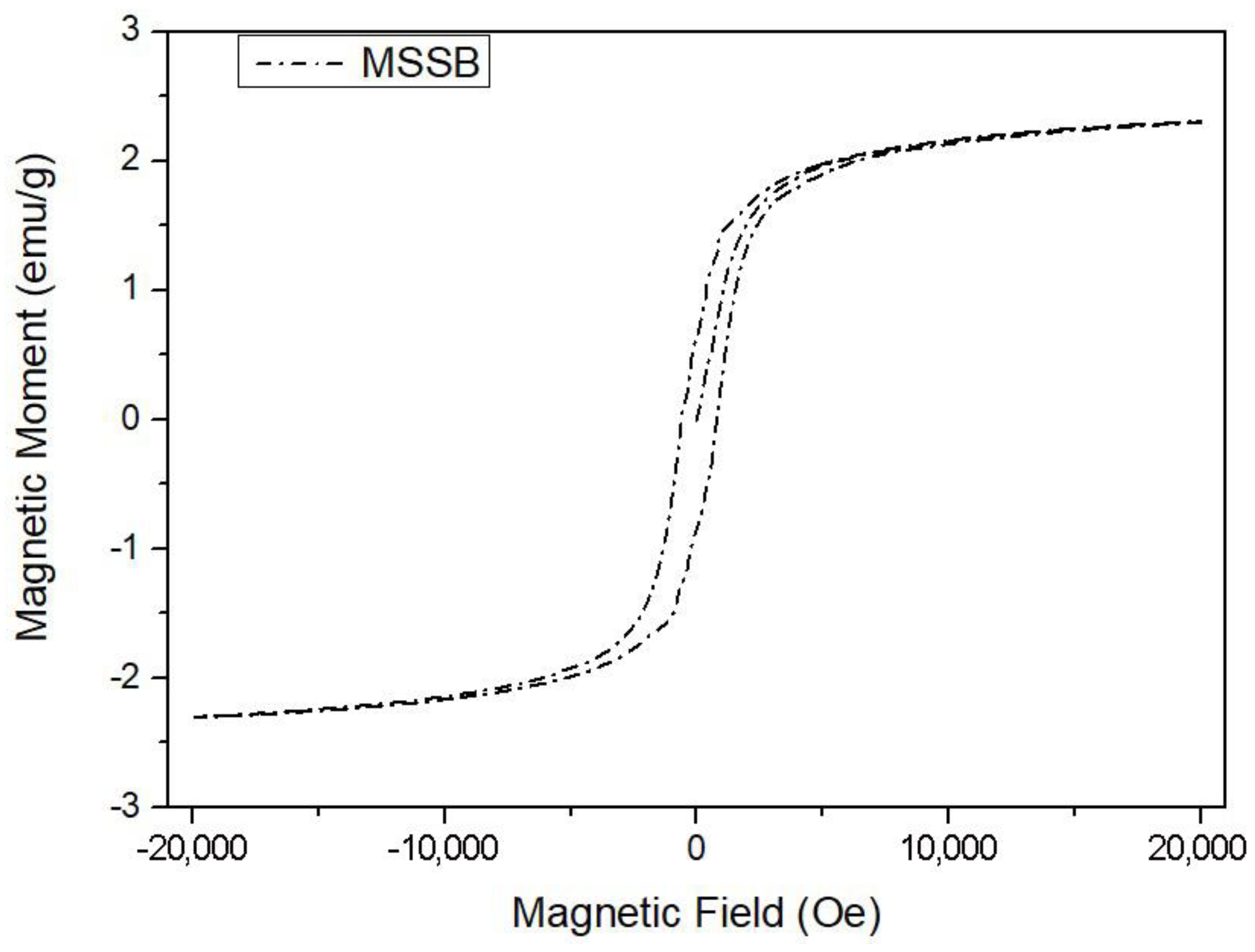
3.1. Physicochemical Properties of MSSB
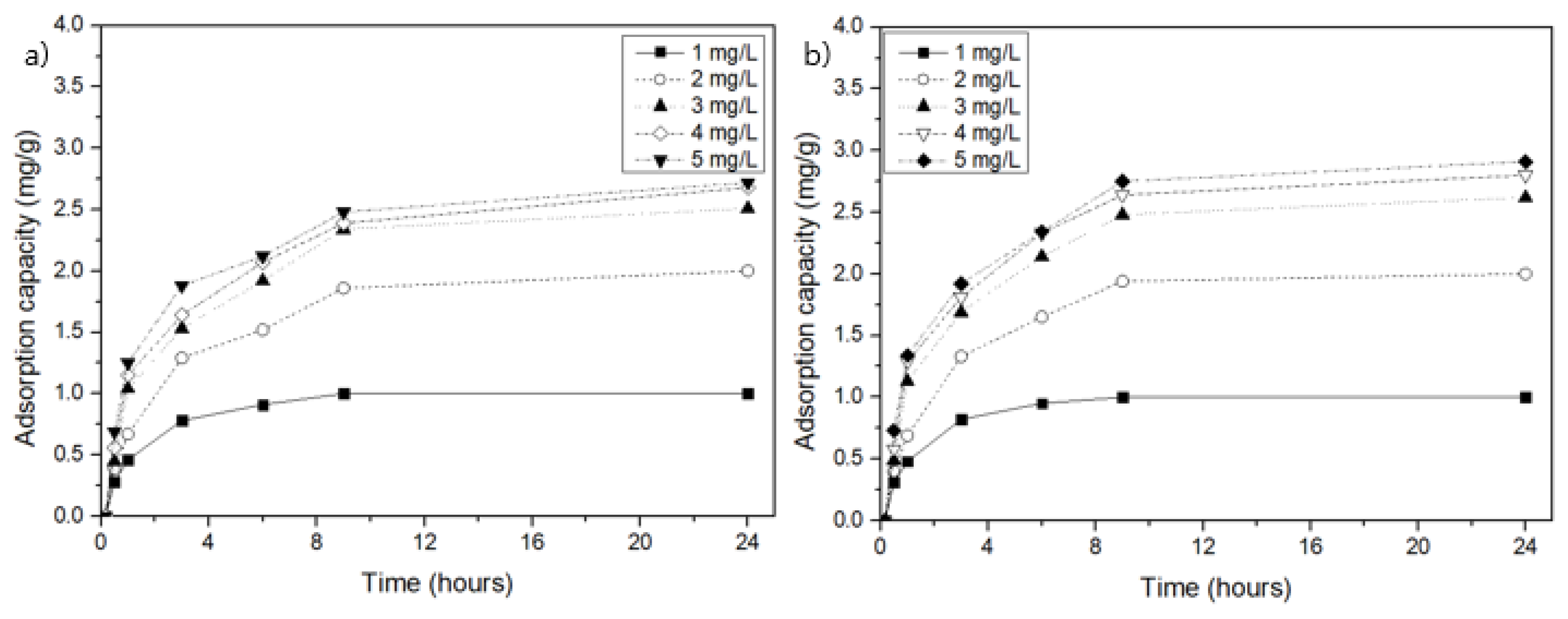
3.2. NH4-N Adsorption by MSSB in Aqueous Solution
3.3. Kinetics and Isotherms of MSSB
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korea Iron & Steel Association Crude Steel Production. Available online: https://www.kosa.or.kr/eng/statistics/production_2011.jsp (accessed on 4 November 2020).
- Min, T.; Kim, H.; Lim, H. Characteristics of Electric Arc Furnace Oxidized Slag and Domestic and International Research Trend. Mag. RCR 2019, 14, 40–47. [Google Scholar]
- Choi, J.-S. The Status and Utilization Prospect of Steel Making Slag. Rev. Archit. Build. Sci. 2012, 56, 18–21. [Google Scholar]
- Xue, P.; He, D.; Xu, A.; Gu, Z.; Yang, Q.; Engström, F.; Björkman, B. Modification of Industrial BOF Slag: Formation of MgFe2O4 and Recycling of Iron. J. Alloy. Compd. 2017, 712, 640–648. [Google Scholar] [CrossRef]
- Kim, W. Current State of Recycling and Properties of Steel Slag. Mag. RCR 2014, 9, 11–15. [Google Scholar]
- Öner, M.; Erdogdu, K.; Günlü, A. Effect of Components Fineness on Strength of Blast Furnace Slag Cement. Cem. Concr. Res. 2003, 33, 463–469. [Google Scholar] [CrossRef]
- Bellmann, F.; Stark, J. Activation of Blast Furnace Slag by a New Method. Cem. Concr. Res. 2009, 39, 644–650. [Google Scholar] [CrossRef]
- Naidu, T.S.; Sheridan, C.M.; van Dyk, L.D. Basic Oxygen Furnace Slag: Review of Current and Potential Uses. Miner. Eng. 2020, 149, 106234. [Google Scholar] [CrossRef]
- Wen, T.; Yang, L.; Dang, C.; Yang, M.; Miki, T.; Bai, H.; Nagasaka, T. Effect of Modified Basic Oxygen Furnace Slag on the controlled Release of Nitrate Nitrogen and the Functional Microbial Community in Soil. J. Environ. Manag. 2020, 261, 110191. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A Novel Magnetic Biochar Efficiently Sorbs Organic Pollutants and Phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management, 2nd ed.; Earthscan: London, UK, 2009. [Google Scholar]
- Lee, Y.-G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Cho, K.H.; Chon, K. Effects of NaOH Activation on Adsorptive Removal of Herbicides by Biochars Prepared from Ground Coffee Residues. Energies 2021, 14, 1297. [Google Scholar] [CrossRef]
- Zhao, S.; Ta, N.; Wang, X. Absorption of Cu(II) and Zn(II) from Aqueous Solutions onto Biochars Derived from Apple Tree Branches. Energies 2020, 13, 3498. [Google Scholar] [CrossRef]
- Park, M.H.; Jeong, S.; Kim, J.Y. Adsorption of NH3-N onto Rice Straw-Derived Biochar. J. Environ. Chem. Eng. 2019, 7, 103039. [Google Scholar] [CrossRef]
- Hestrin, R.; Enders, A.; Lehmann, J. Ammonia Volatilization from Composting with Oxidized Biochar. J. Environ. Qual. 2020, 49, 1690–1702. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.; Jang, E.S.; Hong, S.G.; Lee, S.R.; Ravindran, B. Adsorption Characteristics of Ammonium Nitrogen and Plant Responses to Biochar Pellet. Sustainability 2018, 10, 1331. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ho, S.-H.; Hua, T.; Zhou, Q.; Li, F.; Tang, J. Sustainable Biochar as Electrocatalysts for the Oxygen Reduction Reaction in Microbial Fuel Cells. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic Biochar Decorated with ZnS Nanocrytals for Pb (II) Removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Anfar, Z.; Zbair, M.; Ait Ahsiane, H.; Jada, A.; El Alem, N. Microwave Assisted Green Synthesis of Fe2O3/Biochar for Ultrasonic Removal of Nonsteroidal Anti-inflammatory Pharmaceuticals. RSC Adv. 2020, 10, 11371–11380. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass Waste Components Significantly Influence the Removal of Cr(VI) Using Magnetic Biochar Derived from Four Types of Feedstocks and Steel Pickling Waste Liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption Characteristics and Mechanism of Bisphenol a by Magnetic Biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef] [Green Version]
- Szatyłowicz, E.; Skoczko, I. The Use of Activated Alumina and Magnetic Field for the Removal Heavy Metals from Water. J. Ecol. Eng. 2018, 19, 61–67. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenburg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Tavlieva, M.P.; Genieva, S.D.; Georgieva, V.G.; Vlaev, L.T. Kinetic Study of Brilliant Green Adsorption from Aqueous Solution onto White rice Husk Ash. J. Colloid Interface Sci. 2013, 409, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second Order Model for Sorption Processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Khandelwal, A.; Narayanan, N.; Varghese, E.; Gupta, S. Linear and Nonlinear Isotherm Models and Error Analysis for the Sorption of Kresoxim-Methyl in Agricultural Soils of India. Bull. Environ. Contam. Toxicol. 2020, 104, 503–510. [Google Scholar] [CrossRef]
- Musapatika, E.T.; Singh, R.; Moodley, K.; Nzila, C.; Onyango, M.S.; Ochieng, A. Cobalt Removal from Wastewater Using Pine Sawdust. Afr. J. Biotechnol. 2012, 11, 9407–9415. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous Removal of Arsenic, Cadmium, and Lead from Soil by Iron-modified Magnetic Biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Cho, D.W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of Magnetic Biochar as a Treatment Medium for As(V) via Pyrolysis of FeCl3-Pretreated Spent Coffee Ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef]
- Saravanakumar, R.; Muthukumaran, K.; Selvaraju, N. Enhanced Pb (II) Ions Removal by Using Magnetic NiO/Biochar Composite. Mater. Res. Express 2019, 6, 105504. [Google Scholar] [CrossRef]
- Stolzenburg, P.; Capdevielle, A.; Teychené, S.; Biscans, B. Struvite Precipitation with MgO as a Precursor: Application to Wastewater Treatment. Chem. Eng. Sci. 2015, 133, 9–15. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH)2 Hydration/Dehydration Reaction for Thermochemical Energy Storage Applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative Distribution of Pb2+ Sorption Mechanisms by Sludge-derived Biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Hina, K.; Hedley, M.; Camps-Arbestain, M.; Hanly, J. Comparison of Pine Bark, Biochar and Zeolite as Sorbents for NH4+-N Removal from Water. CLEAN Soil Air Water 2015, 43, 86–91. [Google Scholar] [CrossRef]
- Hsu, D.; Lu, C.; Pang, T.; Wang, Y.; Wang, G. Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain. Appl. Sci. 2019, 9, 5249. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.A.; Golam Hyder, A.H.M.; Hicklen, Q.; Crocker, T.; Oni, B. Adsorption Characteristics of Ammonium onto Biochar from an Aqueous Solution. J. Water Supply Res. Technol. Aqua 2021, 70, 113–122. [Google Scholar] [CrossRef]
- Fan, R.; Chen, C.L.; Lin, J.Y.; Tzeng, J.H.; Huang, C.; Dong, C.; Huang, C.P. Adsorption Characteristics of Ammonium Ion onto Hydrous Biochars in Dilute Aqueous Solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.S. Phosphorus (V) Oxide. Encycl. Reagents Org. Synth. 2001. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of Ammonium and Nitrate to Biochars is Electrostatic and pH-Dependent. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Safavi, K.; Nakayama, T.A. Influence of Mixing Vehicle on Dissociation of Calcium Hydroxide in Solution. J. Endod. 2000, 26, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of Ammonium in Aqueous Solutions by Pine Sawdust and Wheat Straw Biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.P. On the Comparison of Pseudo-first Order and Pseudo-second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, S.K.; Cho, S.Y.; Kim, H.B.; Kang, Y.; Kim, S.D.; Kim, S.J. Adsorption of Heavy Metals by Brewery Biomass. Korean J. Chem. Eng. 2005, 22, 91–98. [Google Scholar] [CrossRef]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The Role of Physisorption and Chemisorption in the Oscillatory Adsorption of Organosilanes on Aluminium Oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef] [Green Version]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment-Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and Multilayer Adsorption Isotherm Models for Sorption from Aqueous Media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]

| Metal Oxides | Composition (%) |
|---|---|
| Fe2O3 | 34.50 |
| CaO | 35.00 |
| SiO2 | 15.80 |
| Al2O3 | 2.90 |
| MnO | 2.53 |
| MgO | 5.10 |
| Cr2O3 | 0.20 |
| P2O5 | 2.10 |
| Others | 1.87 |
| Material | PB | MSSB |
|---|---|---|
| C (%) | 75.9 | 32.7 |
| H (%) | 3.1 | 2.25 |
| N (%) | 0.61 | 0.51 |
| pH | 8.08 | 9.89 |
| BET surface area [m2/g] | 18.21–19.13 | 11.46–11.96 |
| BJH adsorption/desorption area [m2/g] | 18.80–21.42 /19.11–25.68 | 11.84–15.66 /12.28–21.97 |
| BJH adsorption/desorption pore size [nm] | 4.25–4.31 /4.03–4.61 | 3.94–3.97 /3.82–4.56 |
| Base Materials | Additional Components | Saturation Magnetic Moment [emu/g] | Reference |
|---|---|---|---|
| Rice hull | Fe(C5H7O2)3 | 11.6–14.3 | [20] |
| Red cedar (Thuja plicata) | FeCl3∙6H2O/FeSO4∙7H2O | 10–25 | [33] |
| Spent coffee ground | FeCl3 | 3.89–8.08 | [34] |
| Long-thorn kiawe (Prosopis juliflora) | Ni(NO3)2 | 0.087 | [35] |
| Pine tree sawdust | BOF slag | 2.3 | This study |
| Initial Conc. [mg/L] | qe, exp [mg/g] | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe, cal [mg/g] | k1 [mg/g] | r2 | qe, cal [mg/g] | k2 [g/mg·min] | r2 | ||
| 1.000 | 1.000 | 0.956 | 0.008 | 0.963 | 1.177 | 0.009 | 0.999 |
| 2.000 | 2.000 | 1.800 | 0.004 | 0.958 | 2.276 | 0.003 | 0.999 |
| 3.000 | 2.510 | 2.228 | 0.004 | 0.955 | 3.299 | 0.002 | 0.950 |
| 4.000 | 2.680 | 2.113 | 0.004 | 0.983 | 3.006 | 0.003 | 0.965 |
| 5.000 | 2.720 | 1.991 | 0.004 | 0.958 | 2.898 | 0.004 | 0.983 |
| Langmuir | Freundlich | Temkin | |||
|---|---|---|---|---|---|
| KL [L/mg] | 0.384 | Kf [mg/g] | 1.287 | Ar [L/g] | 2.749 |
| qmax [mg/g] | 4.366 | n | 1.949 | B [J/mol] | 2047.934 |
| r2 | 0.977 | r2 | 0.954 | r2 | 0.981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Kim, Y.M.; Kim, S.M.; Cho, H.U.; Park, J.M. Magnetic Steel Slag Biochar for Ammonium Nitrogen Removal from Aqueous Solution. Energies 2021, 14, 2682. https://doi.org/10.3390/en14092682
Kim G, Kim YM, Kim SM, Cho HU, Park JM. Magnetic Steel Slag Biochar for Ammonium Nitrogen Removal from Aqueous Solution. Energies. 2021; 14(9):2682. https://doi.org/10.3390/en14092682
Chicago/Turabian StyleKim, Gyuhyeon, Young Mo Kim, Su Min Kim, Hyun Uk Cho, and Jong Moon Park. 2021. "Magnetic Steel Slag Biochar for Ammonium Nitrogen Removal from Aqueous Solution" Energies 14, no. 9: 2682. https://doi.org/10.3390/en14092682






