Ionic Liquid and Ionanofluid-Based Redox Flow Batteries—A Mini Review
Abstract
:1. Introduction
- Increased system complexity;
- Low power and energy densities;
- High expensiveness;
- Complexity of handling corrosive redox active species and electrolytes.
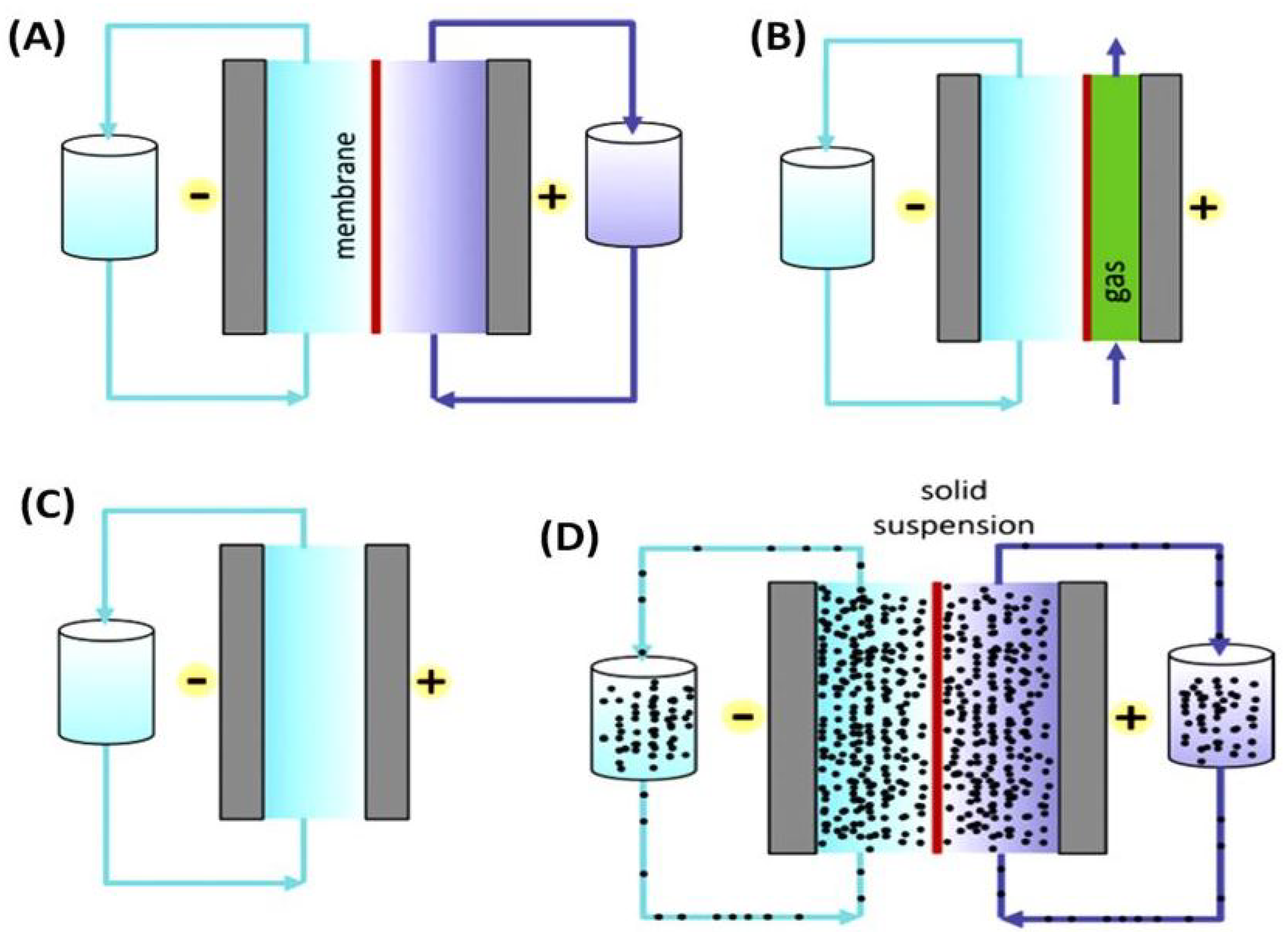
2. Working Mechanism of a Conventional Redox Flow Batteries
3. Ionic Liquid Electrolytes
4. Ionic Liquid Redox Couples
5. Metal Ionic Liquids (MetILs) as Solvents and Electrolytes
6. Membranes
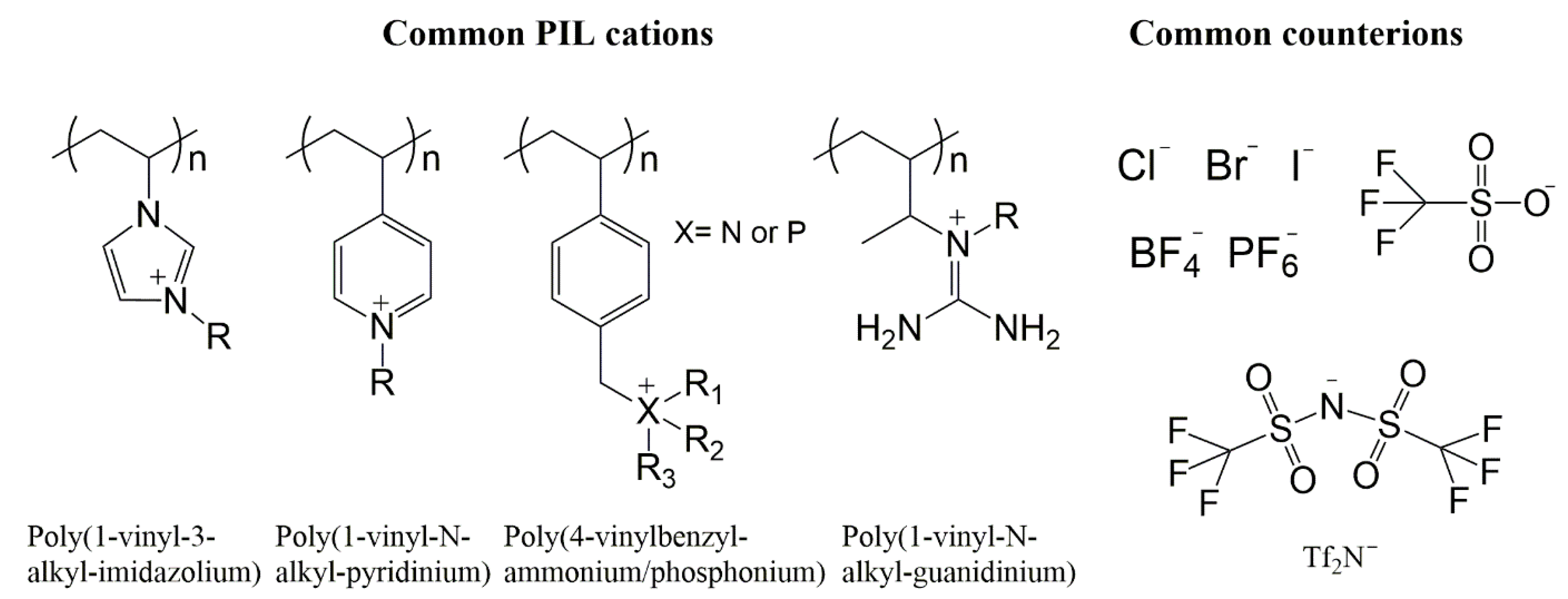
7. Poly(ionic liquids)
8. Ionic Liquid-Based Nanofluids
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL | Ionic liquid |
| FB | Flow battery |
| RFB | Redox flow battery |
| VRB or VRFB | Vanadium redox flow battery |
| ZBFBs | Zinc-bromine redox flow batteries |
| SSFB | Semi-solid flow Batteries |
| AORFBs | Aqueous organic redox flow batteries |
| MAFBs | Metal air flow batteries |
| SRFBs | Solar redox flow batteries |
| SMFB | Solid targeted/mediated/boosted flow batteries |
| ILRFB | Ionic liquid-based redox flow battery |
| UNSW | University of New South Wales |
| E | Potential difference of the two half cells |
| RAP | Redox active polymers |
| PYR14TFSI | N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide |
| IEM | Ion-exchange membranes |
| NARFBs | Non-aqueous redox flow batteries |
| PDADMA | Poly(diallyldimethylammonium) |
References
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Ding, Y.; Peng, S.; Guo, X.; Zhao, Y.; He, G.; Yu, G. Progress and prospects of next-generation redox flow batteries. Energy Storage Mater. 2018, 15, 324–350. [Google Scholar] [CrossRef]
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox flow batteries: Role in modern electric power industry and comparative characteristics of the main types. Russ. Chem. Rev. 2021, 90, 677. [Google Scholar] [CrossRef]
- Kapoor, M.; Verma, A. Technical benchmarking and challenges of kilowatt scale vanadium redox flow battery. Wiley Interdiscip. Rev. Energy Environ. 2022, e439. [Google Scholar] [CrossRef]
- Arenas, L.F.; de León, C.P.; Walsh, F.C. Redox flow batteries for energy storage: Their promise, achievements and challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Rychcik, M.; Robins, R.G.; Fane, A.; Green, M. New all-vanadium redox flow cell. J. Electrochem. Soc. 1986, 133, 1057. [Google Scholar] [CrossRef]
- Navalpotro, P.; Palma, J.; Anderson, M.A.; Marcilla, R. A Membrane-Free Redox Flow Battery with Two Immiscible Electrolytes. ECS Meet. Abstr. 2018, 31. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J. Electrochem. Soc. 2015, 163, A5023. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent advances with UNSW vanadium-based redox flow batteries. Int. J. Energy Res. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Chen, R.; Kim, S.; Chang, Z. Redox flow batteries: Fundamentals and applications. In Redox: Principles and Advance Applications; IntechOpen: London, UK, 2017; pp. 103–118. [Google Scholar]
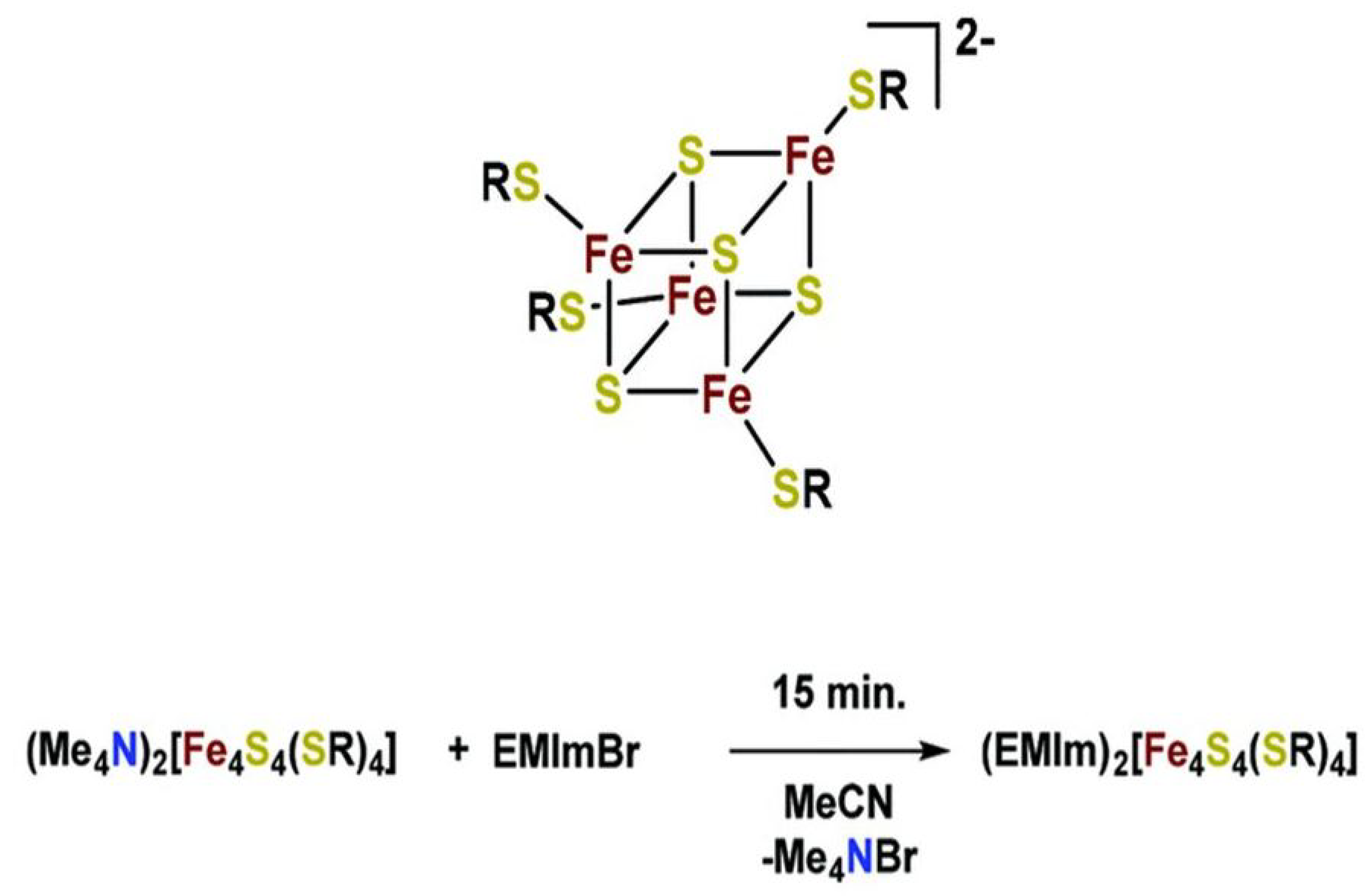
- Modrzynski, C.; Burger, P. Energy storage inspired by nature–ionic liquid iron–sulfur clusters as electrolytes for redox flow batteries. Dalton Trans. 2019, 48, 1941–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navalpotro, P.; Palma, J.; Anderson, M.; Marcilla, R. A membrane-free redox flow battery with two immiscible redox electrolytes. Angew. Chem. 2017, 129, 12634–12639. [Google Scholar] [CrossRef] [Green Version]
- Minea, A.A.; Murshed, S.S. A review on development of ionic liquid based nanofluids and their heat transfer behavior. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]
- Faizan, M.; Ahmed, R.; Ali, H.M. A critical review on thermophysical and electrochemical properties of Ionanofluids (nanoparticles dispersed in ionic liquids) and their applications. J. Taiwan Inst. Chem. Eng. 2021, 124, 391–423. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Boncel, S. Rheology of ionanofluids—A review. J. Mol. Liq. 2020, 302, 112568. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Fabre, E.; Murshed, S.S. A comprehensive review of thermophysical properties and prospects of ionanocolloids in thermal energy applications. Renew. Sustain. Energy Rev. 2021, 151, 111593. [Google Scholar] [CrossRef]
- Castro, C.A.; Murshed, S.; Lourenço, M.J.; Santos, F.J.; Lopes, M.L.; França, J.M. Ionanofluids: New heat transfer fluids for green processes development. In Green Solvents I; Springer: Dordrecht, The Netherlands, 2012; pp. 233–249. [Google Scholar]
- Das, L.; Rubbi, F.; Habib, K.; Aslfattahi, N.; Saidur, R.; Saha, B.B.; Algarni, S.; Irshad, K.; Alqahtani, T. State-of-the-art ionic liquid & ionanofluids incorporated with advanced nanomaterials for solar energy applications. J. Mol. Liq. 2021, 336, 116563. [Google Scholar]
- Minea, A.A.; Sohel Murshed, S. Ionic liquids-based nanocolloids—A review of progress and prospects in convective heat transfer applications. Nanomaterials 2021, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bhat, A.H.; Sharma, D.K.; Usmani, M.A.; Khan, F. Overview of Nanofluids to Ionanofluids: Applications and Challenges. In Nanomaterials for Healthcare, Energy and Environment; Springer: Singapore, 2019; pp. 199–227. [Google Scholar]
- Park, S.K.; Shim, J.; Yang, J.; Shin, K.H.; Jin, C.S.; Lee, B.S.; Lee, Y.S.; Jeon, J.D. Electrochemical properties of a non-aqueous redox battery with all-organic redox couples. Electrochem. Commun. 2015, 59, 68–71. [Google Scholar] [CrossRef]
- Liu, T.; Wei, X.; Nie, Z.; Sprenkle, V.; Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte. Adv. Energy Mater. 2016, 6, 1501449. [Google Scholar] [CrossRef]
- Wei, X.; Duan, W.; Huang, J.; Zhang, L.; Li, B.; Reed, D.; Xu, W.; Sprenkle, V.; Wang, W. A high-current, stable nonaqueous organic redox flow battery. ACS Energy Lett. 2016, 1, 705–711. [Google Scholar] [CrossRef]
- Wei, X.; Xu, W.; Huang, J.; Zhang, L.; Walter, E.; Lawrence, C.; Vijayakumar, M.; Henderson, W.A.; Liu, T.; Cosimbescu, L.; et al. Radical compatibility with nonaqueous electrolytes and its impact on an all-organic redox flow battery. Angew. Chem. Int. Ed. 2015, 54, 8684–8687. [Google Scholar] [CrossRef]
- Clemente, A.; Ramos, G.A.; Costa-Castelló, R. Voltage H control of a vanadium redox flow battery. Electronics 2020, 9, 1567. [Google Scholar] [CrossRef]
- Anderson, T.M.; Pratt, H. Ionic Liquid Flow Battery Materials and Prototyping; Technical Report; Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2015. [Google Scholar]
- Ejigu, A.; Greatorex-Davies, P.A.; Walsh, D.A. Room temperature ionic liquid electrolytes for redox flow batteries. Electrochem. Commun. 2015, 54, 55–59. [Google Scholar] [CrossRef]
- Suttil, J.; Kucharyson, J.; Escalante-Garcia, I.; Cabrera, P.; James, B.; Savinell, R.; Sanford, M.; Thompson, L. Metal acetylacetonate complexes for high energy density non-aqueous redox flow batteries. J. Mater. Chem. A 2015, 3, 7929–7938. [Google Scholar] [CrossRef]
- Chen, R.; Hempelmann, R. Ionic liquid-mediated aqueous redox flow batteries for high voltage applications. Electrochem. Commun. 2016, 70, 56–59. [Google Scholar] [CrossRef]
- Vafiadis, H.; Skyllas-Kazacos, M. Evaluation of membranes for the novel vanadium bromine redox flow cell. J. Membr. Sci. 2006, 279, 394–402. [Google Scholar] [CrossRef]
- Schaltin, S.; Li, Y.; Brooks, N.R.; Sniekers, J.; Vankelecom, I.F.; Binnemans, K.; Fransaer, J. Towards an all-copper redox flow battery based on a copper-containing ionic liquid. Chem. Commun. 2016, 52, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Periyapperuma, K.; Zhang, Y.; MacFarlane, D.R.; Forsyth, M.; Pozo-Gonzalo, C.; Howlett, P.C. Towards Higher Energy Density Redox-Flow Batteries: Imidazolium Ionic Liquid for Zn Electrochemistry in Flow Environment. ChemElectroChem 2017, 4, 1051–1058. [Google Scholar] [CrossRef]
- Bahadori, L.; Boyd, R.; Warrington, A.; Shafeeyan, M.; Nockemann, P. Evaluation of ionic liquids as electrolytes for vanadium redox flow batteries. J. Mol. Liq. 2020, 317, 114017. [Google Scholar] [CrossRef]
- Miller, M.; Wainright, J.; Savinell, R. Communication—Iron ionic liquid electrolytes for redox flow battery applications. J. Electrochem. Soc. 2016, 163, A578. [Google Scholar] [CrossRef] [Green Version]
- Takechi, K.; Kato, Y.; Hase, Y. A highly concentrated catholyte based on a solvate ionic liquid for rechargeable flow batteries. Adv. Mater. 2015, 27, 2501–2506. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Zhao, Y.; Tkacheva, A.; Liu, Z.; Yan, K.; Guo, X.; McDonagh, A.M.; Shanmukaraj, D.; Wang, C.; et al. A versatile functionalized ionic liquid to boost the solution-mediated performances of lithium-oxygen batteries. Nat. Commun. 2019, 10, 602. [Google Scholar] [CrossRef]
- Small, L.J.; Anderson, T.M.; Pratt, H. Method for Maximizing Energy Density in Redox Flow Battery Electrolytes. U.S. Patent 20180337419, 22 November 2018. [Google Scholar]
- Song, X.; Ding, L.; Wang, L.; He, M.; Han, X. Polybenzimidazole membranes embedded with ionic liquids for use in high proton selectivity vanadium redox flow batteries. Electrochim. Acta 2019, 295, 1034–1043. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, R.; Zhong, Y.; Zhang, Y.; Jiang, F. Asymmetric porous membranes with ultra-high ion selectivity for vanadium redox flow batteries. J. Membr. Sci. 2020, 595, 117614. [Google Scholar] [CrossRef]
- Hudak, N.S.; Small, L.J.; Pratt, H.D.; Anderson, T.M. Through-plane conductivities of membranes for nonaqueous redox flow batteries. J. Electrochem. Soc. 2015, 162, A2188. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Shao-Horn, Y.; Hashaikeh, R.; Almheiri, S. Cyclable membraneless redox flow batteries based on immiscible liquid electrolytes: Demonstration with all-iron redox chemistry. Electrochim. Acta 2018, 267, 41–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yuan, Z.; Li, X.; Zhang, H.; Vankelecom, I.F. Advanced charged sponge-like membrane with ultrahigh stability and selectivity for vanadium flow batteries. Adv. Funct. Mater. 2016, 26, 210–218. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Sun, J.K.; Antonietti, M.; Yuan, J. Poly (ionic liquid) s with engineered nanopores for energy and environmental applications. Polymer 2020, 202, 122640. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.K.; Yuan, J. Poly (ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [Green Version]
- Mecerreyes, D.; Porcarelli, L.; Casado, N. Innovative Polymers for Next-Generation Batteries. Macromol. Chem. Phys. 2020, 221, 1900490. [Google Scholar] [CrossRef]
- Hernández, G.; Işik, M.; Mantione, D.; Pendashteh, A.; Navalpotro, P.; Shanmukaraj, D.; Marcilla, R.; Mecerreyes, D. Redox-active poly(ionic liquid)s as active materials for energy storage applications. J. Mater. Chem. A 2017, 5, 16231–16240. [Google Scholar] [CrossRef]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. High–energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane. Sci. Adv. 2015, 1, e1500886. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, E.V.; Katsoudas, J.P.; Segre, C.U.; Singh, D. Rechargeable nanofluid electrodes for high energy density flow battery. NSTI-Nanotech 2013, 2, 679–682. [Google Scholar]
- Cheng, J.; Zhang, L.; Yang, Y.S.; Wen, Y.H.; Cao, G.P.; Wang, X.D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Yu, W.; Wang, L.; Xie, H. Ternary molten salt energy storage coupled with graphene oxide-TiN nanofluids for direct absorption solar collector. Energy Build. 2021, 253, 111481. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. Electroactive graphene nanofluids for fast energy storage. 2D Mater. 2016, 3, 031004. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Latibari, S.T.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Mubeen, S.; Jun, Y.s.; Lee, J.; McFarland, E.W. Solid suspension flow batteries using earth abundant materials. ACS Appl. Mater. Interfaces 2016, 8, 1759–1765. [Google Scholar] [CrossRef]
- Lobato, J.; Mena, E.; Millán, M. Improving a redox flow battery working under realistic conditions by using of graphene based nanofluids. ChemistrySelect 2017, 2, 8446–8450. [Google Scholar] [CrossRef] [Green Version]
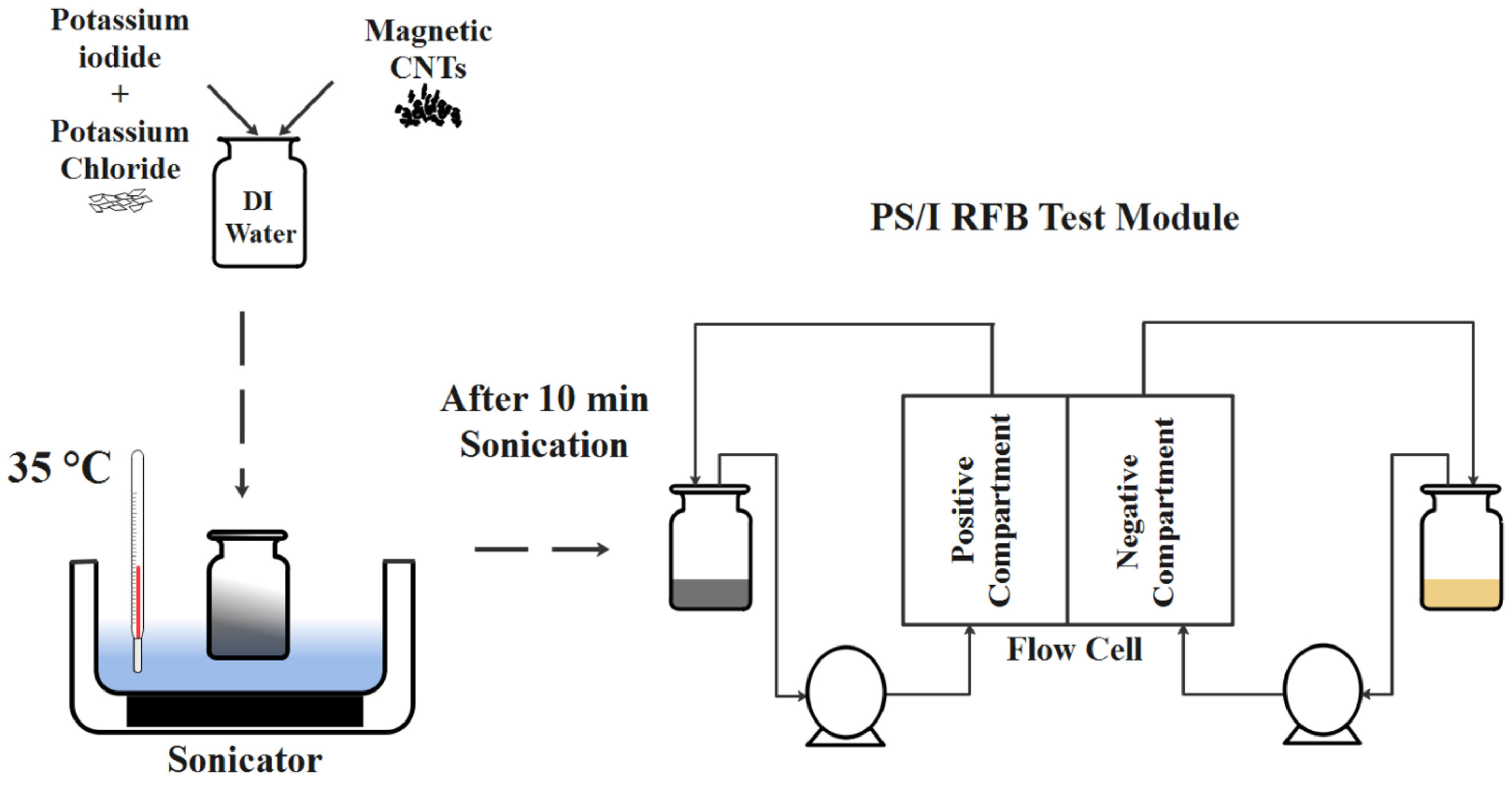
- Rahimi, M.; Dehkordi, A.M.; Roberts, E.P. Magnetic nanofluidic electrolyte for enhancing the performance of polysulfide/iodide redox flow batteries. Electrochim. Acta 2021, 369, 137687. [Google Scholar] [CrossRef]
- Lobato, J.; Oviedo, J.; Cañizares, P.; Rodrigo, M.A.; Millán, M. Impact of carbonaceous particles concentration in a nanofluidic electrolyte for vanadium redox flow batteries. Carbon 2020, 156, 287–298. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Murshed, S.S.; de Castro, C.N.; Lourenço, M.; Lopes, M.; Santos, F. Current research and future applications of nano-and ionano-fluids. J. Phys. Conf. Ser. 2012, 395, 012117. [Google Scholar] [CrossRef]
- Li, B.; Liu, J. Progress and directions in low-cost redox-flow batteries for large-scale energy storage. Natl. Sci. Rev. 2017, 4, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.; Xavier, M.M.; Fal, J.; Żyła, G.; Sasi, S.; Nair, P.R.; Padmanabhan, A.; Mathew, S. Synthesis and electrochemical characterization of electroactive IoNanofluids with high dielectric constants from hydrated ferrous sulphate. Chem. Commun. 2019, 55, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.; Bose, P.; Bhattacharya, S. Gel-polymer electrolytes plasticized with pyrrolidinium-based ionanofluid for lithium battery applications. Ionics 2021, 27, 123–136. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Bejan, D.; Ibanescu, C.; Danu, M.; Minea, A.A. Ionanofluids with [C2mim][CH3SO3] ionic liquid and alumina nanoparticles: An experimental study on viscosity, specific heat and electrical conductivity. Chem. Eng. Sci. 2021, 229, 116140. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, A.; Sobczak, J.; Żyła, G.; Mathew, S. Ionic Liquid and Ionanofluid-Based Redox Flow Batteries—A Mini Review. Energies 2022, 15, 4545. https://doi.org/10.3390/en15134545
Joseph A, Sobczak J, Żyła G, Mathew S. Ionic Liquid and Ionanofluid-Based Redox Flow Batteries—A Mini Review. Energies. 2022; 15(13):4545. https://doi.org/10.3390/en15134545
Chicago/Turabian StyleJoseph, Aswathy, Jolanta Sobczak, Gaweł Żyła, and Suresh Mathew. 2022. "Ionic Liquid and Ionanofluid-Based Redox Flow Batteries—A Mini Review" Energies 15, no. 13: 4545. https://doi.org/10.3390/en15134545





