Parametric Study of Methyl Orange Removal Using Metal–Organic Frameworks Based on Factorial Experimental Design Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Adsorption Experiments
2.4. Factorial Design Analysis
3. Results and Discussion
3.1. Characterization
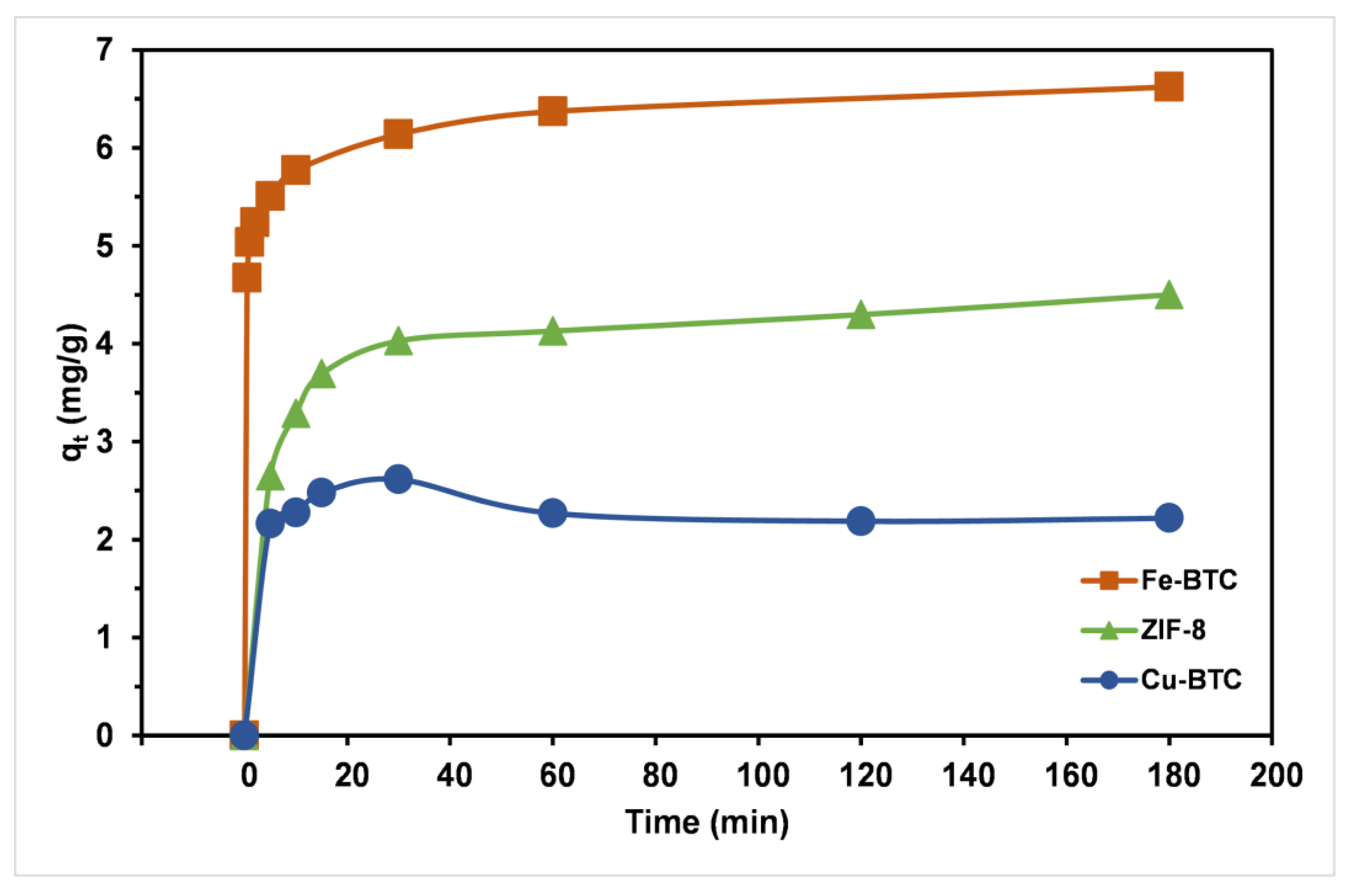
3.2. Removal Efficiencies
3.3. Initial pH (pHinitial) Effect and Adsorption Mechanism
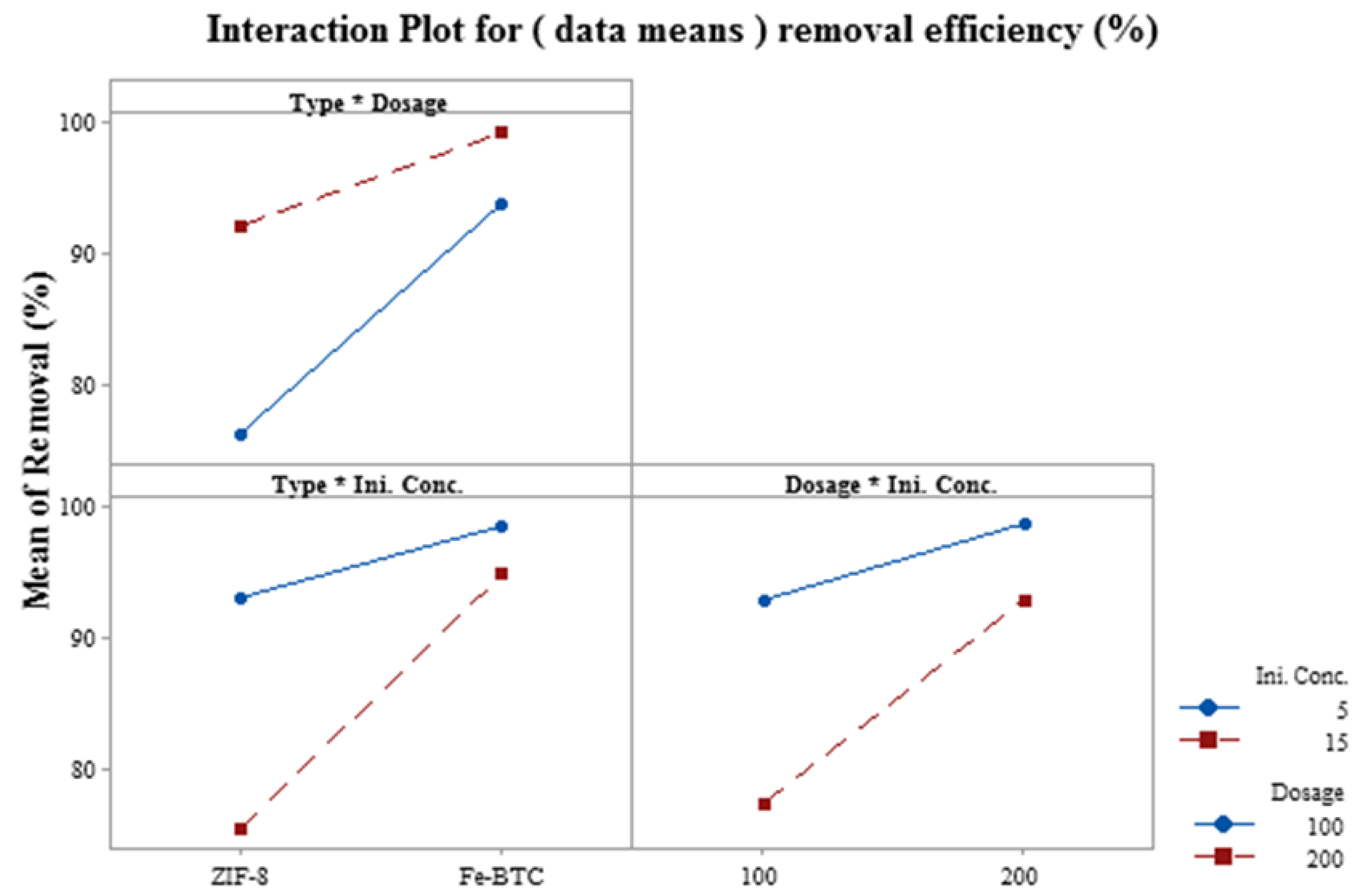
3.4. Factorial Design Analysis
3.5. Response Surface of Independent Variables and Their Interaction
3.6. Process Optimization Curve
3.7. Comparison of Adsorption Capacity with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Molinos-Senante, M.; Maziotis, A. Evaluation of energy efficiency of wastewater treatment plants: The influence of the technology and aging factors. Appl. Energy 2022, 310, 118535. [Google Scholar] [CrossRef]
- Di Fraia, S.; Massarotti, N.; Vanoli, L. A novel energy assessment of urban wastewater treatment plants. Energy Convers. Manag. 2018, 163, 304–313. [Google Scholar] [CrossRef]
- Hernández-Chover, V.; Bellver-Domingo, Á.; Hernández-Sancho, F. Efficiency of wastewater treatment facilities: The influence of scale economies. J. Environ. Manag. 2018, 228, 77–84. [Google Scholar] [CrossRef]
- International Energy Agency. Water Energy Nexus—Excerpt from the World Energy Outlook 2016; International Energy Agency: Paris, France, 2016; ISBN 978-92-64-26494-6. [Google Scholar]
- Bodík, I.; Kubaská, M. Energy and sustainability of operation of a wastewater treatment plant. Environ. Prot. Eng. 2013, 39, 15–24. [Google Scholar] [CrossRef]
- Wakeel, M.; Chen, B.; Hayat, T.; Alsaedi, A.; Ahmad, B. Energy consumption for water use cycles in different countries: A Review. Appl. Energy 2016, 178, 868–885. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Hao, X.; Liu, R.; Huang, X. Evaluation of the potential for operating carbon neutral wwtps in China. Water Res. 2015, 87, 424–431. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernandez-Sancho, F.; Sala-Garrido, R. Benchmarking in wastewater treatment plants: A tool to save operational costs. Clean Technol. Environ. Policy 2013, 16, 149–161. [Google Scholar] [CrossRef]
- Ananda, J. Productivity implications of the water-energy-emissions nexus: An empirical analysis of the drinking water and wastewater sector. J. Clean. Prod. 2018, 196, 1097–1105. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Fatima, M.; Farooq, R.; Lindström, R.W.; Saeed, M. A review on biocatalytic decomposition of azo dyes and electrons recovery. J. Mol. Liq. 2017, 246, 275–281. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Metal organic frameworks as adsorbents for Dye adsorption: Overview, prospects and future challenges. Toxicol. Environ. Chem. 2012, 94, 1846–1863. [Google Scholar] [CrossRef]
- Karami, A.; Sabouni, R.; Al-Sayah, M.H.; Aidan, A. Adsorption potentials of iron-based metal–organic framework for methyl orange removal: Batch and fixed-bed column studies. Int. J. Environ. Sci. Technol. 2021, 18, 3597–3612. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Optimization of rambutan peel based activated carbon preparation conditions for Remazol Brilliant Blue R Removal. Chem. Eng. J. 2011, 168, 280–285. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A Review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Karami, A.; Sabouni, R.; Ghommem, M. Experimental investigation of competitive co-adsorption of naproxen and diclofenac from water by an aluminum-based metal-organic framework. J. Mol. Liq. 2020, 305, 112808. [Google Scholar] [CrossRef]
- Konaganti, V.K.; Kota, R.; Patil, S.; Madras, G. Adsorption of anionic dyes on chitosan grafted poly(alkyl methacrylate)s. Chem. Eng. J. 2010, 158, 393–401. [Google Scholar] [CrossRef]
- Özbay, N.; Yargıç, A.Ş.; Yarbay-Şahin, R.Z.; Önal, E. Full factorial experimental design analysis of reactive dye removal by carbon adsorption. J. Chem. 2013, 2013, 234904. [Google Scholar] [CrossRef] [Green Version]
- Mtaallah, S.; Marzouk, I.; Hamrouni, B. Factorial experimental design applied to adsorption of cadmium on activated alumina. J. Water Reuse Desalin. 2017, 8, 76–85. [Google Scholar] [CrossRef]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.-S.; Jegal, J.; Jhung, S.H. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpää, M. Emerging adsorptive removal of azo dye by metal–organic frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.F.; Kulandainathan, M.A.; Katsounaros, I.; Rassaei, L.; Burrows, A.D.; Raithby, P.R.; Marken, F. Electrocatalytic activity of BasoliteTM F300 metal-organic-framework structures. Electrochem. Commun. 2010, 12, 632–635. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3 (TMA)2 (H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Centrone, A.; Santiso, E.E.; Hatton, T.A. Separation of chemical reaction intermediates by metal-organic frameworks. Small 2011, 7, 2356–2364. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-benzenetricarboxylic metal–organic coordination polymers prepared by SOLVOTHERMAL method and their application in efficient as(v) removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Wang, Q.M.; Shen, D.; Bülow, M.; Ling Lau, M.; Deng, S.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Hamon, L.; Jolimaître, E.; Pirngruber, G.D. CO2 and CH4 separation by adsorption using Cu-BTC metal−organic framework. Ind. Eng. Chem. Res. 2010, 49, 7497–7503. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekbala, M.R.; Khan, M.A.; Hosseini, S.; Abdullah, L.C.; Choong, T.S.Y. Adsorption/desorption of cationic dye on surfactant modified mesoporous carbon coated monolith: Equilibrium, kinetic and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 369–377. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Khan, M.A.; Siddiqui, M.R.; Otero, M.; Jeon, B.-H.; Alothman, Z.A.; Hakami, A.A. Oxygenated functionalities enriched mwcnts decorated with silica coated spinel ferrite—A nanocomposite for potentially rapid and efficient de-colorization of aquatic environment. J. Mol. Liq. 2020, 317, 113916. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2008, 7, 79–84. [Google Scholar] [CrossRef]
- Hosseini, S.; Khan, M.A.; Malekbala, M.R.; Cheah, W.; Choong, T.S.Y. Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and Desorption Studies. Chem. Eng. J. 2011, 171, 1124–1131. [Google Scholar] [CrossRef]
- Ranjan, D.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Alshaeef, S.A. Optimization of Bromate adsorption onto Fe-CNTS nanocomposite using response surface methodology. Water SA 2021, 47, 423–429. [Google Scholar]
- Najafi Nobar, S. Cu-BTC Synthesis, characterization and preparation for adsorption studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound CU3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Yang, Y.; Shukla, P.; Wang, S.; Rudolph, V.; Chen, X.-M.; Zhu, Z. Significant improvement of surface area and CO2 adsorption of Cu–BTC via Solvent Exchange Activation. RSC Adv. 2013, 3, 17065. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, S.; Qian, J.-F.; Fan, W.; He, M.-Y.; Chen, Q.; Zhang, Z.-H. Effective adsorption separation of n-hexane/2-methylpentane in facilely synthesized zeolitic imidazolate frameworks zif-8 and zif-69. Ind. Eng. Chem. Res. 2016, 55, 10751–10757. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. Zif-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Kim, J.; Kim, S.-N.; Ahn, W.-S. High yield 1-L scale synthesis of ZIF-8 via a sonochemical route. Microporous Mesoporous Mater. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Torres, N.; Galicia, J.; Plasencia, Y.; Cano, A.; Echevarría, F.; Desdin-Garcia, L.F.; Reguera, E. Implications of structural differences between Cu-BTC and Fe-BTC on their hydrogen storage capacity. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 138–146. [Google Scholar] [CrossRef]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable fe-BTC catalyst for efficient removal of mehylene blue by advanced Fenton Oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Horcajada, P.; Gibson, E.; Vishnuvarthan, M.; Vimont, A.; Grenèche, J.-M.; Serre, C.; Daturi, M.; Garcia, H. Comparison of porous iron trimesates basolite F300 and mil-100(fe) as heterogeneous catalysts for Lewis acid and oxidation reactions: Roles of structural defects and stability. ACS Catal. 2012, 2, 2060–2065. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous fe(btc). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Autie-Castro, G.; Autie, M.A.; Rodríguez-Castellón, E.; Aguirre, C.; Reguera, E. Cu-BTC and Fe-BTC Metal-organic frameworks: Role of the materials structural features on their performance for volatile hydrocarbons separation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 351–357. [Google Scholar] [CrossRef]
- Dathe, H.; Peringer, E.; Roberts, V.; Jentys, A.; Lercher, J.A. Metal organic frameworks based on Cu2+ and benzene-1,3,5-tricarboxylate as host for SO2 trapping agents. C. R. Chim. 2005, 8, 753–763. [Google Scholar] [CrossRef]
- Chowdhury, P.; Bikkina, C.; Meister, D.; Dreisbach, F.; Gumma, S. Comparison of adsorption isotherms on Cu-BTC Metal Organic Frameworks synthesized from different routes. Microporous Mesoporous Mater. 2009, 117, 406–413. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.-D.; Ozgur Yazaydin, A. A comparative study of CO2, CH4 and N2 adsorption in zif-8, zeolite-13x and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of zif-8 under oxidative and Inert Environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on Metal Organic Frameworks of cu-BTC, Fe-BTC and zif-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.; de Asua, I.; Ruano, D.; Diaz, K. Direct synthesis, structural features, and enhanced catalytic activity of the basolite F300-like Semiamorphous Fe-BTC Framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Chevreau, H.; Horcajada, P.; Devic, T.; Serre, C.; Garcia, H. Iron(III) metal–organic frameworks as solid Lewis acids for the isomerization of α-pinene oxide. Catal. Sci. Technol. 2012, 2, 324–330. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Ma, H.; Xu, R.; Liu, H.; Li, D.; Tian, Z. Synthesis of zif-8 in a deep eutectic solvent using cooling-induced crystallisation. Microporous Mesoporous Mater. 2014, 195, 50–59. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Liu, Q.; Wang, K.; Chen, F.; Wang, H. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous Mesoporous Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (zif-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- McKinstry, C.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Scalable continuous production of high quality HKUST-1 via conventional and microwave heating. Chem. Eng. J. 2017, 326, 570–577. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Dumée, L.F.; Liu, D.; Velleman, L.; She, F.; Banos, C.; Davies, J.B.; Kong, L. Silver nanoparticles prepared by gamma irradiation across metal–organic framework templates. RSC Adv. 2015, 5, 10707–10715. [Google Scholar] [CrossRef]
- Conde-González, J.E.; Peña-Méndez, E.M.; Rybáková, S.; Pasán, J.; Ruiz-Pérez, C.; Havel, J. Adsorption of silver nanoparticles from aqueous solution on copper-based metal organic frameworks (HKUST-1). Chemosphere 2016, 150, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of zif-8 nanoparticles for controlled release of 6-Mercaptopurine Drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Bustamante, E.L.; Fernández, J.L.; Zamaro, J.M. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (zif-8) nanocrystals at room temperature. J. Colloid Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Le, K.K.A.; Phan, N.T.S. A zeolite imidazolate framework zif-8 catalyst for Friedel-Crafts acylation. Chin. J. Catal. 2012, 33, 688–696. [Google Scholar] [CrossRef]
- Luan Tran, B.; Chin, H.-Y.; Chang, B.K.; Chiang, A.S.T. Dye adsorption in zif-8: The importance of external surface area. Microporous Mesoporous Mater. 2019, 277, 149–153. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Yoon, J.W.; Lee, J.S.; Lee, U.-H.; Hwang, Y.K.; Jun, C.-H.; Horcajada, P.; Serre, C.; Chang, J.-S. Large scale fluorine-free synthesis of hierarchically porous iron(iii) trimesate mil-100(fe) with a zeolite MTN topology. Microporous Mesoporous Mater. 2012, 157, 137–145. [Google Scholar] [CrossRef]
- Wu, S.-C.; You, X.; Yang, C.; Cheng, J.-H. Adsorption behavior of methyl orange onto an aluminum-based metal organic framework, MIL-68(AL). Water Sci. Technol. 2017, 75, 2800–2810. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (mofs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.-E.; El Haddad, M. Use of response factorial design for process optimization of basic dye adsorption onto activated carbon derived from Persea species. Microchem. J. 2017, 130, 129–136. [Google Scholar] [CrossRef]
- Mathialagan, T.; Viraraghavan, T. Biosorption of pentachlorophenol by fungal biomass from aqueous solutions: A factorial design analysis. Environ. Technol. 2005, 26, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on go and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Yao, Y.; Bing, H.; Feifei, X.; Xiaofeng, C. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Guan, Q.; Li, W. Kinetic and thermodynamic studies on the adsorption of xylenol orange onto MIL-101(CR). Chem. Eng. J. 2012, 183, 60–67. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.-H.; Yan, X.-P. Metal–organic framework mil-100(fe) for the adsorption of Malachite Green from aqueous solution. J. Mater. Chem. 2012, 22, 7449. [Google Scholar] [CrossRef]
- Embaby, M.S.; Elwany, S.D.; Setyaningsih, W.; Saber, M.R. The adsorptive properties of uio-66 towards organic dyes: A record adsorption capacity for the anionic dye alizarin red s. Chin. J. Chem. Eng. 2018, 26, 731–739. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, R.; Wang, J. Adsorption of methyl orange from aqueous solution using Chitosan/diatomite composite. Water Sci. Technol. 2017, 75, 1633–1642. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ning, J.; Gao, M.; Jiang, W.; Zhou, Z.; Li, G. Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.H.; Chen, J.; Jiang, D.Y.; Guo, X.L.; Chen, H.; Zhang, Y.X. Merging of Memory Effect and anion intercalation: Mnox-decorated mgal-LDO as a high-performance nano-adsorbent for the removal of methyl orange. Dalton Trans. 2016, 45, 10530–10538. [Google Scholar] [CrossRef] [PubMed]
- Patra, G.; Barnwal, R.; Behera, S.K.; Meikap, B.C. Removal of dyes from aqueous solution by sorption with fly ash using a hydrocyclone. J. Environ. Chem. Eng. 2018, 6, 5204–5211. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of uio-66 metal-organic frameworks (mofs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fe-based metal organic framework/graphene oxide composite as an efficient catalyst for Fenton-like degradation of methyl orange. RSC Adv. 2017, 7, 50829–50837. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ghaedi, A.M.; Negintaji, E.; Ansari, A.; Vafaei, A.; Rajabi, M. Random forest model for removal of bromophenol blue using activated carbon obtained from Astragalus bisulcatus tree. J. Ind. Eng. Chem. 2014, 20, 1793–1803. [Google Scholar] [CrossRef]
- Tong, M.; Liu, D.; Yang, Q.; Devautour-Vinot, S.; Maurin, G.; Zhong, C. Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. J. Mater. Chem. A 2013, 1, 8534. [Google Scholar] [CrossRef]
- Ji, W.-J.; Hao, R.-Q.; Pei, W.-W.; Feng, L.; Zhai, Q.-G. Design of two isoreticular CD-biphenyltetracarboxylate frameworks for Dye adsorption, separation and photocatalytic degradation. Dalton Trans. 2018, 47, 700–707. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Din, A.T.M.; Hameed, B.H.; Ahmad, A.L. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater. 2009, 161, 1522–1529. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Teng, H.; Hsieh, C.-T. Activation Energy for Oxygen Chemisorption on Carbon at Low Temperatures. Ind. Eng. Chem. Res. 1999, 38, 292–297. [Google Scholar] [CrossRef]
- Piasecki, W.; Rudziński, W. Application of the statistical rate theory of interfacial transport to investigate the kinetics of divalent metal ion adsorption onto the energetically heterogeneous surfaces of oxides and activated carbons. Appl. Surf. Sci. 2007, 253, 5814–5817. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Mittal, A.; Malviya, A.; Kaur, D.; Mittal, J.; Kurup, L. Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials. J. Hazard. Mater. 2007, 148, 229–240. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009. [CrossRef]
- Machado, F.M.; Bergmann, C.P.; Lima, E.C.; Royer, B.; de Souza, F.E.; Jauris, I.M.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Blue 4 dye from water solutions by carbon nanotubes: Experiment and theory. Phys. Chem. Chem. Phys. 2012, 14, 11139. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic oxidation of cycloalkenes catalyzed by iron metal organic framework containing N-hydroxyphthalimide. J. Catal. 2012, 289, 259–265. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.-J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Basar, C. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Najafi, F. Synthesis, amine functionalization and dye removal ability of titania/silica nano-hybrid. Microporous Mesoporous Mater. 2012, 156, 153–160. [Google Scholar] [CrossRef]
- Shen, T.; Luo, J.; Zhang, S.; Luo, X. Hierarchically mesostructured MIL-101 metal–organic frameworks with different mineralizing agents for adsorptive removal of methyl orange and methylene blue from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1372–1383. [Google Scholar] [CrossRef]
- Haque, E.; Lo, V.; Minett, A.I.; Harris, A.T.; Church, T.L. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal–organic framework, amino-MIL-101(Al). J. Mater. Chem. A 2014, 2, 193–203. [Google Scholar] [CrossRef]
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef]
- Kara, M.; Yuzer, H.; Sabah, E.; Celik, M. Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res. 2003, 37, 224–232. [Google Scholar] [CrossRef]








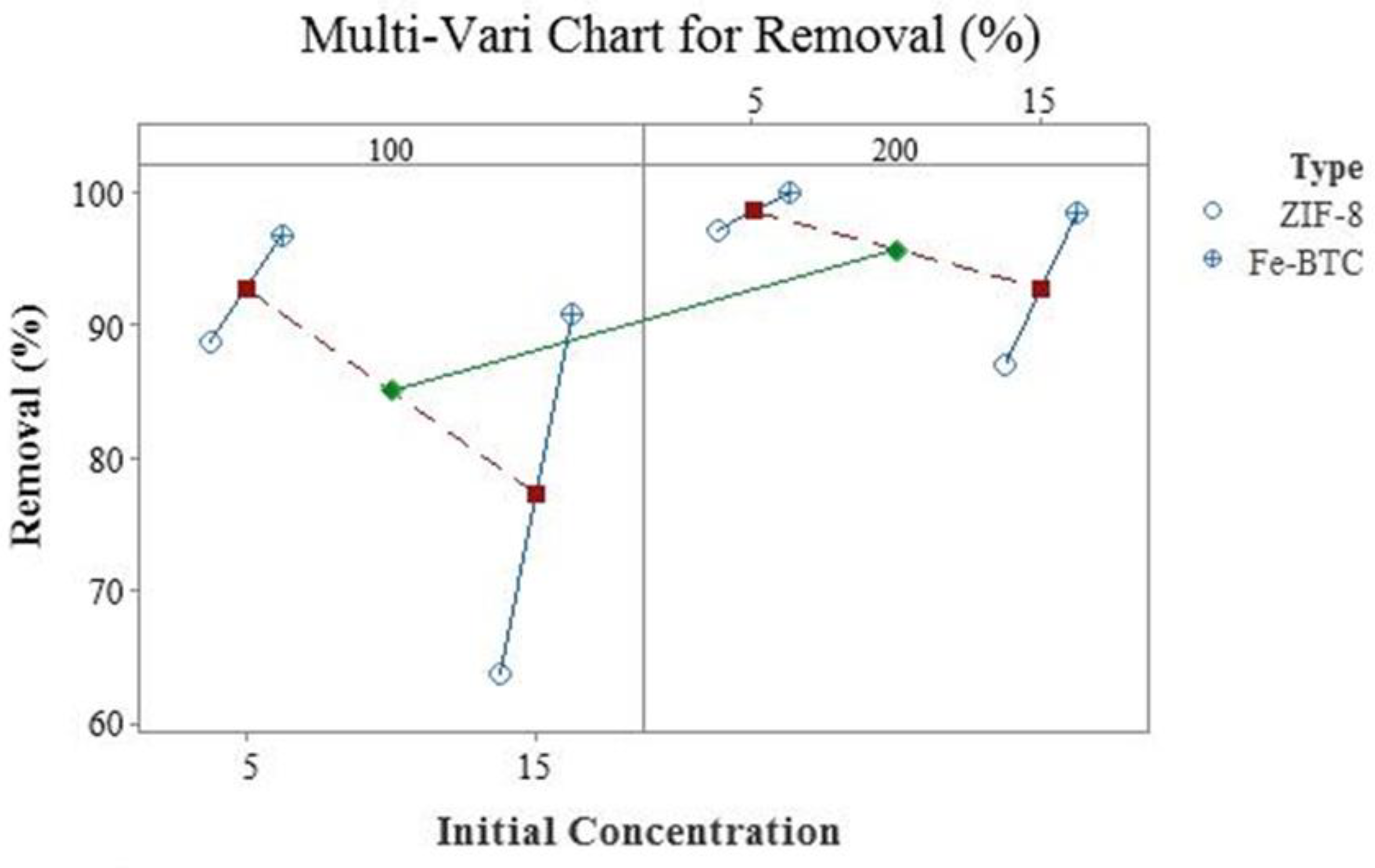

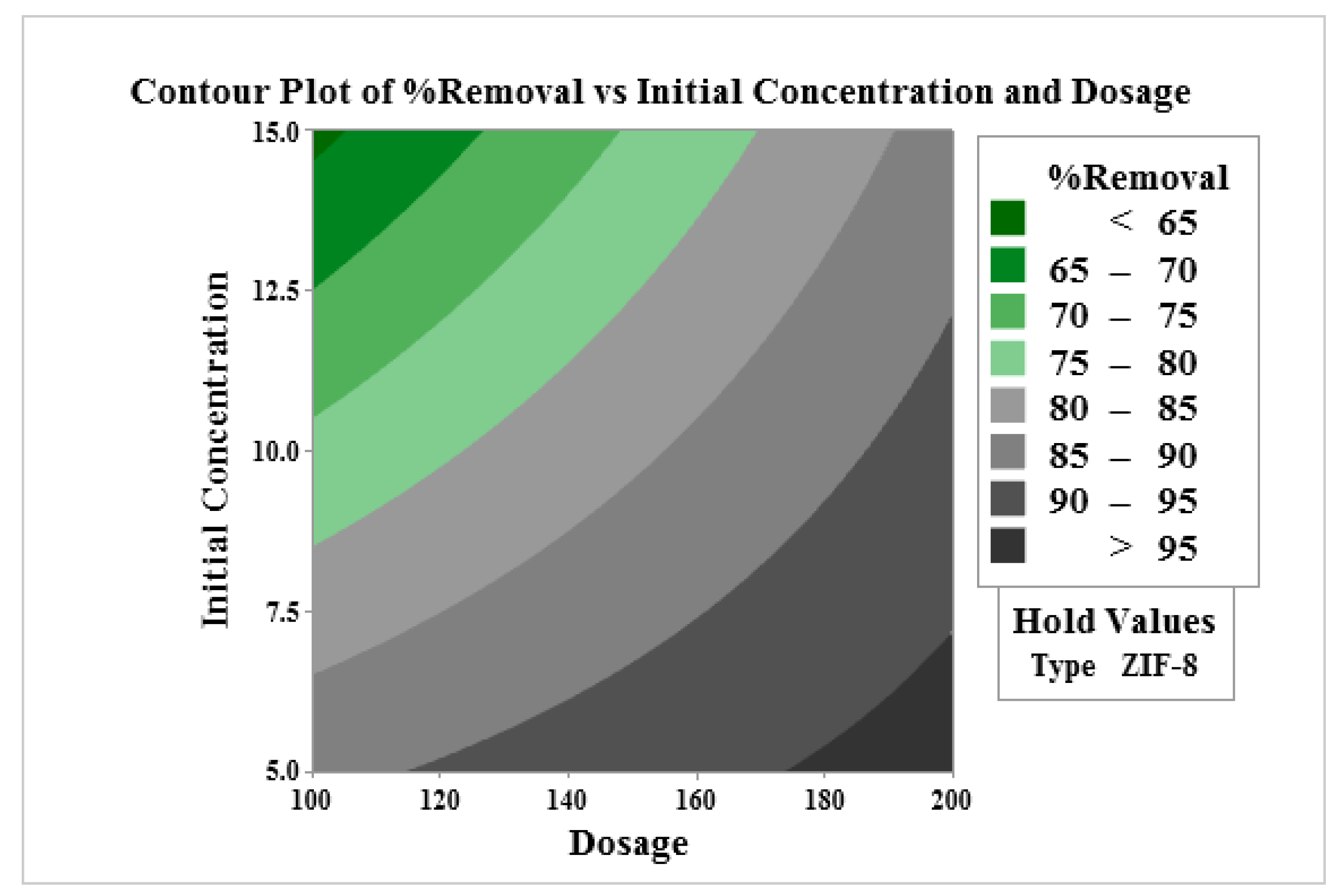

| Factor | Code | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| Type of MOF | A | ZIF-18 | Fe-BTC |
| Dosage (mg) | B | 100 | 200 |
| Initial Concentration (mg/L) | C | 5 | 15 |
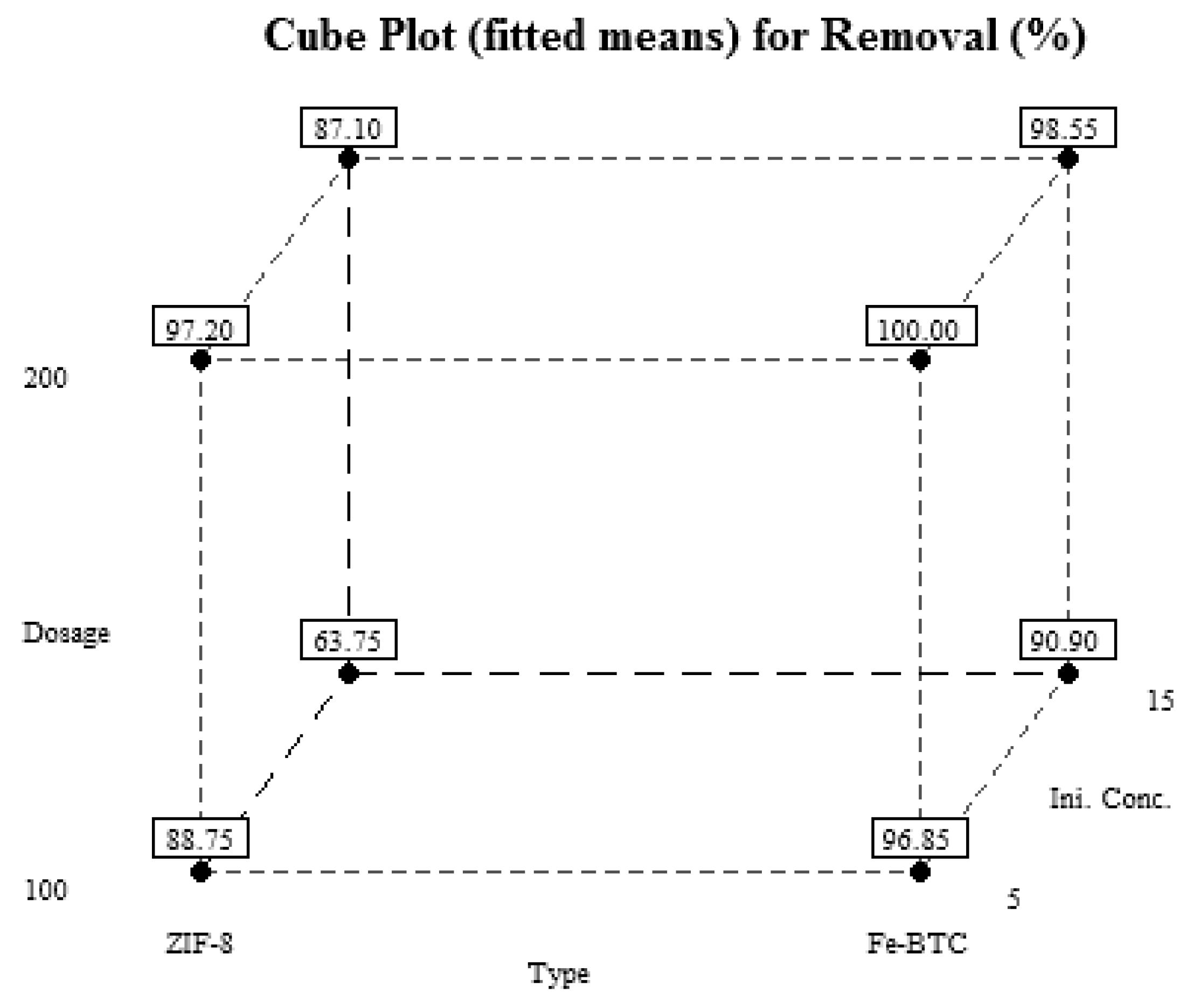
| Runs | A | B | C | MO Removal Efficiency (%) |
|---|---|---|---|---|
| 1 | −1 | +1 | +1 | 87.3 |
| 2 | −1 | −1 | −1 | 88.9 |
| 3 | +1 | +1 | +1 | 99 |
| 4 | +1 | −1 | −1 | 96.9 |
| 5 | −1 | −1 | +1 | 61.5 |
| 6 | −1 | +1 | −1 | 97.2 |
| 7 | −1 | −1 | −1 | 88.6 |
| 8 | +1 | −1 | +1 | 90.9 |
| 9 | −1 | +1 | +1 | 86.9 |
| 10 | +1 | +1 | −1 | 100 |
| 11 | +1 | −1 | −1 | 96.8 |
| 12 | +1 | +1 | −1 | 100 |
| 13 | +1 | +1 | +1 | 98.1 |
| 14 | +1 | −1 | +1 | 90.9 |
| 15 | −1 | −1 | +1 | 66 |
| 16 | −1 | +1 | −1 | 97.2 |
| Source | DF | Adjusted Sum of Squares (Adj. SS) | Adjusted Mean Squares (Adj. MS) | F-Value | p-Value | Contribution |
|---|---|---|---|---|---|---|
| A | 1 | 612.6 | 612.6 | 459.7 | 0.000 | 31.39% |
| B | 1 | 453.7 | 453.7 | 340.5 | 0.000 | 23.25% |
| C | 1 | 451.6 | 451.6 | 338.9 | 0.000 | 23.14% |
| AB | 1 | 110.3 | 110.3 | 82.74 | 0.000 | 5.650% |
| AC | 1 | 191.8 | 191.8 | 144.0 | 0.000 | 9.830% |
| BC | 1 | 94.09 | 94.09 | 70.61 | 0.000 | 4.820% |
| ABC | 1 | 27.04 | 27.04 | 20.29 | 0.002 | 1.390% |
| Error | 8 | 10.66 | 1.332 | |||
| Total | 7 | 1951.68 | ||||
| Main effects | 7 | 1941.02 | 277.288 | 208.10 | 0.000 | |
| 2-way interactions | 3 | 396.16 | 132.054 | 99.10 | 0.000 | |
| 3-way interaction | 1 | 27.04 | 27.040 | 20.29 | 0.002 |
| Term | Effect | Coefficient | Standard Error Coefficient | T-Statistic | p-Value |
|---|---|---|---|---|---|
| Constant | 90.39 | 0.289 | 313.21 | 0.000 | |
| A | 12.38 | 6.188 | 0.289 | 21.44 | 0.000 |
| B | 10.65 | 5.325 | 0.289 | 18.45 | 0.000 |
| C | −10.63 | −5.312 | 0.289 | −18.41 | 0.000 |
| AB | −5.250 | −2.625 | 0.289 | −9.10 | 0.000 |
| AC | 6.925 | 3.463 | 0.289 | 12.00 | 0.000 |
| BC | 4.850 | 2.425 | 0.289 | 8.40 | 0.000 |
| ABC | −2.600 | −1.300 | 0.289 | −4.50 | 0.002 |
| S | 1.154 | ||||
| R-Sq | 99.45% | ||||
| R-Sq (adj.) | 98.98% | ||||
| Adsorbent Type | pH | Time (Min) | Initial Concentration (mg/L) | Adsorption Capacity (%) | References |
|---|---|---|---|---|---|
| Zirconium MOF | 31 | [79] | |||
| chitosan/diatomite composite | 5 | 40 | 50 | 88.3 | [80] |
| Polyethyleneimine-modified persimmon tannin | 4 | 120 | 30 | 97.6 | [81] |
| MnOx-decorated MgAl | 20 | 40 | 90.5 | [82] | |
| Fly ash | 8.5 | 60 | 66 | [83] | |
| UiO-66 MOF | 5.5 | 180 | 10 | 87.4 | [84] |
| Fe-based MOF/graphene oxide | 3 | 240 | 50 | 98 | [85] |
| Fe-BTC | 6 | 60 | 15 | 99 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, A.; Shomal, R.; Sabouni, R.; Al-Sayah, M.H.; Aidan, A. Parametric Study of Methyl Orange Removal Using Metal–Organic Frameworks Based on Factorial Experimental Design Analysis. Energies 2022, 15, 4642. https://doi.org/10.3390/en15134642
Karami A, Shomal R, Sabouni R, Al-Sayah MH, Aidan A. Parametric Study of Methyl Orange Removal Using Metal–Organic Frameworks Based on Factorial Experimental Design Analysis. Energies. 2022; 15(13):4642. https://doi.org/10.3390/en15134642
Chicago/Turabian StyleKarami, Abdollah, Reem Shomal, Rana Sabouni, Mohammad H. Al-Sayah, and Ahmed Aidan. 2022. "Parametric Study of Methyl Orange Removal Using Metal–Organic Frameworks Based on Factorial Experimental Design Analysis" Energies 15, no. 13: 4642. https://doi.org/10.3390/en15134642