Recent Studies on the Environmentally Benign Alkaline-Earth Silicide Mg2Si for Middle-Temperature Thermoelectric Applications
Abstract
:1. Introduction
2. Improvements in the TE Performance of Mg2Si by Lightly Doping It with Isoelectronic Impurities
2.1. TE Performance of Mg2Si Codoped with Sb and the Isoelectronic Impurity Zn
2.1.1. Energetic Stabilities
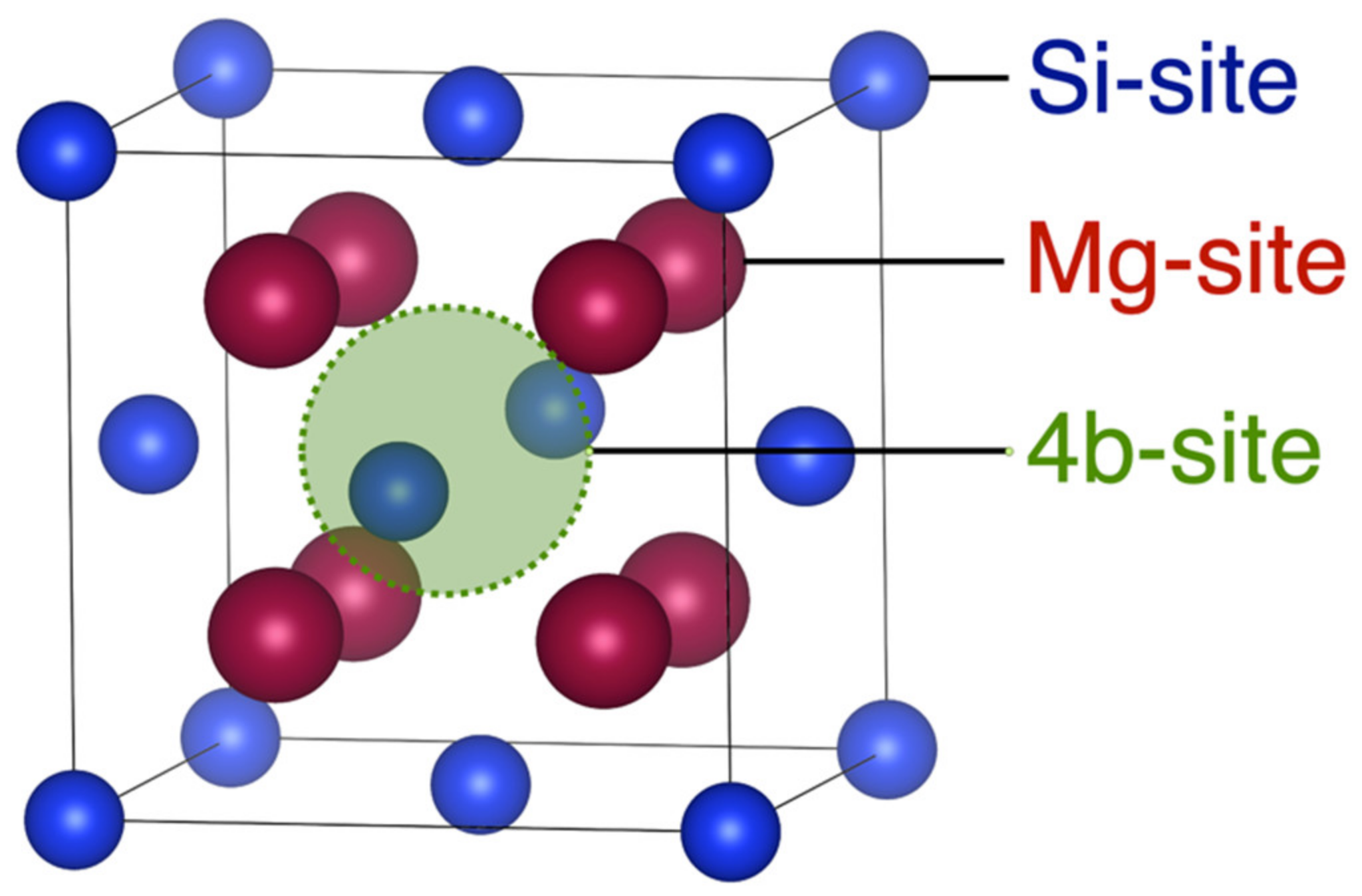
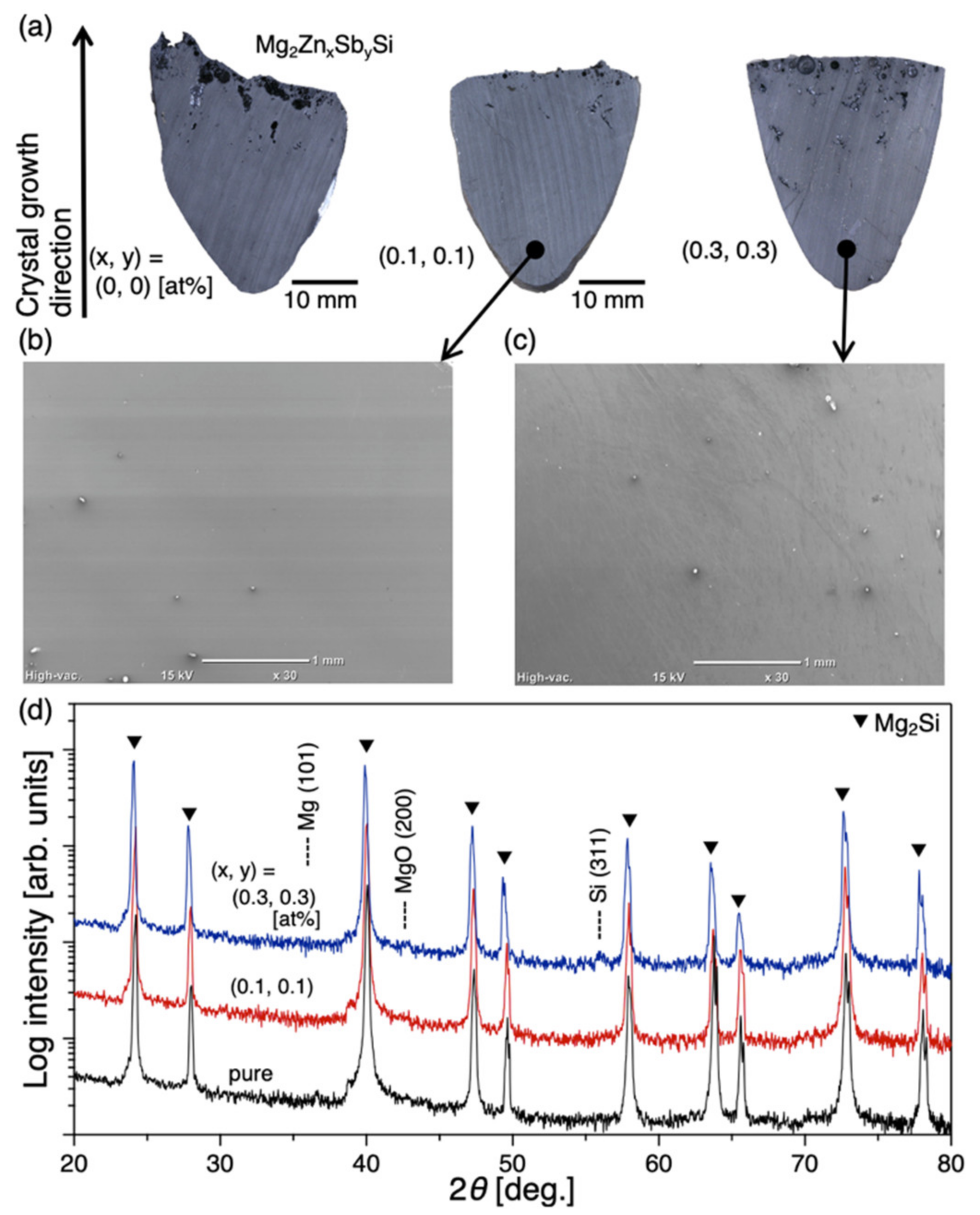
2.1.2. Crystal Structure
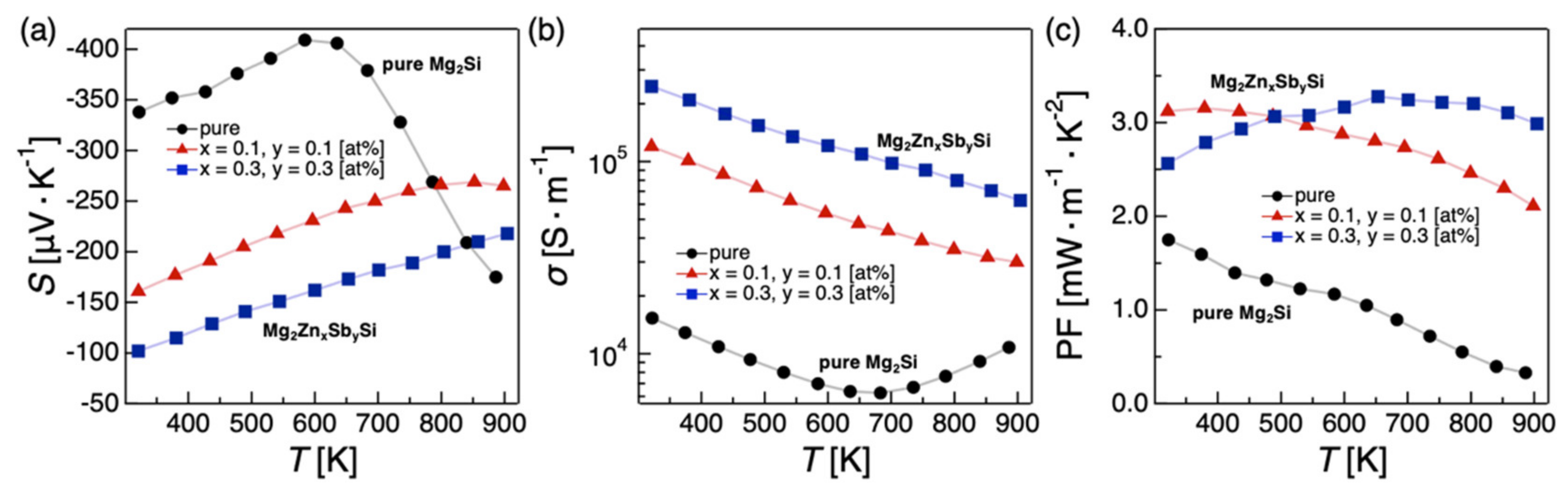
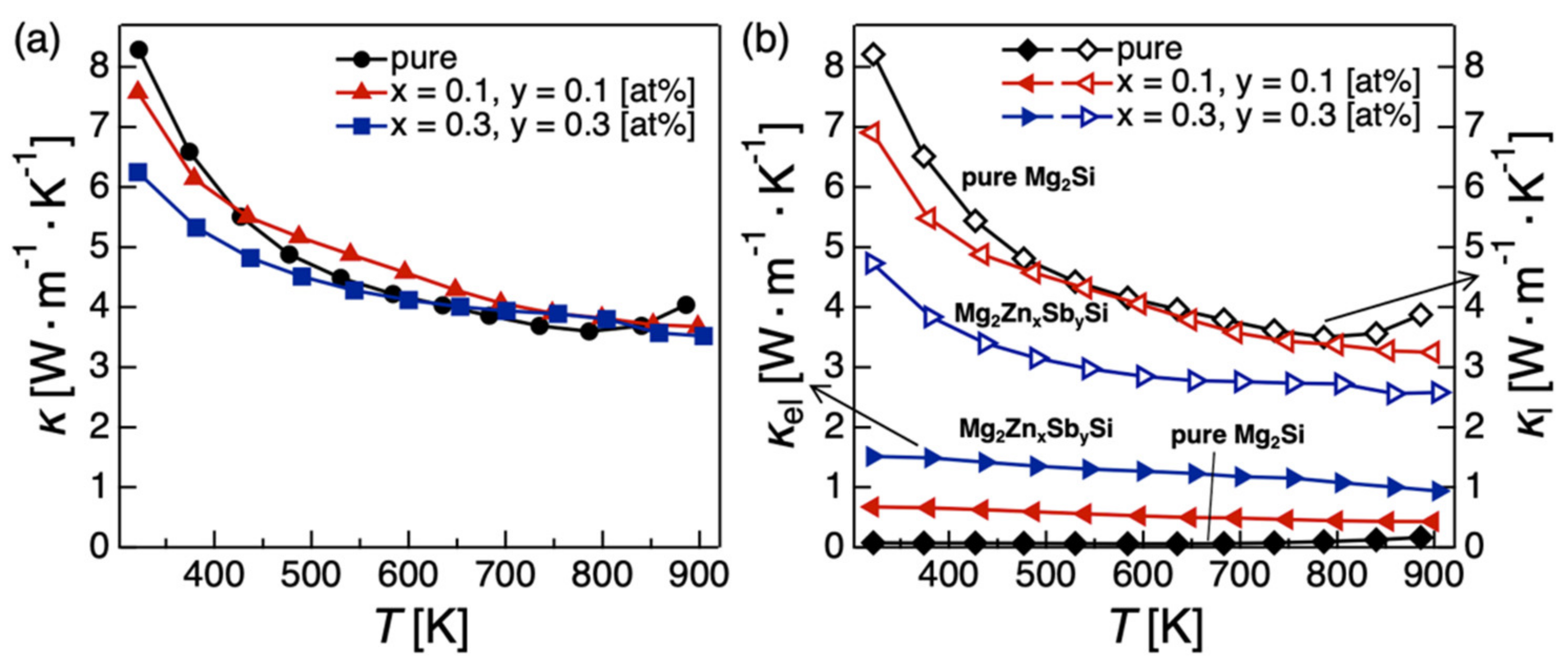
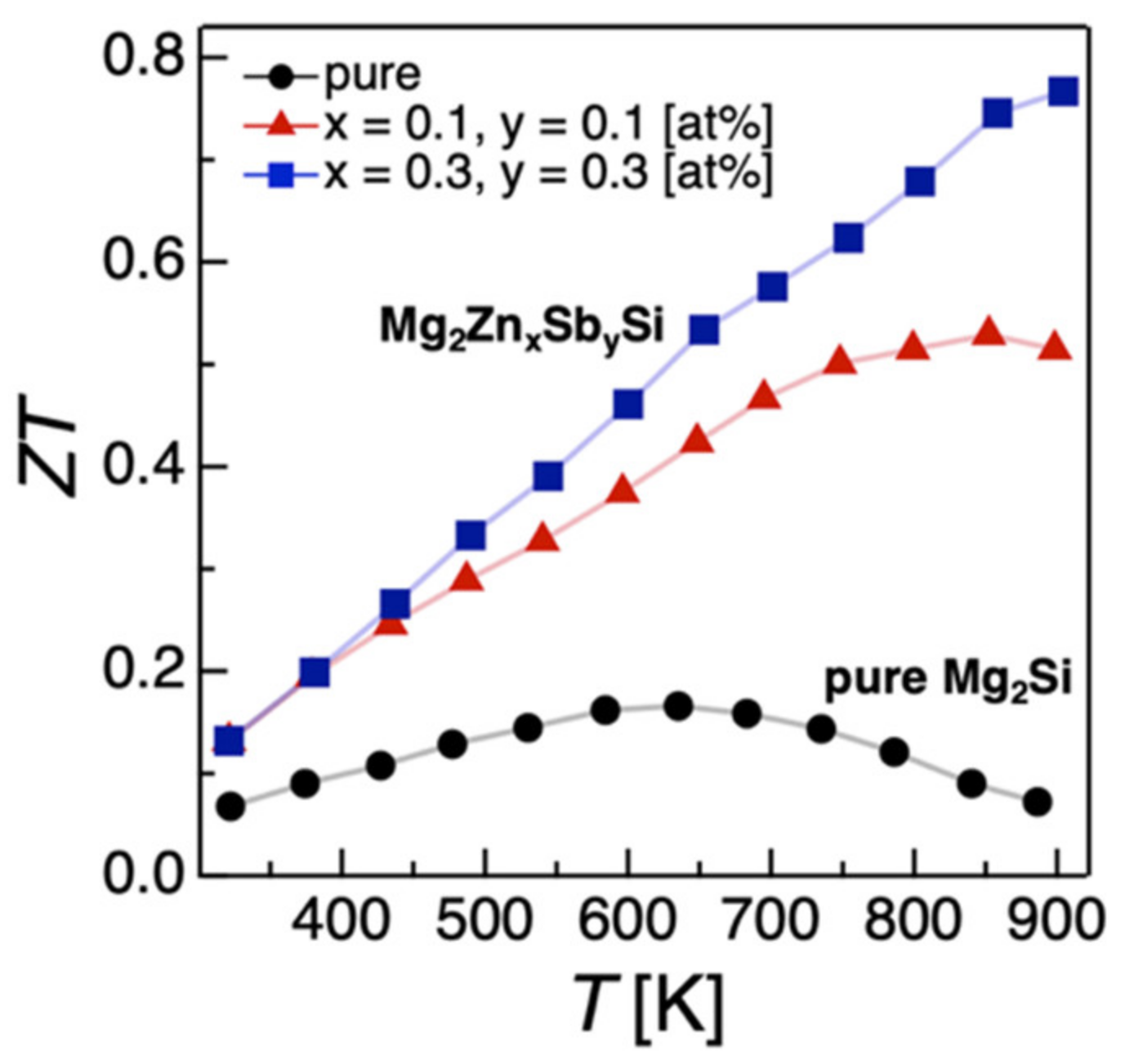
2.1.3. Codoping Effects on the Electronic, Thermal, and TE Properties
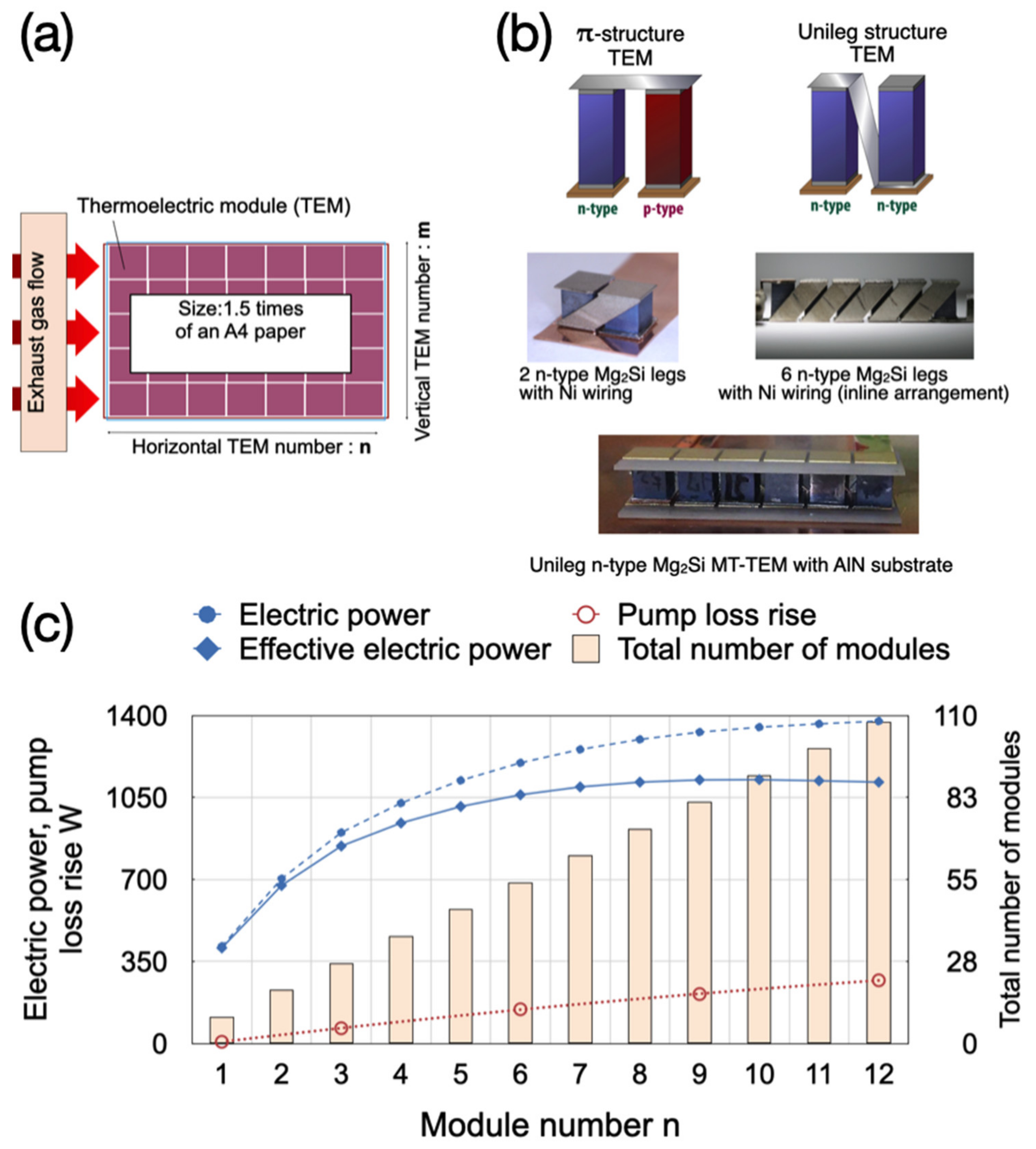
2.2. Electrodes
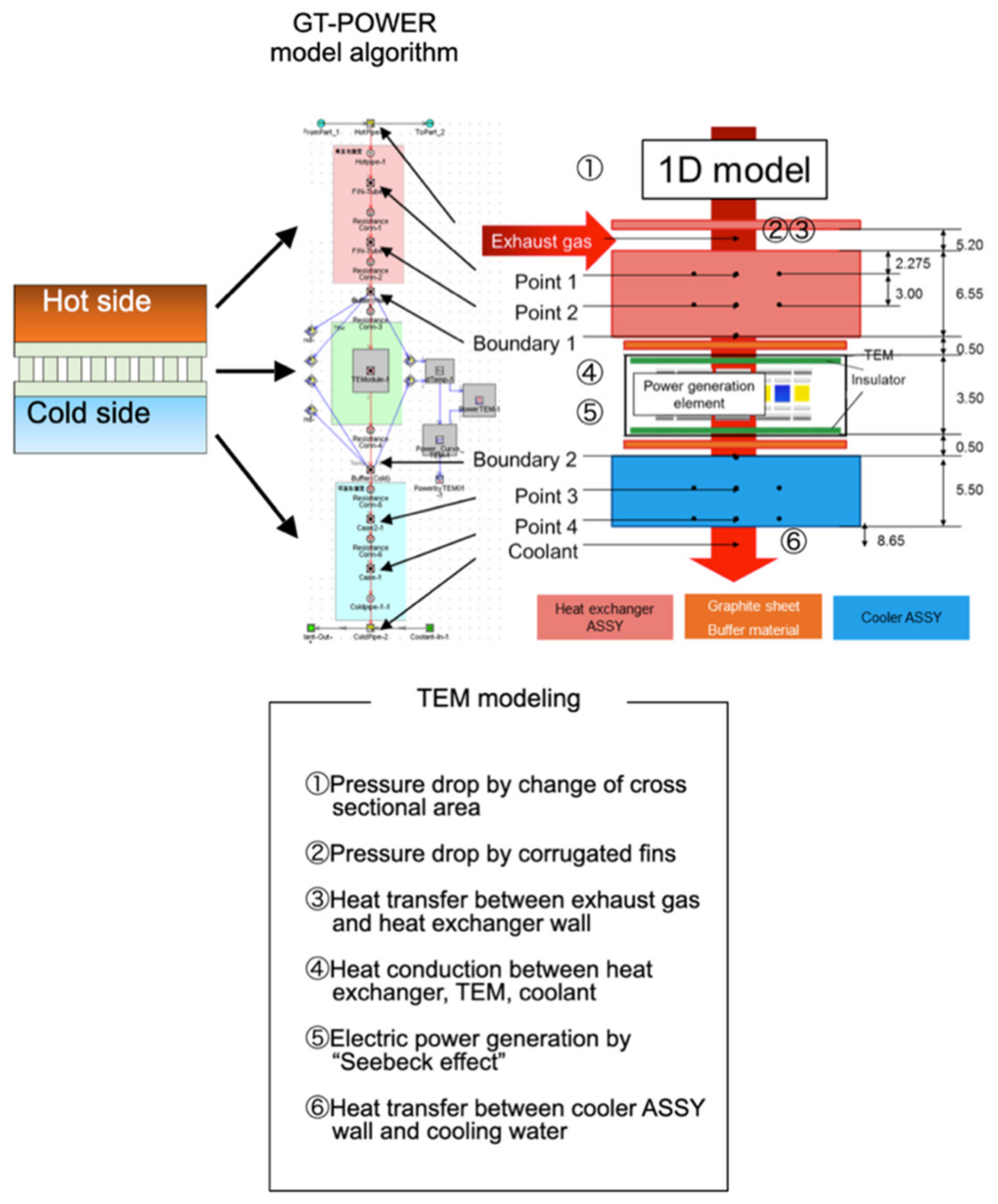
3. Thermoelectric Generator Modeling in Combustion Engine Simulator
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, 1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. Engl. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Radio Corporation of America, Astro-Electronics Division; U.S. Atomic Energy Commission. High-Temperature Radioisotope Thermoelectric Generator for Space Applications; Radio Corporation of America, Astro-Electronics Division; U.S. Atomic Energy Commission: Washington, DC, USA, 1964. [Google Scholar]
- Goldsmid, H.J. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmid, H.J.; Douglas, R.W. The use of semiconductors in thermoelectric refrigeration. Br. J. Appl. Phys. 1954, 5, 386–390. [Google Scholar] [CrossRef]
- Liu, Z.; Sato, N.; Gao, W.; Yubuta, K.; Kawamoto, N.; Mitome, M.; Kurashima, K.; Owada, Y.; Nagase, K.; Lee, C.-H.; et al. Demonstration of ultrahigh thermoelectric efficiency of ∼7.3% in Mg3Sb2/MgAgSb module for low-temperature energy harvesting. Joule 2021, 5, 1196–1208. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Pedersen, S.H.; Yin, H.; Hung, L.T.; Iversen, B.B. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Commun. 2017, 8, 13901. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Mao, J.; Sui, J.; Ren, Z. High thermoelectric performance of α-MgAgSb for power generation. Energy Environ. Sci. 2018, 11, 23–44. [Google Scholar] [CrossRef]
- Wei, T.-R.; Qin, Y.; Deng, T.; Song, Q.; Jiang, B.; Liu, R.; Qiu, P.; Shi, X.; Chen, L. Copper chalcogenide thermoelectric materials. Sci. China Mater. 2019, 62, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, X.; Ren, Z.; Zhang, Q. Recent progress towards high performance of tin chalcogenide thermoelectric materials. J. Mater. Chem. 2018, A6, 2432–2448. [Google Scholar] [CrossRef]
- Qiu, P.; Shi, X.; Chen, L. Cu-based thermoelectric materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Ohtaki, M. Recent aspects of oxide thermoelectric materials for power generation from mid-to-high temperature heat source. J. Ceram. Soc. Jpn. 2011, 119, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Nozariasbmarz, A.; Agarwal, A.; Coutant, Z.A.; Hall, M.J.; Liu, J.; Liu, R.; Malhotra, A.; Norouzzadeh, P.; Öztürk, M.C.; Ramesh, V.P.; et al. Thermoelectric silicides: A review. Jpn. J. Appl. Phys. 2017, 56, 05DA04. [Google Scholar] [CrossRef]
- Hou, Z.; Takagiwa, Y.; Shinohara, Y.; Xu, Y.; Tsuda, K. First-principles study of electronic structures and elasticity of Al2Fe3Si3. J. Phys. Condens. Matter. 2021, 33, 195501. [Google Scholar] [CrossRef]
- Takagiwa, Y.; Isoda, Y.; Goto, M.; Shinohara, Y. Conduction type control and power factor enhancement of the thermoelectric material Al2Fe3Si3. J. Phys. Chem. Solids 2018, 118, 95–98. [Google Scholar] [CrossRef]
- Hirayama, N.; Imai, Y.; Hamada, N. Conduction band engineering of Mg2Si by isotropic strain for enhancement of thermoelectric performance: A first-principles study. J. Appl. Phys. 2020, 127, 205107. [Google Scholar] [CrossRef]
- Hirayama, N.; Iida, T.; Sakamoto, M.; Nishio, K.; Hamada, N. Substitutional and interstitial impurity p-type doping of thermoelectric Mg2Si: A theoretical study. Sci. Technol. Adv. Mater. 2019, 20, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Masuoka, Y.; Mito, Y.; Ogino, A.; Nakamura, T.; Amano, K.; Asahi, R. Collaborative effects of Zn and Sb co-doping in magnesium silicide for thermoelectric applications. J. Alloys Compd. 2019, 781, 606–612. [Google Scholar] [CrossRef]
- Imai, Y.; Hirayama, N.; Yamamoto, A.; Iida, T.; Takarabe, K. Exploratory Study of Substitutional Elements in Mg2Si for Inducing State of Negative Chemical Pressure. Mater. Trans. 2018, 59, 1417–1422. [Google Scholar] [CrossRef]
- Imai, Y.; Mori, Y.; Nakamura, S.; Takarabe, K. Consideration about the synthesis pressure effect on lattice defects of Mg2Si using first-principle calculations. J. Alloys Compd. 2016, 664, 377–569. [Google Scholar] [CrossRef]
- Imai, Y.; Sohma, M.; Suemasu, T. Effect of oxygen incorporation in the Mg2Si lattice on its conductivity type—A possible reason of the p-type conductivity of postannealed Mg2Si thin film. J. Alloys Compd. 2016, 676, 91–95. [Google Scholar] [CrossRef]
- Kubouchi, M.; Hayashi, K.; Miyazaki, Y. Electronic structure and thermoelectric properties of boron doped Mg2Si. Scr. Mater. 2016, 123, 59–63. [Google Scholar] [CrossRef]
- Hirayama, N.; Iida, T.; Funashima, H.; Morioka, S.; Sakamoto, M.; Nishio, K.; Kogo, Y.; Takanashi, K.; Hamada, N. First-principles study on structural and thermoelectric properties of Al- and Sb-doped Mg2Si. J. Electron. Mater. 2015, 44, 1656–1662. [Google Scholar] [CrossRef]
- Oto, Y.; Iida, T.; Sakamoto, T.; Miyahara, R.; Natsui, A.; Nishio, K.; Kogo, Y.; Hirayama, N.; Takanashi, Y. Thermoelectric properties and durability at elevated temperatures of impurity doped n-type Mg2Si. Phys. Status Solidi C 2013, 10, 1857–1861. [Google Scholar] [CrossRef]
- Imai, Y.; Mori, Y.; Nakamura, S.; Takarabe, K. Energetic consideration of the conduction type of Mg2Si doped with Cu, Ag, or Au using first-principle calculations. J. Alloys Compd. 2013, 549, 175–178. [Google Scholar] [CrossRef]
- Meng, Q.S.; Fan, W.H.; Chen, R.X.; Munir, Z.A. Thermoelectric properties of Sc- and Y-doped Mg2Si prepared by field-activated and pressure-assisted reactive sintering. J. Alloys Compd. 2011, 509, 7922–7926. [Google Scholar] [CrossRef]
- Sakamoto, T.; Iida, T.; Matsumoto, A.; Honda, Y.; Nemoto, T.; Sato, J.; Nakajima, T.; Taguchi, H.; Takanashi, Y. Thermoelectric characteristics of a commercialized Mg2Si source doped with Al, Bi, Ag, and Cu. J. Electron. Mater. 2010, 39, 1708–1713. [Google Scholar] [CrossRef] [Green Version]
- Akasaka, M.; Iida, T.; Matsumoto, A.; Yamanaka, K.; Takanashi, K.; Imai, T.; Hamada, N. The thermoelectric properties of bulk crystalline n- and p-type Mg2Si prepared by the vertical Bridgman method. J. Appl. Phys. 2008, 104, 013703. [Google Scholar] [CrossRef]
- Imai, Y.; Watanabe, A.; Mukaida, M. Electronic Structures of Semiconducting Alkaline-Earth Metal Silicides. J. Alloys Compd. 2013, 358, 257. [Google Scholar] [CrossRef]
- Fiameni, S.; Battiston, S.; Soldrini, S.; Famengo, A.; Agresti, F.; Barison, S.; Fabrizio, M. Synthesis and characterization of Bi-doped Mg2Si thermoelectric materials. J. Solid State Chem. 2012, 193, 142–146. [Google Scholar] [CrossRef]
- You, S.W.; Kim, I.H. Solid-state synthesis and thermoelectric properties of Bi-doped Mg2Si compounds. Curr. Appl. Phys. 2011, 11, S392–S395. [Google Scholar] [CrossRef]
- Tani, J.; Kido, H. Thermoelectric Properties of Bi-Doped Mg2Si Semiconductors. Phys. B Condens. Matter 2005, 364, 218–224. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Cai, B.; Chen, C.; Zhang, Q.; Zhao, Z.; Zhang, L.; Yu, F.; Yu, D.; Tian, Y.; et al. Enhanced thermoelectric performance of high pressure synthesized Sb-doped Mg2Si. J. Alloys Compd. 2018, 741, 1148–1152. [Google Scholar] [CrossRef]
- Kambe, K.; Udono, H. Convenient melt-growth method for thermoelectric Mg2Si. J. Electron. Mater. 2015, 43, 2212–2217. [Google Scholar] [CrossRef]
- Tani, J.I.; Kido, H. Thermoelectric properties of Sb-doped Mg2Si semiconductors. Intermetallics 2007, 15, 1202–1207. [Google Scholar] [CrossRef]
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Satyala, N.; Vashaee, D. The effect of crystallite size on thermoelectric properties of bulk nanostructured magnesium silicide (Mg2Si) compounds. Appl. Phys. Lett. 2012, 100, 073107. [Google Scholar] [CrossRef]
- Satyala, N.; Vashaee, D. Detrimental influence of nanostructuring on the thermoelectric properties of magnesium silicide. J. Appl. Phys. 2012, 112, 093716. [Google Scholar] [CrossRef]
- Liu, W.; Tan, X.; Yin, K.; Liu, H.; Tang, X.; Shi, J.; Zhang, Q.; Uher, C. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si1−xSnx solid solutions. Phys. Rev. Lett. 2012, 108, 166601. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, V.Z.; Fedorov, M.I.; Gurieva, E.A.; Eremin, I.S.; Konstantinov, P.P.; Samunin, A.Y.; Vedernikov, M.V. Highly effective Mg2Si1−xSnx thermoelectrics. Phys. Rev. B 2006, 74, 045207. [Google Scholar] [CrossRef]
- Labotz, R.J.; Mason, D.R.; O’Kane, D.F. The thermoelectric properties of mixed crystals of Mg2GexSi1−x. J. Electrochem. Soc. 1963, 110, 127–134. [Google Scholar] [CrossRef]
- Skomedal, G.; Burkov, A.; Samunin, A.; Haugsrud, R.; Middleton, H. High temperature oxidation of Mg2(Si-Sn). Corros. Sci. 2016, 111, 325–333. [Google Scholar] [CrossRef]
- Shiojiri, D.; Iida, T.; Hamba, H.; Kodama, T.; Yamaguchi, M.; Hirayama, N.; Imai, Y. Enhanced thermoelectric performance of vertical Bridgman-grown Mg2Si by codoping with Sb and Zn. J. Electron. Mater. 2022, 51, 1311–1321. [Google Scholar] [CrossRef]
- Kadono, T.; Hirayama, N.; Nishio, T.; Yamazawa, S.; Oki, N.; Takahashi, Y.; Takikawa, N.; Yasui, A.; Nitta, K.; Sekizawa, O.; et al. Investigation of local structures and electronic states of Sb-doped Mg2Si by fluorescence XAFS and HAXPES. Appl. Phys. Lett. 2020, 117, 143901. [Google Scholar] [CrossRef]
- Kubouchi, M.; Ogawa, Y.; Hayashi, K.; Takamatsu, T.; Miyazaki, Y. Effect of interstitial Mg in Mg2+xSi on electrical conductivity and Seebeck coefficient. J. Electron. Mater. 2016, 45, 1589–1593. [Google Scholar] [CrossRef]
- Kubouchi, M.; Hayashi, K.; Miyazaki, Y. Quantitative analysis of interstitial Mg in Mg2Si studied by single crystal X-ray diffraction. J. Alloys Compd. 2014, 617, 389–392. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Prosek, T.; Nazarov, A.; Bexell, U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corros. Sci. 2008, 50, 2216. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Imai, Y.; Hirayama, N.; Yamamoto, A.; Takarabe, K. Changes of the band structure of Mg2Si induced by interstitial doping with nonmetallic elements. Jpn. J. Appl. Phys. 2020, 59, SFFC04. [Google Scholar] [CrossRef]
- Akasaka, M.; Iida, T.; Nemoto, T.; Soga, J.; Sato, J.; Makino, K.; Fukano, M.; Takanashi, Y. Non-wetting crystal growth of Mg2Si by vertical Bridgman method and thermoelectric characteristics. J. Cryst. Growth 2007, 304, 196. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef] [Green Version]
- Iida, T.; Inoue, R.; Shiojiri, D.; Hirayma, N.; Hamada, N.; Kogo, Y. Thermoelectric Energy Conversion: Theories and Mechanisms, Materials, Devices, and Applications, Chapter 2.16: Silicide Materials: Thermoelectric, Mechanical Properties, and Durability for Mg-Si and Mn-Si; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128185353. [Google Scholar] [CrossRef]
- Imai, Y.; Sugawara, H.; Mori, Y.; Nakamura, S.; Yamamoto, A.; Takarabe, K. Energetic consideration of compounds at Mg2Si–Ni electrode interlayer produced by spark-plasma sintering. Jpn. J. Appl. Phys. 2017, 56, 05DC03. [Google Scholar] [CrossRef]





| Pmax (mW) | Pdensity (W/cm2) | Efficiency (%) | Internal Resistance (mΩ) | Contact Resistance (10−9 Ω·m2) | |
|---|---|---|---|---|---|
| Monobloc sintering | 110 | 1.20 | 3.0 | 7.0 | 0.264 |
| Paste Printing | 157 | 1.62 | 3.5 | 5.6 | 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiojiri, D.; Iida, T.; Hirayama, N.; Imai, Y.; Sugawara, H.; Kusaka, J. Recent Studies on the Environmentally Benign Alkaline-Earth Silicide Mg2Si for Middle-Temperature Thermoelectric Applications. Energies 2022, 15, 4859. https://doi.org/10.3390/en15134859
Shiojiri D, Iida T, Hirayama N, Imai Y, Sugawara H, Kusaka J. Recent Studies on the Environmentally Benign Alkaline-Earth Silicide Mg2Si for Middle-Temperature Thermoelectric Applications. Energies. 2022; 15(13):4859. https://doi.org/10.3390/en15134859
Chicago/Turabian StyleShiojiri, Daishi, Tsutomu Iida, Naomi Hirayama, Yoji Imai, Hiroharu Sugawara, and Jin Kusaka. 2022. "Recent Studies on the Environmentally Benign Alkaline-Earth Silicide Mg2Si for Middle-Temperature Thermoelectric Applications" Energies 15, no. 13: 4859. https://doi.org/10.3390/en15134859






