Effects of Bioliquid Recirculation on Hydrothermal Carbonization of Lignocellulosic Biomass
Abstract
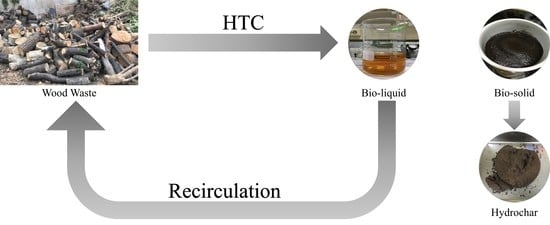
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Wood Wastes
2.1.2. Hydrothermal Carbonization (HTC) Reactor
2.2. Experiment
2.2.1. Production of Hydrochar and Bioliquid
2.2.2. Recirculation
2.3. Analyses
2.3.1. Bioliquid Analysis
2.3.2. Hydrochar Analysis
3. Results and Discussion
3.1. Characteristics of Bioliquid
3.1.1. Production and pH of Bioliquid
3.1.2. Organic Acid and Total Organic Carbon Analyses
3.1.3. Intermediate Analysis of Bioliquid
3.2. Characteristics of Hydrochar
3.2.1. Fourier Transform Infrared Analysis of Hydrochar
3.2.2. Mass Yield of Hydrochar
3.2.3. Proximate Analysis of Hydrochar
3.2.4. Changes in the Properties of Hydrochar Fuel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenhouse Gas Inventory and Research Center of Korea. National Inventory Report 2021; Ministry of Environment: Seoul, Korea, 2021.
- Korea Forest Service. Forestry Statistics 2020; Korea Forest Service: Daejeon, Korea, 2021.
- Ministry of Environment Republic of Korea. Enforcement Rule of The Wastes Control Act. Available online: https://law.go.kr/lsSc.do?section=&menuId=1&subMenuId=15&tabMenuId=81&eventGubun=060101&query=%ED%8F%90%EA%B8%B0%EB%AC%BC%EA%B4%80%EB%A6%AC%EB%B2%95#J14551159 (accessed on 21 July 2017).
- Intergovernmental Panel on Climate Change. Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2000. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Yan, W.; Hastings, J.T.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Mass and Energy Balances of Wet Torrefaction of Lignocellulosic Biomass. Energy Fuels 2010, 24, 4738–4742. [Google Scholar] [CrossRef]
- Boutaieb, M.; Román, S.; Ledesma, B.; Sabio, E.; Guiza, M.; Ouederni, A. Towards a more efficient Hydrothermal Carbonization: Processing water recirculation under different conditions. Waste Manag. 2021, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kabadayi Catalkopru, A.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef]
- Xu, C.; Leppänen, A.-S.; Eklund, P.; Holmlund, P.; Sjöholm, R.; Sundberg, K.; Willför, S. Acetylation and characterization of spruce (Picea abies) galactoglucomannans. Carbohydr. Res. 2010, 345, 810–816. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Wang, J.; Zheng, C. Effects of aqueous phase recirculation in hydrothermal carbonization of sweet potato waste. Bioresour. Technol. 2018, 267, 167–174. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Paulke, E.; Mumme, J.; Nehls, I. Hydrothermal Carbonization of Biomass: Major Organic Components of the Aqueous Phase. Chem. Eng. Technol. 2014, 37, 511–518. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Uddin, M.H.; Reza, M.T.; Lynam, J.G.; Coronella, C.J. Effects of water recycling in hydrothermal carbonization of loblolly pine. Environ. Prog. Sustain. Energy 2013, 33, 1309–1315. [Google Scholar] [CrossRef]
- Köchermann, J.; Görsch, K.; Wirth, B.; Mühlenberg, J.; Klemm, M. Hydrothermal carbonization: Temperature influence on hydrochar and aqueous phase composition during process water recirculation. J. Environ. Chem. Eng. 2018, 6, 5481–5487. [Google Scholar] [CrossRef]
- Weiner, B.; Poerschmann, J.; Wedwitschka, H.; Koehler, R.; Kopinke, F.-D. Influence of Process Water Reuse on the Hydrothermal Carbonization of Paper. ACS Sustain. Chem. Eng. 2014, 2, 2165–2171. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Gu, C.; Han, Y.; Zan, S.; Wu, S. Effects of process water recirculation on solid and liquid products from hydrothermal carbonization of Laminaria. Bioresour. Technol. 2019, 292, 121996. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Morita, S.; Ozaki, Y. Study on Temperature-Dependent Changes in Hydrogen Bonds in Cellulose Iβ by Infrared Spectroscopy with Perturbation-Correlation Moving-Window Two-Dimensional Correlation Spectroscopy. Biomacromolecules 2006, 7, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zheng, W.; Chen, H.; Zhang, Y.-H.P.J. Upgrade of wood sugar d-xylose to a value-added nutraceutical by in vitro metabolic engineering. Metab. Eng. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Oliveira, P.C.; Canhaci, S.J.; Gabriel, C.B.; Moreira, C.R.; de Farias, A.M.D.; Fraga, M.A. Conversion of xylose to bioproducts on bifunctional supported platinum-group metals catalysts. Curr. Res. Green Sustain. Chem. 2022, 5, 100305. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Oefner, P.J.; Lanziner, A.H.; Bonn, G.; Bobleter, O. Quantitative studies on furfural and organic acid formation during hydrothermal, acidic and alkaline degradation of D-xylose. Monatsh. Chem. 1992, 123, 547–556. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Sci. Technol. 2021, 56, 87–109. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Vanapalli, K.R.; Cheela, V.S.; Venna, S.; Dubey, B. Study on the process wastewater reuse and valorisation during hydrothermal co-carbonization of food and yard waste. Sci. Total Environ. 2022, 806, 150748. [Google Scholar] [CrossRef] [PubMed]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Mccollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by Fischer–Tropsch-Type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef]
- Yu, J.; Savage, P.E. Decomposition of Formic Acid under Hydrothermal Conditions. Ind. Eng. Chem. Res. 1998, 37, 2–10. [Google Scholar] [CrossRef]
- Lange, J.-P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefining 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Schwiderski, M.; Kruse, A.; Grandl, R.; Dockendorf, D. Comparison of the influence of a Lewis acid AlCl3 and a Brønsted acid HCl on the organosolv pulping of beech wood. Green Chem. 2014, 16, 1569–1578. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Yemis, O.; Mazza, G. Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 2011, 102, 7371–7378. [Google Scholar] [CrossRef]
- Parajo, J.C.; Arbeit, D. Hydrothermal processing of lignocellulosic materials. Eur. J. Wood Prod. 1999, 57, 191–202. [Google Scholar]
- Leng, S.; Li, W.; Han, C.; Chen, L.; Chen, J.; Fan, L.; Lu, Q.; Li, J.; Leng, L.; Zhou, W. Aqueous phase recirculation during hydrothermal carbonization of microalgae and soybean straw: A comparison study. Bioresour. Technol. 2020, 298, 122502. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, E. Treatment of urban sludge by hydrothermal carbonization. Bioresour. Technol. 2017, 238, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Leng, S.; Leng, L.; Chen, L.; Chen, J.; Chen, J.; Zhou, W. The effect of aqueous phase recirculation on hydrothermal liquefaction/carbonization of biomass: A review. Bioresour. Technol. 2020, 318, 124081. [Google Scholar] [CrossRef] [PubMed]




| Proximate Analysis, wt% | Fixed Carbon, % | |||
|---|---|---|---|---|
| Moisture Content | Ash Content | Volatile Matter | ||
| WW | 6.03 ± 0.16 | 1.84 ± 0.24 | 76.94 ± 0.31 | 15.19 ± 0.42 |
| H1 | 2.68 ± 0.04 | 2.35 ± 0.27 | 68.47 ± 0.04 | 26.51 ± 0.34 |
| H2 | 2.54 ± 0.14 | 2.31 ± 0.24 | 66.91 ± 0.11 | 28.24 ± 0.21 |
| H3 | 2.00 ± 0.07 | 2.37 ± 0.35 | 67.90 ± 0.19 | 27.72 ± 0.13 |
| H4 | 1.57 ± 0.04 | 2.89 ± 0.03 | 67.46 ± 1.17 | 28.08 ± 1.10 |
| H5 | 1.28 ± 0.05 | 3.09 ± 0.06 | 66.76 ± 0.09 | 28.86 ± 0.11 |
| H6 | 2.30 ± 0.02 | 2.71 ± 0.10 | 66.06 ± 0.38 | 28.93 ± 0.29 |
| H7 | 1.98 ± 0.07 | 2.85 ± 0.12 | 65.62 ± 0.27 | 29.55 ± 0.33 |
| H8 | 2.21 ± 0.07 | 2.86 ± 0.18 | 65.18 ± 0.25 | 29.74 ± 0.19 |
| H9 | 2.75 ± 0.02 | 2.88 ± 0.01 | 66.32 ± 0.45 | 28.05 ± 0.46 |
| H10 | 2.44 ± 0.01 | 3.07 ± 0.08 | 65.86 ± 0.42 | 28.63 ± 0.36 |
| Ultimate Analysis, wt% | |||||
|---|---|---|---|---|---|
| C | H | N | S | O | |
| WW | 41.8 ± 2.13 | 6.2 ± 0.23 | 0.8 ± 0.02 | 0.05 ± 0.00 | 49.3 ± 0.06 |
| H1 | 55.7 ± 3.02 | 5.9 ± 1.41 | 0.5 ± 0.03 | 0.05 ± 0.00 | 35.5 ± 0.07 |
| H2 | 58.4 ± 0.86 | 5.9 ± 1.23 | 0.6 ± 0.03 | 0.05 ± 0.00 | 32.7 ± 0.03 |
| H3 | 59.3 ± 1.08 | 7.0 ± 1.80 | 0.6 ± 0.01 | 0.05 ± 0.00 | 30.6 ± 0.04 |
| H4 | 59.2 ± 0.24 | 7.2 ± 0.87 | 0.6 ± 0.02 | 0.05 ± 0.00 | 30.1 ± 0.01 |
| H5 | 59.7 ± 0.18 | 6.9 ± 0.95 | 0.6 ± 0.01 | 0.05 ± 0.00 | 29.7 ± 0.01 |
| H6 | 59.1 ± 0.39 | 6.7 ± 0.86 | 0.6 ± 0.02 | 0.05 ± 0.00 | 30.9 ± 0.02 |
| H7 | 58.7 ± 0.68 | 6.5 ± 1.11 | 0.6 ± 0.02 | 0.05 ± 0.00 | 31.3 ± 0.02 |
| H8 | 59.1 ± 0.74 | 6.8 ± 0.91 | 0.6 ± 0.01 | 0.05 ± 0.00 | 30.6 ± 0.03 |
| H9 | 59.0 ± 0.20 | 6.9 ± 1.11 | 0.6 ± 0.03 | 0.05 ± 0.00 | 30.6 ± 0.02 |
| H10 | 59.2 ± 0.66 | 7.5 ± 1.17 | 0.6 ± 0.01 | 0.05 ± 0.00 | 29.7 ± 0.03 |
| HHV, MJ/kg | LHV, MJ/kg | ED | EY, % | |
|---|---|---|---|---|
| WW | 18.02 ± 0.35 | 16.47 ± 0.47 | - | - |
| H1 | 22.67 ± 0.36 | 21.27 ± 0.16 | 1.26 ± 0.04 | 80.03 ± 2.27 |
| H2 | 23.24 ± 0.54 | 21.84 ± 0.32 | 1.29 ± 0.05 | 89.59 ± 3.10 |
| H3 | 23.49 ± 0.29 | 21.85 ± 0.60 | 1.30 ± 0.02 | 88.78 ± 1.34 |
| H4 | 23.63 ± 0.17 | 21.96 ± 0.06 | 1.31 ± 0.03 | 88.56 ± 2.28 |
| H5 | 23.73 ± 0.07 | 22.14 ± 0.23 | 1.32 ± 0.02 | 89.63 ± 1.48 |
| H6 | 24.52 ± 0.30 | 22.94 ± 0.11 | 1.36 ± 0.03 | 92.81 ± 2.02 |
| H7 | 24.55 ± 0.18 | 23.04 ± 0.34 | 1.36 ± 0.02 | 92.26 ± 1.10 |
| H8 | 24.63 ± 0.08 | 23.04 ± 0.28 | 1.37 ± 0.03 | 92.50 ± 1.72 |
| H9 | 24.53 ± 0.01 | 22.90 ± 0.26 | 1.36 ± 0.03 | 92.92 ± 1.74 |
| H10 | 24.78 ± 0.06 | 23.03 ± 0.25 | 1.38 ± 0.02 | 94.38 ± 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Oh, M.-A.; Oh, S.-J.; Cho, N.-H.; Kang, Y.-Y.; Lee, J.-Y. Effects of Bioliquid Recirculation on Hydrothermal Carbonization of Lignocellulosic Biomass. Energies 2022, 15, 4903. https://doi.org/10.3390/en15134903
Lee S-J, Oh M-A, Oh S-J, Cho N-H, Kang Y-Y, Lee J-Y. Effects of Bioliquid Recirculation on Hydrothermal Carbonization of Lignocellulosic Biomass. Energies. 2022; 15(13):4903. https://doi.org/10.3390/en15134903
Chicago/Turabian StyleLee, Sun-Ju, Min-Ah Oh, Seung-Jin Oh, Na-Hyeon Cho, Young-Yeul Kang, and Jai-Young Lee. 2022. "Effects of Bioliquid Recirculation on Hydrothermal Carbonization of Lignocellulosic Biomass" Energies 15, no. 13: 4903. https://doi.org/10.3390/en15134903