Evolution of the Pseudo-Components of Heavy Oil during Low Temperature Oxidation Processes
Abstract
:1. Introduction
2. Apparatus and Methodology
2.1. Materials
2.2. Apparatus
2.3. Kinetic Cell Experiment
2.4. FT-ICR MS Characterization
2.5. Total Oxygen Consumption and Carbon Oxides Production
3. Results and Discussion
3.1. Oxidation Degree at Different Temperature Regions
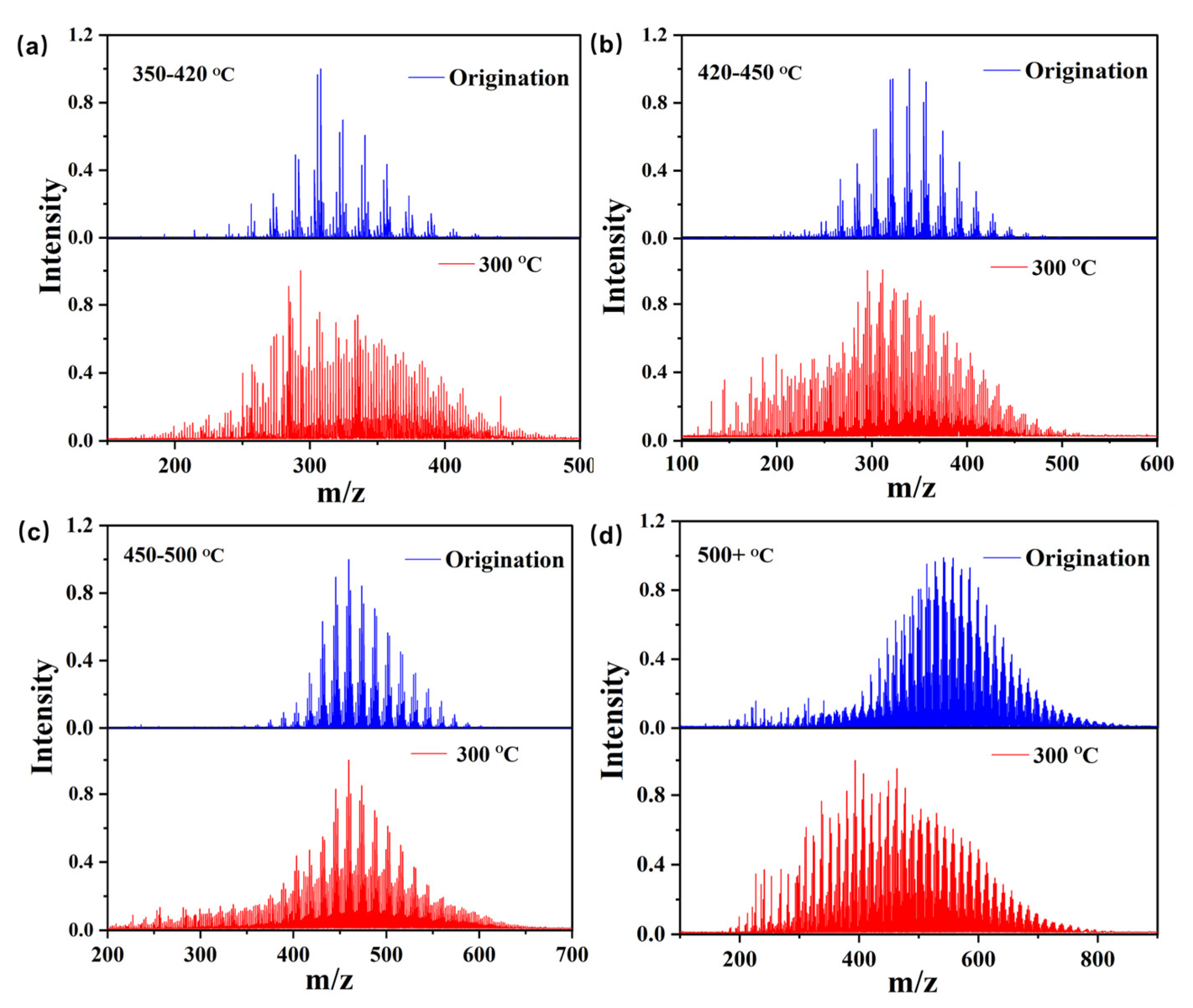
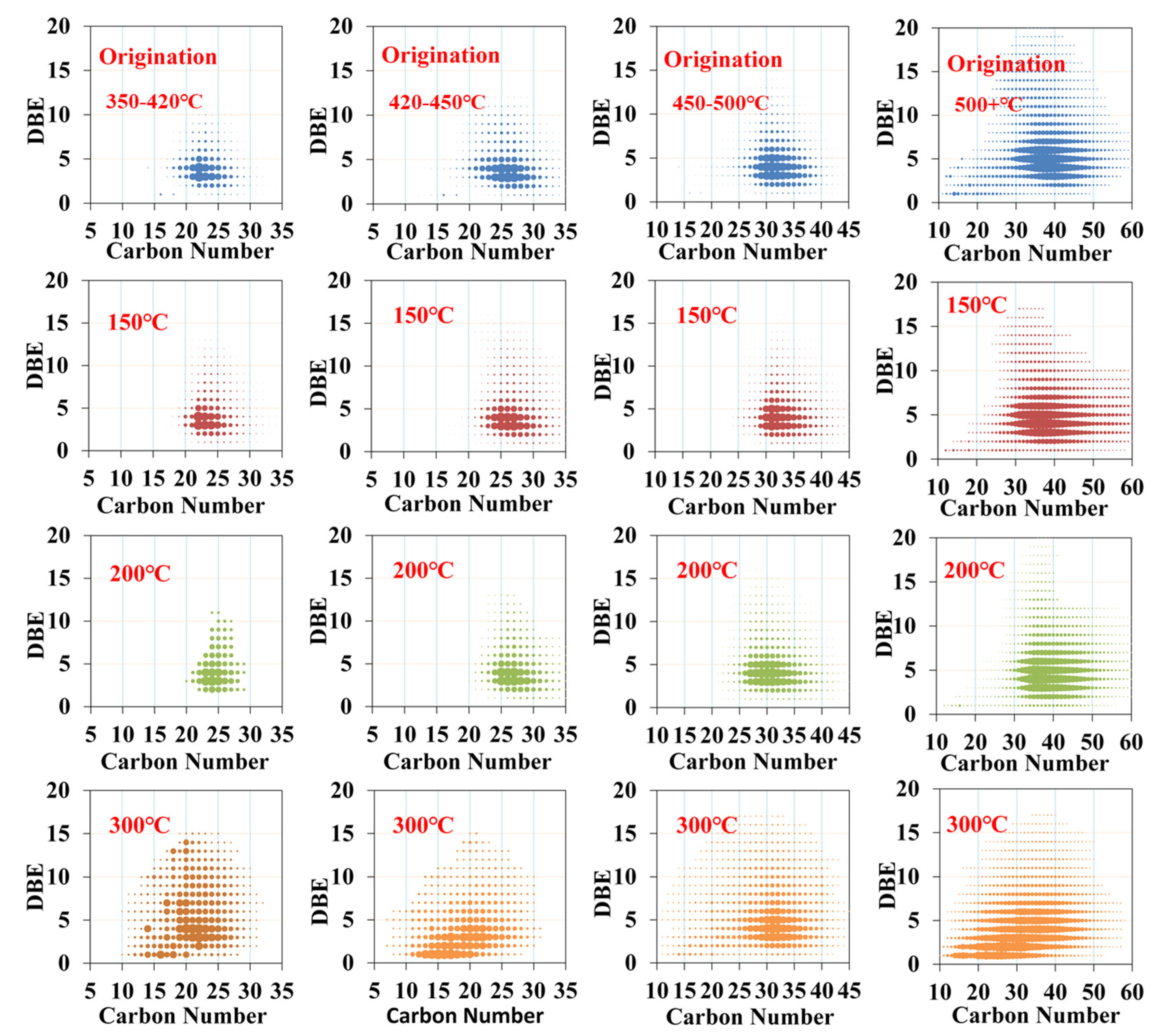
3.2. Evolution of Relative Molecular Weight Distribution
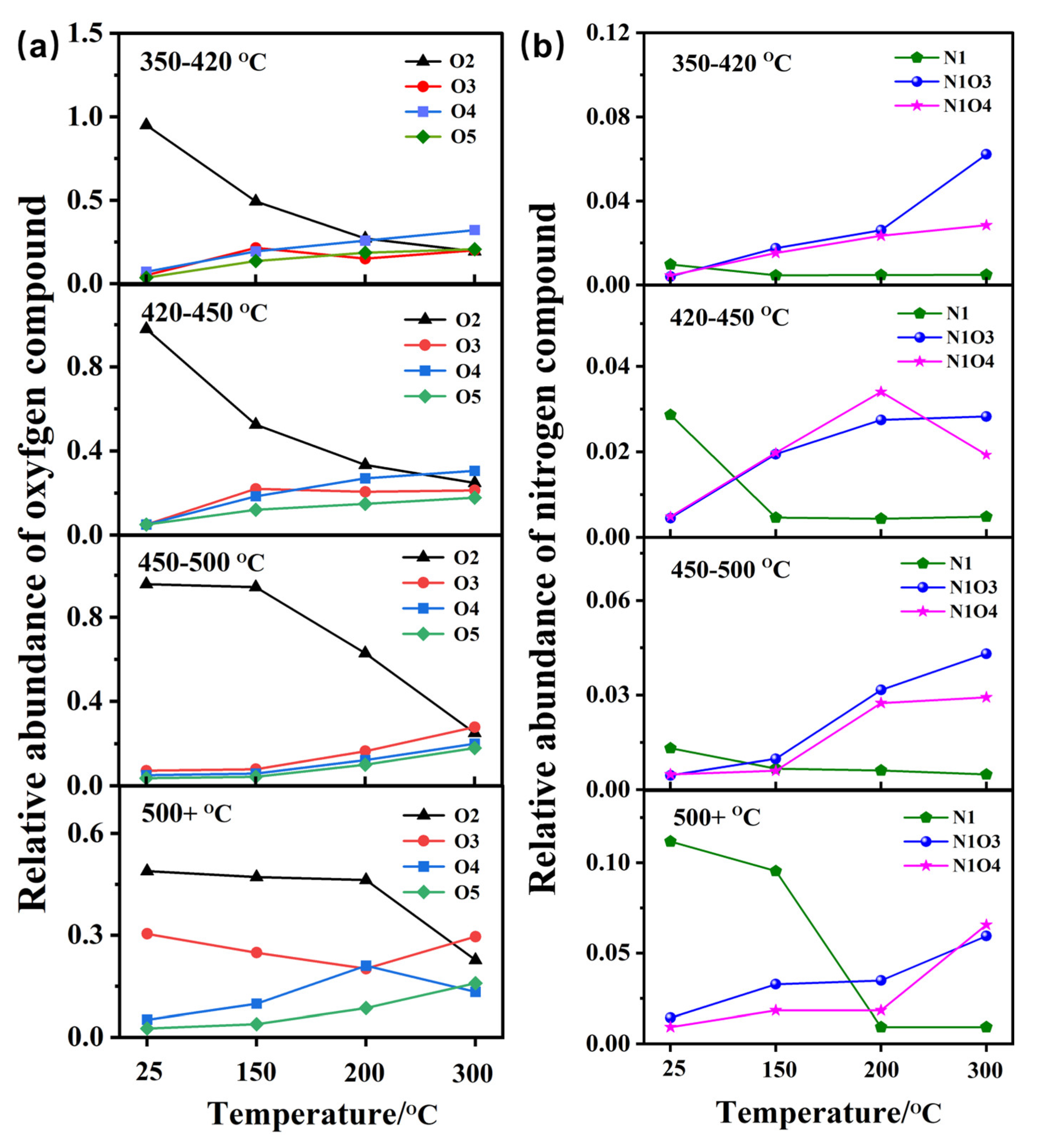
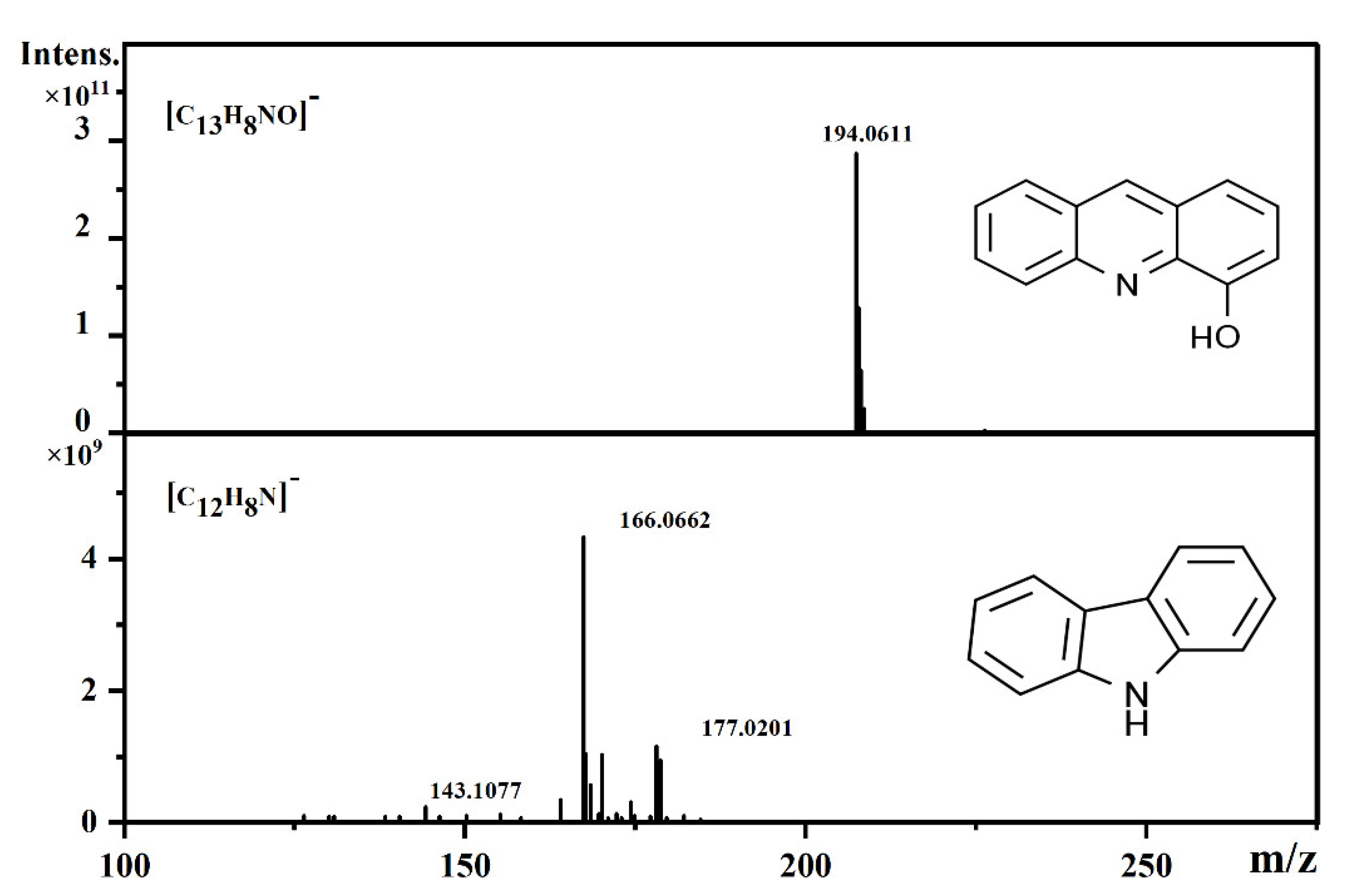
3.3. Evolution of Functional Groups of Oxygen and Nitrogen-Containing Compounds
4. Conclusions
- (1)
- The pseudo-components were subjected to two different reactions during the LTO process: oxygen addition and thermal cracking. The two reactions were detected and both occurred sequentially and simultaneously in different pseudo-components.
- (2)
- The pseudo-components with a low boiling point range generated high molecular weight compounds through the oxygen addition process. Then the fractions of high boiling point range could be oxidized into other compounds with greater molecular weight through oxygen addition reactions. As the temperature increased, the products continued to endure cracking reactions, through which new kinds of compounds with lower molecular weight were generated.
- (3)
- The variation in functional groups serves as an proximate indicator of the reaction scheme. N1O3- and N1O4-containing compounds are generated through oxygen addition of basic N1-containing compounds rather than of neutral N1-containing compounds.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lovett, S.; Monmont, F.; Nikiforakis, N. An experimentally-based in-situ combustion model with adaptive meshing. Combust. Flame 2015, 162, 960–977. [Google Scholar] [CrossRef]
- Ashraf, U.; Zhang, H.; Anees, A.; Mangi, H.N.; Ali, M.; Zhang, X.; Imraz, M.; Abbasi, S.S.; Abbas, A.; Ullah, Z.; et al. A core logging, machine learning and geostatistical modeling interactive approach for subsurface imaging of lenticular geobodies in a clastic depositional system, SE pakistan. Nat. Resour. Res. 2021, 30, 2807–2830. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Thanh, H.V.; Dashtgoli, D.S.; Yasin, Q.; Radwan, A.E.; Ashraf, U.; Lee, K. Application of robust intelligent schemes for accurate modelling interfacial tension of CO2 brine systems: Implications for structural CO2 trapping. Fuel 2022, 319, 123821. [Google Scholar] [CrossRef]
- Zhao, R.B.; Xia, X.T.; Luo, W.W.; Shi, Y.L.; Diao, C.J. Alteration of heavy oil properties under in-situ combustion: A field study. Energy Fuels 2015, 29, 6839–6848. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Fan, S.; Lang, X.; Li, G. Influence of low temperature oxidation on heavy oil coking by thermal pyrolysis during in situ combustion process. Fuel 2022, 316, 123314. [Google Scholar] [CrossRef]
- Khansari, Z.; Gates, I.D.; Mahinpey, N. Detailed study of low-temperature oxidation of an alaska heavy oil. Energy Fuels 2012, 26, 1592–1597. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Yin, H.; He, D.-L.; Gong, H.-F.; Liu, Z.-Z.; Liu, Y.-Q.; Zhang, X.-M.; Pu, W.-F. Low temperature oxidized coke of the ultra-heavy oil during in-situ combustion process: Structural characterization and evolution elucidation. Fuel 2022, 313, 122676. [Google Scholar] [CrossRef]
- Akkutlu, I.Y.; Yortsos, Y.C. The dynamics of in-situ combustion fronts in porous media. Combust. Flame 2003, 134, 229–247. [Google Scholar] [CrossRef]
- Khansari, Z.; Gates, I.D.; Mahinpey, N. Low-temperature oxidation of lloydminster heavy oil: Kinetic study and product sequence estimation. Fuel 2014, 115, 534–538. [Google Scholar] [CrossRef]
- Bissada, K.K.; Tan, J.; Szymczyk, E.; Darnell, M.; Mei, M. Group-type characterization of crude oil and bitumen. Part I: Enhanced separation and quantification of saturates, aromatics, resins and asphaltenes (SARA). Org. Geochem. 2016, 95, 21–28. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Varfolomeev, M.A.; Yuan, C.; Rodionov, A.A. Integrative investigation of low-temperature oxidation characteristics and mechanisms of heavy crude oil. Ind. Eng. Chem. Res. 2019, 58, 14595–14602. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Yang, W.; Yang, D. Quantification of low-temperature oxidation of light oil and its SAR fractions with TG-DSC and TG-FTIR analysis. Energy. Sci. Eng. 2020, 8, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Pu, W.; Varfolomeev, M.A.; Liu, Y.; Liu, Z. Oxidation characteristics of heavy oil and its SARA fractions during combustion using TG-FTIR. J. Petrol. Sci. Eng. 2020, 192, 107331. [Google Scholar] [CrossRef]
- Akin, S.; Kok, M.V.; Bagci, S.; Karacan, O. Oxidation of heavy oil and their SARA fractions: Itsrole in modeling in-situ combustion. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1 October 2000. [Google Scholar]
- Aleksandrov, D.; Kudryavtsev, P.; Hascakir, B. Variations in in-situ combustion performance due to fracture orientation. J. Petrol. Sci. Eng. 2017, 154, 488–494. [Google Scholar] [CrossRef]
- Lopes, M.S.; Gonçalves, F.d.; Lopes, M.S.; Perna, R.F.; Filho, R.M. Extending the true boiling point curve of a heavy crude oil by means of molecular distillation and characterization of the products obtained. Petrol. Sci. Technol. 2017, 35, 1523–1529. [Google Scholar] [CrossRef]
- Zhao, R.B.; Wei, Y.G.; Wang, Z.M.; Yan, W.; Yang, H.J.; Liu, S.J. Kinetics of low-temperature oxidation of light crude oil. Energy Fuels 2016, 30, 2647–2654. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Huan, R.; Castanier, L.M.; Kovscek, A.R. An experimental investigation of the in-situ combustion behavior of Karamay crude oil. J. Petrol. Sci. Eng. 2015, 127, 82–92. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Wang, Y.; Li, P. GC-MS used in study on the mechanism of the viscosity reduction of heavy oil through aquathermolysis catalyzed by aromatic sulfonic H3PMo12O40. Energy 2010, 35, 3454–3460. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Gani, A.; Hourani, N.; Emwas, A.-H.; Sarathy, S.M.; Roberts, W.L. TG/DTG, FT-ICR mass spectrometry, and NMR spectroscopy study of heavy fuel oil. Energy Fuels 2015, 29, 7825–7835. [Google Scholar] [CrossRef] [Green Version]
- Freitag, N.P. Chemical reaction mechanisms that govern oxidation rates during in-situ combustion and high-pressure air injection. SPE Res. Eval. Eng. 2016, 19, 645–654. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Che, H. Parameters determination during in-situ combustion of Liao he heavy oil. Energy Fuels 2013, 27, 3416–3426. [Google Scholar] [CrossRef]
- Trejo, F.; Rana, M.S.; Ancheyta, J. Thermogravimetric determination of coke from asphaltenes, resins and sediments and coking kinetics of heavy crude asphaltenes. Catal. Today 2010, 150, 272–278. [Google Scholar] [CrossRef]
- Meléndez, L.V.; Lache, A.; Orrego-Ruiz, J.A.; Pachón, Z.; Mejía-Ospino, E. Prediction of the SARA analysis of colombian crude oils using ATR-FTIR spectroscopy and chemometric methods. J. Petrol. Sci. Eng. 2012, 90, 56–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Xu, Z.; Zhang, N.; Chung, K.H.; Zhao, S.; Xu, C.; Shi, Q. Molecular characterization of heavy petroleum and its supercritical fluid extraction fractions by FT-ICR MS with various ionization methods. Energy Fuels 2014, 28, 7448–7456. [Google Scholar] [CrossRef]
- Cho, Y.; Witt, M.; Kim, Y.H.; Kim, S. Characterization of crude oils at the molecular level by use of laser desorption ionization fourier-transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2012, 84, 8587–8594. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Rodgers, R.P.; Rahimi, P.; Teclemariam, A.; Marshall, A.G. Effect of thermal treatment on acidic organic species from athabasca bitumen heavy vacuum gas oil, analyzed by negative-ion electrospray Fourier Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometry. Energy Fuels 2009, 23, 314–319. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, J.; Fang, Q.; Wei, Y.; Song, G.; Xu, C.; Hsu, C.S.; Shi, Q. Evolution of acidic compounds in crude oil during in-situ combustion. Energy Fuels 2017, 31, 5926–5932. [Google Scholar] [CrossRef]
- Cinar, M.; Castanier, L.M.; Kovscek, A.R. Combustion kinetics of heavy oils in porous media. Energy Fuels 2011, 25, 4438–4451. [Google Scholar] [CrossRef]
- Cinar, M.; Hascakir, B.; Castanier, L.M.; Kovscek, A.R. Predictability of crude oil in-situ combustion by the iso conversional kinetic approach. Soc. Petrol. Eng. J. 2011, 16, 537–547. [Google Scholar] [CrossRef]
- Burger, J.G.; Sahuquet, B.C. Laboratory research on wet combustion. J. Petrol. Technol. 2013, 25, 1137–1146. [Google Scholar] [CrossRef]
- Kapadia, P.; Gates, I.D.; Mahinpey, N.; Gates, I.D. Kinetic models for low temperature oxidation subranges based on reaction products. In Proceedings of the SPE Heavy Oil Conference-Canada, Calgary, AB, Canada, 11 June 2013; pp. 11–13. [Google Scholar] [CrossRef]
- Zhao, R.B.; Li, B.C.; Yang, J.; Gao, B.; Zhang, C.; Fu, J.Y.; Wang, R. Temperature prediction via reaction heat calculation of burned pseudo-componentss during in-situ combustion. Energy Fuels 2009, 23, 314–319. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, J.; Wang, L.; Chen, Z.; Ren, S.; Hu, C.; Zhang, S. Low-temperature oxidation characteristics and its effect on the critical coking temperature of heavy oils. Energy Fuels 2015, 29, 538–545. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Jia, H.; Pu, W.-F.; Wang, L.-L.; Peng, H. Sensitivity studies on the oxidation behavior of crude oil in porous media. Energy Fuels 2012, 26, 6815–6823. [Google Scholar] [CrossRef]
- Castro, L.V.; Vazquez, F. Fractionation and characterization of Mexican crude oils. Energy Fuels 2009, 23, 1603–1609. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, P.; Wang, Y.Q.; He, J. Change of carbazole compounds in heavy oil by catalytic aquathermolysis and the catalytic mechanism of viscosity reduction. J. Fuel. Chem. Technol. 2011, 39, 271–277. [Google Scholar]



| Boiling Range (°C) | Content (%) |
|---|---|
| <200 | 1.312 |
| 200–300 | 9.544 |
| 300–350 | 9.783 |
| 350–420 | 11.436 |
| 420–450 | 9.277 |
| 450–500 | 14.163 |
| 500+ | 44.485 |
| Inlet Pressure (MPa) | Outlet Pressure (MPa) | Gas Injection Rate (L/min) | Heating Rate (°C/min) | Temperature Range (°C) | Oil Sand Quality (g) |
|---|---|---|---|---|---|
| 1.70 | 1.50 | 2.00 | 3.83 | 25–150 25–200 25–300 | 10.50 |
| Boiling Range | 350–420 °C | 420–450 °C | 450–500 °C | 500+ °C | |
|---|---|---|---|---|---|
| Temperature Range | |||||
| Room temperature—150 °C | 1.33 × 10−4 | 5.42 × 10−5 | 4.73 × 10−5 | 0 | |
| Room temperature—200 °C | 6.36 × 10−3 | 6.56 × 10−3 | 1.64 × 10−4 | 3.60 × 10−5 | |
| Room temperature—300 °C | 0.0247 | 0.0259 | 0.0266 | 0.0119 | |
| Boiling Range | 350–420 °C | 420–450 °C | 450–500 °C | 500+ °C | |
|---|---|---|---|---|---|
| Temperature Range | |||||
| Room temperature—150 °C | 2.96 × 10−5 | 9.23 × 10−6 | 0 | 0 | |
| Room temperature—200 °C | 3.73 × 10−4 | 1.19 × 10−4 | 4.12 × 10−5 | 1.19 × 10−5 | |
| Room temperature—300 °C | 0.0116 | 0.0124 | 0.0135 | 0.00581 | |
| Boiling Range | 350–420 °C | 420–450 °C | 450–500 °C | 500+ °C | |
|---|---|---|---|---|---|
| Temperature Range | |||||
| Room temperature—150 °C | 0.223 | 0.170 | 0 | 0 | |
| Room temperature—200 °C | 0.059 | 0.018 | 0.251 | 0.330 | |
| Room temperature—300 °C | 0.470 | 0.478 | 0.508 | 0.488 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wang, T.; Chen, L.; Pan, J.; Li, S.; Zhao, D.; Chen, L.; Wang, J. Evolution of the Pseudo-Components of Heavy Oil during Low Temperature Oxidation Processes. Energies 2022, 15, 5201. https://doi.org/10.3390/en15145201
Zhao R, Wang T, Chen L, Pan J, Li S, Zhao D, Chen L, Wang J. Evolution of the Pseudo-Components of Heavy Oil during Low Temperature Oxidation Processes. Energies. 2022; 15(14):5201. https://doi.org/10.3390/en15145201
Chicago/Turabian StyleZhao, Renbao, Tiantian Wang, Lijuan Chen, Jingjun Pan, Shutong Li, Dong Zhao, Long Chen, and Jiaying Wang. 2022. "Evolution of the Pseudo-Components of Heavy Oil during Low Temperature Oxidation Processes" Energies 15, no. 14: 5201. https://doi.org/10.3390/en15145201






