Impact of Exposure to Supercritical Carbon Dioxide on Reservoir Caprocks and Inter-Layers during Sequestration
Abstract
:1. Introduction
1.1. Interaction of Supercritical Carbon Dioxide with Rocks
1.2. Overview
- CO2 dissolved in brine;
- Dry CO2;
- Wet CO2 (brine water dissolved in CO2 phase); and
- Concentrated brine (due to the dissolution of dry scCO2 into brine water).
1.3. Supercritical Carbon Dioxide (scCO2) Dissolved in Brine
- -
- The geometry of the plume front and saturation within the plume (contact surface area);
- -
- Plume migration before becoming stable;
- -
- The presence of any hydrological flows within the reservoir; and
- -
- The development of convection currents due to brine densification when scCO2 is dissolved.
1.4. Dry and Variably Wet scCO2
1.5. Concentrated Brine
1.6. Kinetics of Interactions with Rocks
1.7. Link to Porosity and Permeability
1.8. Experimental Studies of Mineral Reactions
1.9. Single Mineral Interactions
1.9.1. Illite
1.9.2. Mica
1.9.3. Feldspars
1.9.4. Quartz
1.10. Shale Interactions (Multi-Mineral)
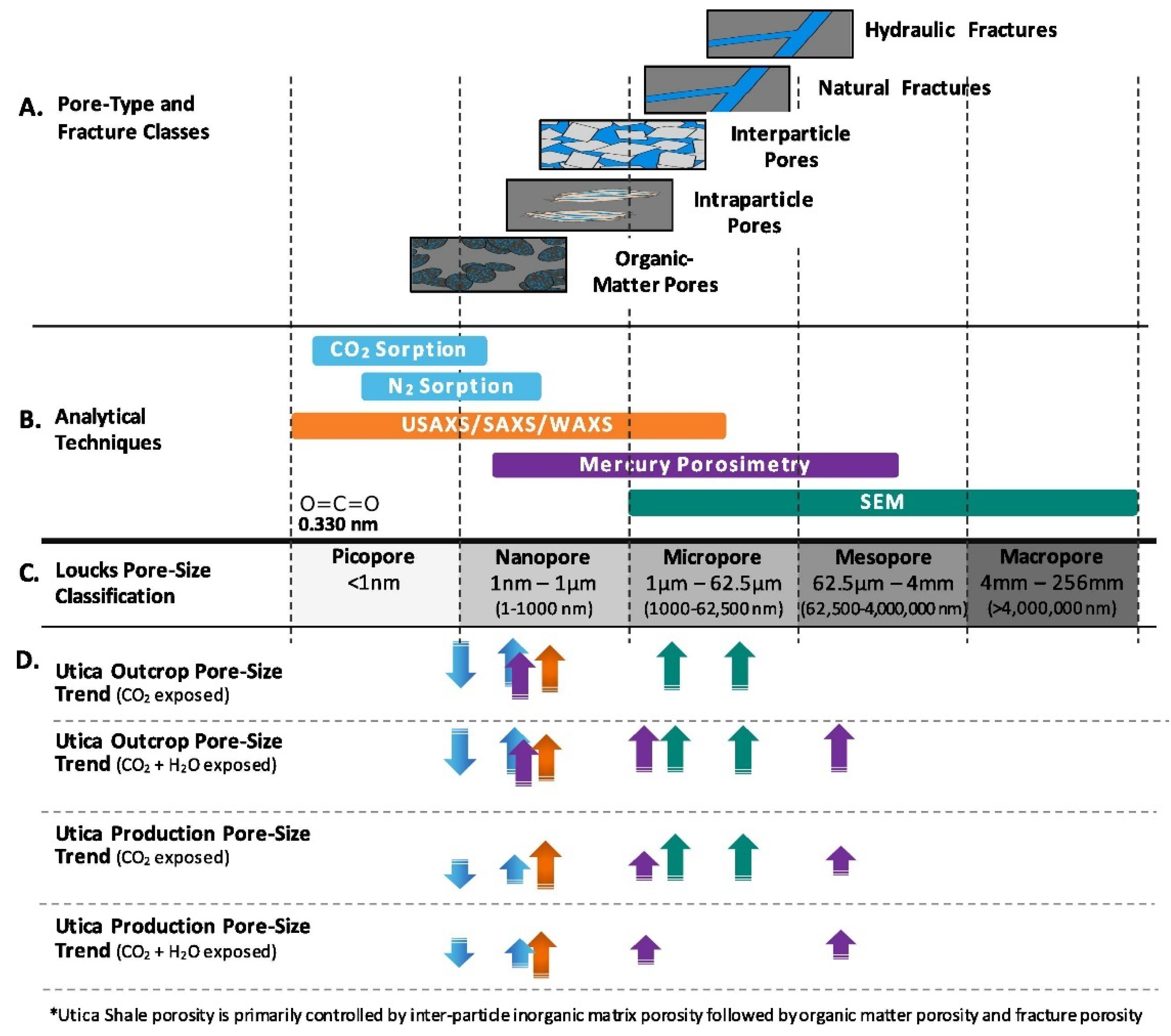
2. Characterisation of the Structural Evolution of Rocks Following Exposure to Carbon Dioxide
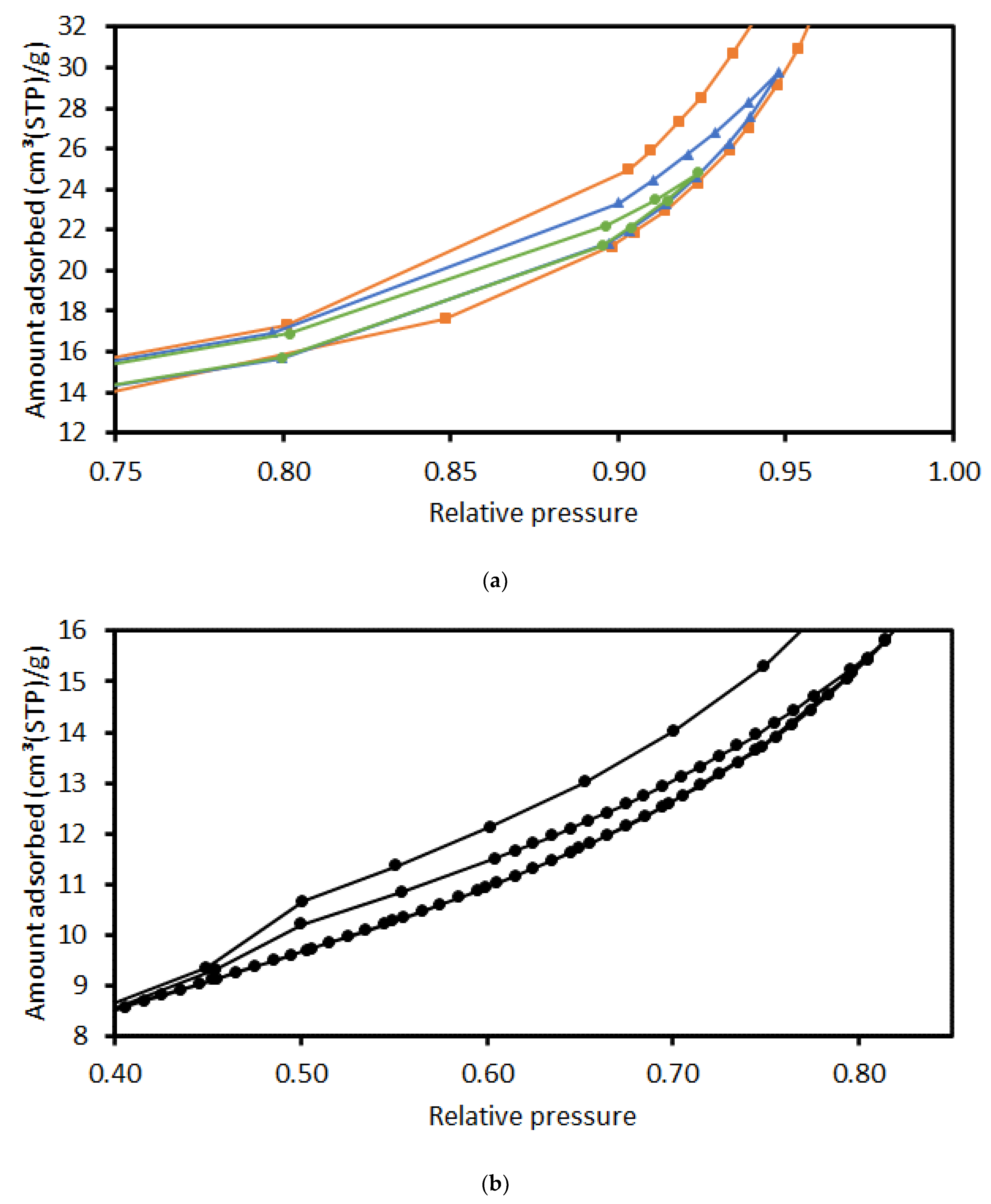
2.1. Surface Area Determination
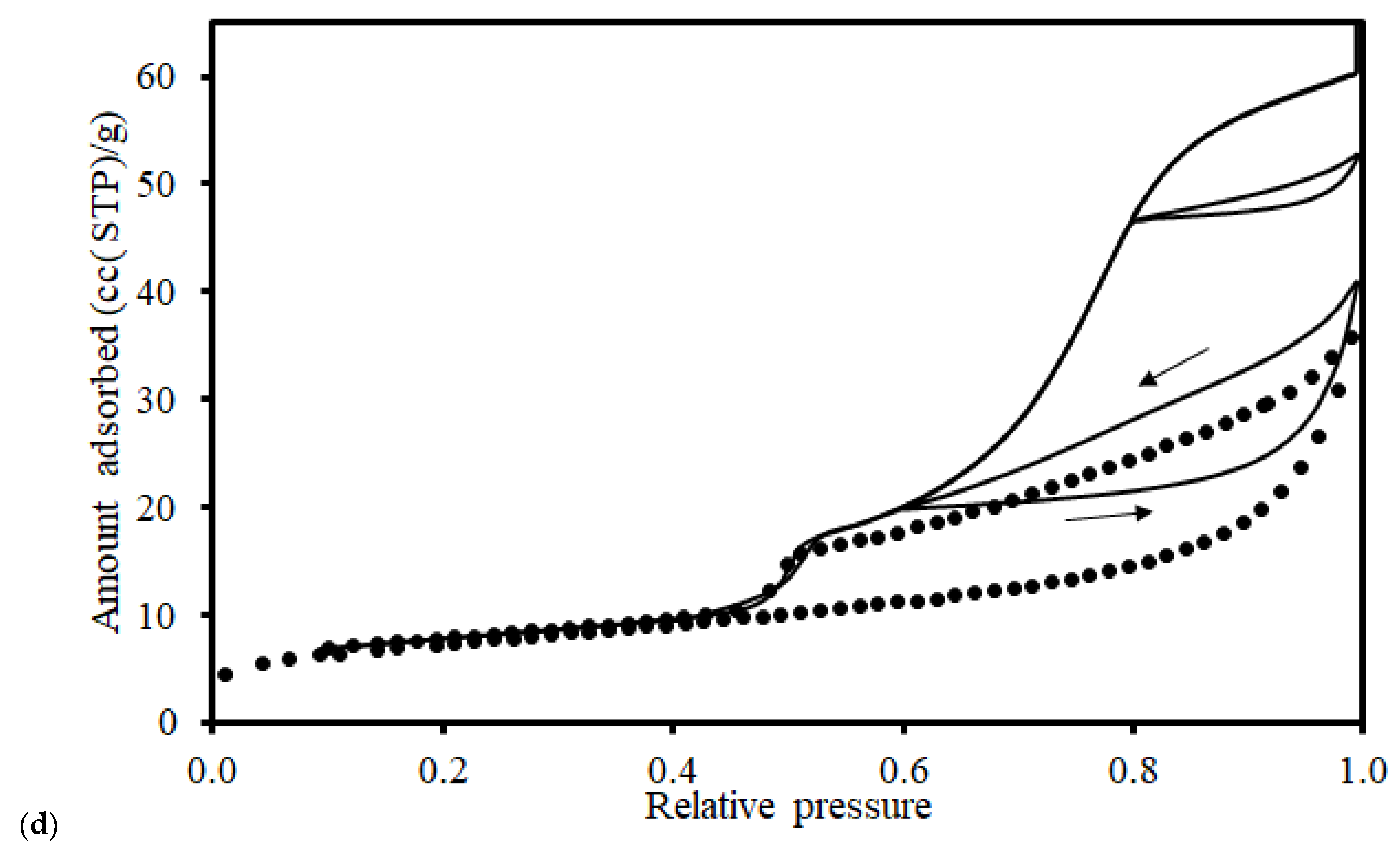
2.2. Pore Volume Determination
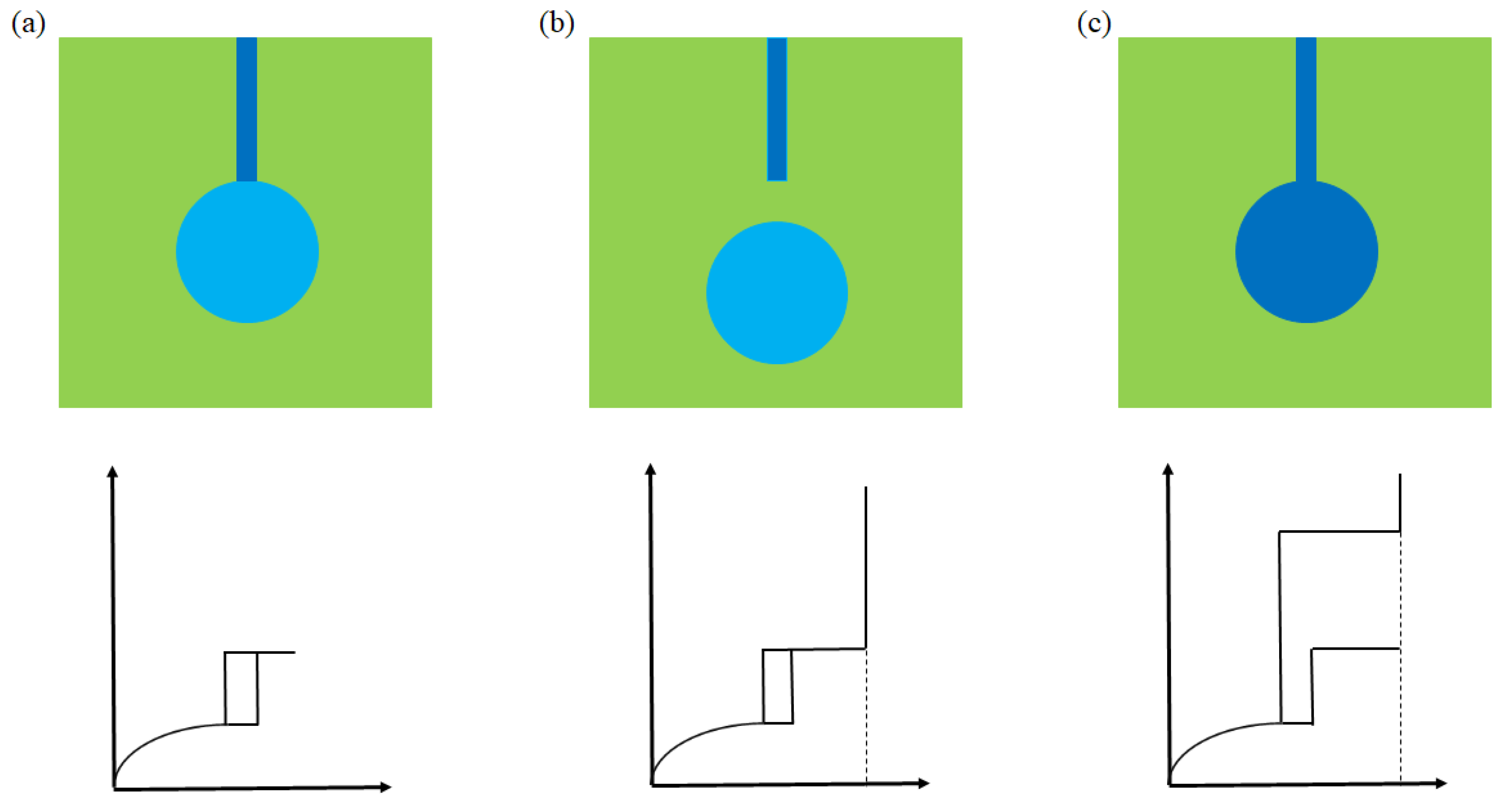
2.3. Void Space Segmentation Schemes
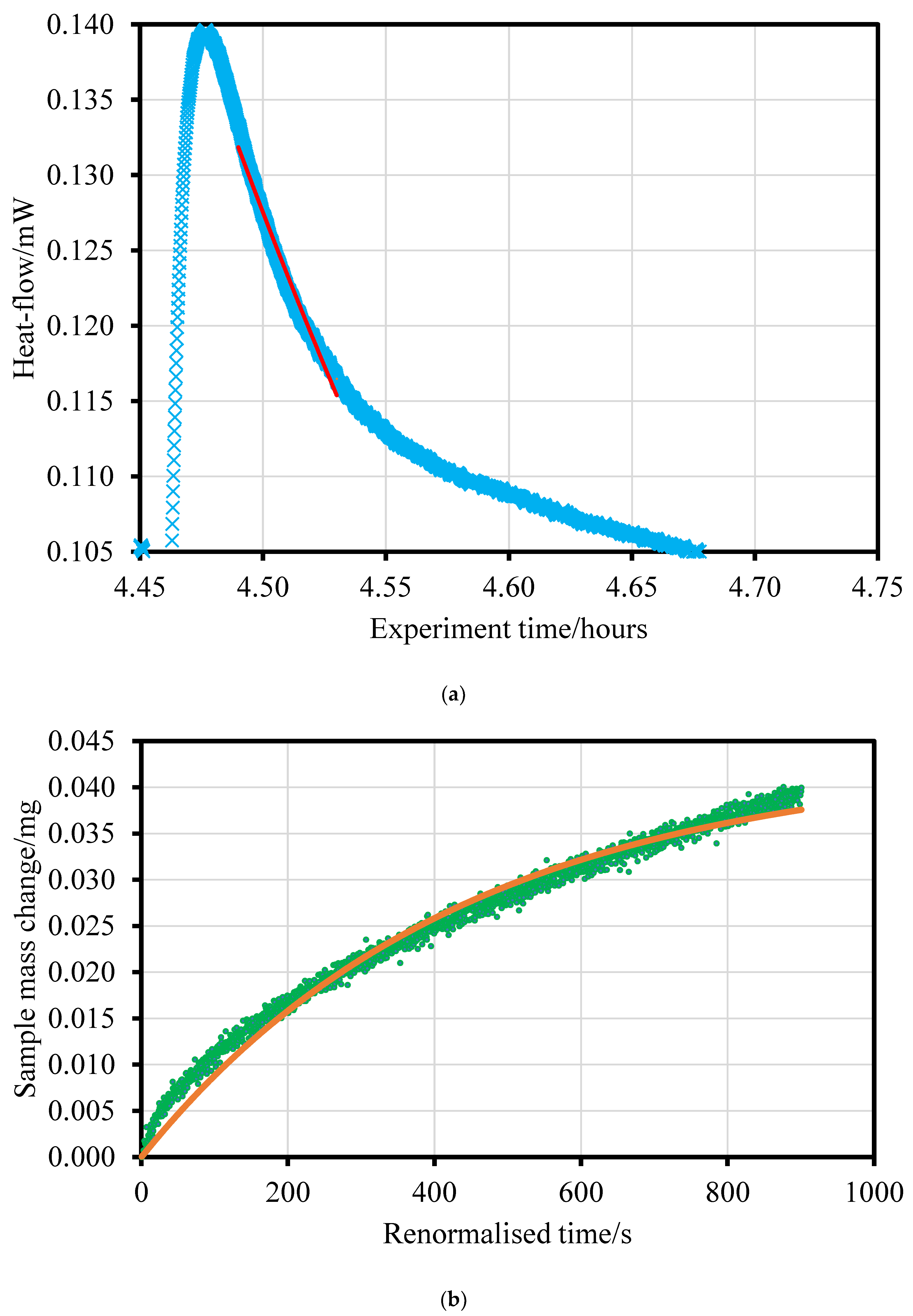
2.4. Adsorption Calorimetry
3. Reservoir-Scale Simulations
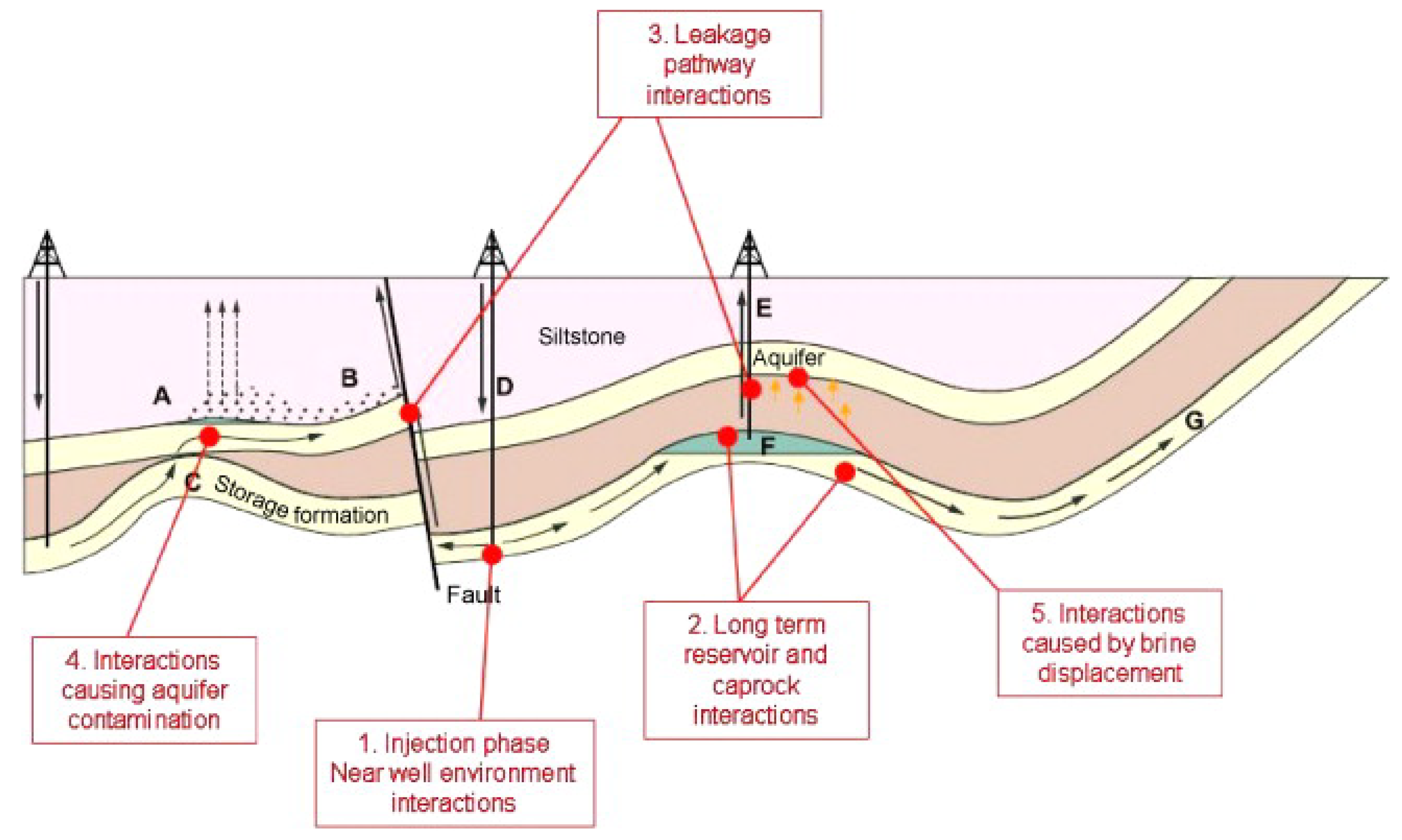
3.1. Seals and Interlayers
3.2. Geomechanical Processes
3.3. Geochemical Processes
3.4. Coupled Flow-Geomechanical-Geochemical Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mollaei, A. Forecasting of Isothermal Enhanced Oil Recovery (EOR) and Waterflood Processes. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2011. [Google Scholar]
- Dai, Z.; Middleton, R.; Viswanathan, H.; Fessenden-Rahn, J.; Bauman, J.; Pawar, R.; Lee, S.-Y.; McPherson, B. An integrated framework for optimizing CO2 sequestration and enhanced oil recovery. Environ. Sci. Technol. Lett. 2014, 1, 49–54. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.-Y.; McPherson, B.; et al. CO2 accounting and risk analysis for CO2 sequestration at enhanced oil recovery sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage. 2005. Available online: https://www.ipcc.ch/report/carbon-dioxide-capture-and-storage/ (accessed on 1 May 2021).
- Fitts, J.P.; Peters, C.A. Caprock fracture dissolution and CO2 leakage. Rev. Mineral. Geochem. 2013, 77, 459–479. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Than, H.V.; Da, Z.; Mehbodniya, A.; Rahimi, M.; Ashraf, U.; Radwan, A.E. Exploring the power of machine learning to predict carbon dioxide trapping efficiency in saline aquifers for carbon geological storage project. J. Clean. Prod. 2022, 372, 133778. [Google Scholar] [CrossRef]
- Alalimi, A.; AlRassas, A.M.; Thanh, H.V.; Al-qaness, M.A.A.; Pan, L.; Ashraf, U.; Al-Alimi, D.; Moharam, S. Developing the efficiency-modeling framework to explore the potential of CO2 storage capacity of S3 reservoir, Tahe oilfield, China. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 128. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Thanh, H.V.; Dashtgoli, D.S.; Yasind, Q.; Radwan, A.E.; Ashraf, U.; Lee, K.-K. Application of robust intelligent schemes for accurate modelling interfacial tension of CO2 brine systems: Implications for structural CO2 trapping. Fuel 2022, 319, 123821. [Google Scholar] [CrossRef]
- Thanh, H.V.; Sugai, Y.; Sasaki, K. Application of artificial neural network for predicting the performance of CO2 enhanced oil recovery and storage in residual oil zones. Sci. Rep. 2020, 10, 18204. [Google Scholar] [CrossRef]
- Thanh, H.V.; Sugai, Y.; Nguele, R.; Sasaki, K. Integrated workflow in 3D geological model construction for evaluation of CO2 storage capacity of a fractured basement reservoir in Cuu Long Basin, Vietnam. Int. J. Greenh. Gas Control 2019, 90, 102826. [Google Scholar] [CrossRef]
- Thanh, H.V.; Sugai, Y.; Nguele, R.; Sasaki, K. Robust optimization of CO2 sequestration through a water alternating gas process under geological uncertainties in Cuu Long Basin, Vietnam. J. Nat. Gas Sci. Eng. 2020, 76, 103208. [Google Scholar] [CrossRef]
- Goodman, A.; Sanguinito, S.; Tkach, M. Investigating the role of water on CO2-Utica Shale interactions for carbon storage and shale gas extraction activities—Evidence for pore scale alterations. Fuel 2019, 242, 744–755. [Google Scholar] [CrossRef]
- Czernichowski-Lauriol, I.; Rochelle, C.; Gaus, I.; Azaroual, M.; Pearce, J.; Durst, P. Geochemical Interactions Between CO2, Pore-Waters and Reservoir Rocks; Springer: Dordrecht, Germany, 2006. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Spanakos, D.; Rigby, S.P. Evaluation of impact of surface diffusion on methane recovery via carbon dioxide injection in shale reservoirs. Fuel 2022, 307, 121928. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.; Tang, J.; Chen, Y.; Li, H. Investigation on the physics structure and chemical properties of the shale treated by supercritical CO2. J. CO2 Util. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Harrison, A.L.; Jew, A.D.; Dustin, M.K.; Thomas, D.L.; Joe-Wong, C.M.; Bargar, J.R.; Johnson, N.; Brown, G.E.; Maher, K. Element release and reaction-induced porosity alteration during shale-hydraulic fracturing fluid interactions. Appl. Geochem. 2017, 82, 47–62. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Karstens, J.; Ahmed, W.; Berndt, C.; Class, H. Focused fluid flow and the sub-seabed storage of CO2: Evaluating the leakage potential of seismic chimney structures for the Sleipner CO2 storage operation. J. Mar. Petrol. Geol. 2017, 88, 81–93. [Google Scholar] [CrossRef]
- Loring, J.S.; Miller, Q.R.S.; Thompson, C.J.; Schaef, H.T. Experimental Studies of Reactivity and Transformations of Rocks and Minerals in Water-Bearing Supercritical CO2. In Science of Carbon Storage in Deep Saline Formations; Newell, P., Ilgen, A.G., Eds.; Elsevier: Cham, The Netherlands, 2019; pp. 63–88. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. Numerical modelling of CO2-water-basalt interaction. Mineral. Mag. 2008, 72, 55–59. [Google Scholar] [CrossRef]
- Hövelmann, J.; Austrheim, H.; Jamtveit, B. ‘Microstructure and porosity evolution during experimental carbonation of a natural peridotite’, Chemical Geology. Chem. Geol. 2012, 334, 254–265. [Google Scholar] [CrossRef]
- Hovorka, S.D.; Lu, J. Field observations of geochemical response to CO2 injection at the reservoir scale. In Science of Carbon Storage in Deep Saline Formations; Newell, P., Ilgen, A.G., Eds.; Elsevier: Cham, The Netherlands, 2019; pp. 33–61. [Google Scholar] [CrossRef]
- Goodman, A.; Sanguinito, S.; Kutchko, B.; Natesakhawat, S.; Cvetic, P.; Allen, A.J. Shale pore alteration: Potential implications for hydrocarbon extraction and CO2 storage. Fuel 2020, 89, 116930. [Google Scholar] [CrossRef]
- Kervévan, C.; Azaroual, M.; Durst, P. Improvement of the Calculation Accuracy of Acid Gas Solubility in Deep Reservoir Brines: Application to the Geological Storage of CO2. Oil Gas Sci. Technol. 2005, 60, 357–379. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Zhu, C. In situ silicate reaction rates in sandy aquifers. Geochim. Et Cosmochim. Acta 2006, 70, A753. [Google Scholar] [CrossRef]
- Loring, J.S.; Thompson, C.J.; Wang, Z.; Joly, A.G.; Sklarew, D.S.; Schaef, H.T.; Ilton, E.S.; Rosso, K.M.; Felmy, A.R. In situ infrared spectroscopic study of forsterite carbonation in wet supercritical CO2. Environ. Sci. Technol. 2011, 45, 6204–6210. [Google Scholar] [CrossRef] [PubMed]
- Miller, Q.R.S.; Thompson, C.J.; Loring, J.S.; Windisch, C.F.; Rosso, K.M.; Schaef, H.T. Insights into silicate carbonation processes in water-bearing supercritical CO2 fluids. Int. J. Greenh. Gas Control 2013, 15, 104–118. [Google Scholar] [CrossRef]
- Schaef, H.T.; Thompson, C.J.; Loring, J.S.; Bowden, M.S.; Arey, B.W.; McGrail, B.P.; Rosso, K.M. Silicate carbonation in supercritical CO2 containing dissolved H2O: An in situ high pressure X-Ray diffraction and infrared spectroscopy study. Energy Procedia 2013, 37, 5892–5896. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhao, Y.P.; Wang, X.; Pan, L. Adsorption-induced pore blocking and its mechanisms in nanoporous shale due to interactions with supercritical CO2. J. Pet. Sci. Eng. 2019, 178, 74–81. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Hong, L.; Romanov, V. Experimental studies: Molecular interactions at clay interfaces. In Greenhouse Gases and Clay Minerals; Romanov, V., Ed.; Springer: Cham, Switzerland, 2018; pp. 95–123. [Google Scholar] [CrossRef]
- Lasaga, A.C.; Soler, J.M.; Ganor, J.; Burch, T.E.; Nagy, K.L. Chemical weathering rate laws and global geochemical cycles. Geochim. Et Cosmochim. Acta 1994, 58, 2361–2386. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.; Zhang, Y.; Sun, L.; Wang, K. Experimental Investigation of the Geochemical Interactions between Supercritical CO2 and Shale: Implications for CO2 Storage in Gas-Bearing Shale Formations. Energy Fuels 2018, 32, 1963–1978. [Google Scholar] [CrossRef]
- Ilton, E.S.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R. In situ X-ray diffraction study of Na+ saturated montmorillonite exposed to variably wet super critical CO2. Environ. Sci. Technol. 2012, 46, 4241–4248. [Google Scholar] [CrossRef]
- Schaef, H.T.; Ilton, E.S.; Qafoku, O.; Martin, P.F.; Felmy, A.R.; Rosso, K.M. In situ XRD study of Ca2+ saturated montmorillonite (STX-1) exposed to anhydrous and wet supercritical carbon dioxide. Int. J. Greenh. Gas Control 2012, 6, 220–229. [Google Scholar] [CrossRef]
- Schaef, H.T.; Loring, J.S.; Glezakou, V.-A.; Miller, Q.R.S.; Thompson, C.J. Competitive sorption of CO2 and H2O in 2:1 layer phyllosilicates. Geochim. Et Cosmochim. Acta 2015, 161, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Ray, J.R.; Jun, Y.S. Effects of salinity and the extent of water on supercritical CO2-induced phlogopite dissolution and secondary mineral formation. Environ. Sci. Technol. 2011, 45, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.R.; Dewers, T.A.; Heath, J.E.; Wang, Y.; Matteo, E.N.; Meserole, S.P.; Tallant, D. Fundamental Study of CO2-H2O-Mineral Interactions for Carbon Sequestration, with Emphasis on the Nature of the Supercritical Fluid-Mineral Interface. Available online: http://www.ntis.gov/help/ordermethods.asp?loc=7-4-0#online (accessed on 29 April 2020).
- Schembre, J.M.; Kovscek, A.R. Mechanism of formation damage at elevated temperature’, Journal of Energy Resources Technology. Trans. ASME 2005, 127, 171–180. [Google Scholar] [CrossRef]
- Lin, H.; Fujii, T.; Takisawa, R.; Takahashi, T.; Hashida, T. Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions. J. Mater. Sci. 2008, 43, 2307–2315. [Google Scholar] [CrossRef]
- Schaef, H.T.; McGrail, B.P.; Owen, A.T. Basalt reactivity variability with reservoir depth in supercritical CO2 and aqueous phases. Energy Procedia 2011, 4, 4977–4984. [Google Scholar] [CrossRef] [Green Version]
- Regnault, O.; Lagneau, V.; Catalette, H.; Schneider, H. Étude expérimentale de la réactivité du CO2 supercritique vis-à-vis de phases minérales pures. Implications pour la séquestration géologique de CO2. Comptes Rendus Geosci. 2005, 337, 1331–1339. [Google Scholar] [CrossRef]
- Adler, H.H.; Kerr, P.F. Infrared spectra, symmetry and structure relations of some carbonate minerals. Am. Mineral. J. Earth Planet. Mater. 1963, 48, 839–853. [Google Scholar]
- Edwards, H.G.M.; Jorge Villar, S.E.; Jehlicka, J.; Munshi, T. FT-Raman spectroscopic study of calcium-rich and magnesium-rich carbonate minerals. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2005, 61, 2273–2280. [Google Scholar] [CrossRef]
- Kaszuba, J.; Yardley, B.; Andreani, M. Experimental perspectives of mineral dissolution and precipitation due to carbon dioxide-water-rock interactions. Rev. Mineral. Geochem. 2013, 77, 153–188. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 2016, 6, 19362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinito, S.; Goodman, S.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawata, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying dry supercritical CO2-induced changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Ardakani, O.H.; Sanei, H.; Ghanizadeh, A.; Lavoie, D.; Clarkson, C.R. Do all fractions of organic matter contribute equally in shale porosity? A case study from Upper Ordovician Utica Shale, southern Quebec, Canada. Mar. Pet. Geol. 2018, 92, 794–808. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Nicot, J.P.; Mickler, P.J.; Ribeiro, L.H.; Darvari, R. Alteration of Bakken reservoir rock during CO2-based fracturing—An autoclave reaction experiment. J. Unconv. Oil Gas 2016, 14, 72–85. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, D.; Luo, P.; Zhang, Y.; Zhang, L.; Sun, L. Influences of subcritical and supercritical CO2 treatment on the pore structure characteristics of marine and terrestrial shales. J. CO2 Util. 2018, 28, 152–167. [Google Scholar] [CrossRef]
- Lahann, R.; Masterlerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Hadian, P.; Rezaee, R. The Effect of Supercritical CO2 on Shaly Caprocks. Energies 2019, 13, 149. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.; Alles, S.; Krooss, B.M.; Stanjek, H.; Dewhurst, D. Effects of physical sorption and chemical reactions of CO2 in shaly caprocks. Energy Procedia 2009, 1, 3229–3235. [Google Scholar] [CrossRef] [Green Version]
- Bateman, K.; Rochelle, C.A.; Purser, G.; Kemp, S.J.; Wagner, D. Geochemical Interactions Between CO2 and Minerals within the Utsira Caprock: A 5-year Experimental Study. Energy Procedia 2013, 37, 5307–5314. [Google Scholar] [CrossRef]
- Johnson, J.W.; Nitao, J.J.; Knauss, K.G. Reactive Transport Modelling of CO2 Storage in Saline Aquifers to Elucidate Fundamental Processes, Trapping Mechanisms and Sequestration Partitioning’, Geological Society Special Publication; Geological Society: London, UK, 2004; Volume 233, pp. 107–128. [Google Scholar] [CrossRef] [Green Version]
- Rigby, S.P. Structural Characterisation of Natural and Industrial Porous Materials: A Manual; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Walker WC, Zettlemoyer AC A dual-surface BET adsorption theory. Y. Phys. Colloid Chem. 1948, 52, 47–58. [CrossRef] [PubMed]
- Ng, K.C.; Burhan, M.; Shahzad, M.W.; Bin Ismail, A. A universal isotherm model to capture adsorption uptake and energy distribution of porous heterogeneous surface. Sci. Rep. 2017, 7, 10634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BS ISO 9277; 2010 Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standards Organisation (ISO): Geneva, Switzerland, 2010.
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: London, UK, 1999. [Google Scholar]
- Avnir, D.; Farin, D. Molecular fractal surfaces. Nature 1984, 308, 261–263. [Google Scholar] [CrossRef]
- Mahnke, M.; Mögel, H.J. Fractal Analysis of Physical Adsorption on Material Surfaces. Colloids Surf. A 2003, 216, 215–228. [Google Scholar] [CrossRef]
- Seely, R.; Liddy, T.J.; Rochelle, C.A.; Fletcher, R.S.; Rigby, S.P. Evolution of the mineralogy, pore structure and transport properties of Nordland Shale following exposure to supercritical carbon dioxide. J. Pet. Sci. Eng. 2022, 213, 110466. [Google Scholar] [CrossRef]
- Pitcher, E.G.; Large, D.J.; Fletcher, R.S.; Rigby, S.P. Multi-scale pore structural change across a paleodepositional transition in Utica shale probed by gas sorption overcondensation and scanning. Mar. Pet. Geol. 2021, 134, 105348. [Google Scholar] [CrossRef]
- Rigby, S.P.; Stevens, L.; Meersmann, T.; Pavloskaya, G.E.; Rees, G.J.; Henderson, J.; Bryant, S.J.; Edler, K.J.; Fletcher, R.S. Structural and chemical heterogeneity in ancient glass probed using gas overcondensation, X-ray tomography, and solid-state NMR. Mater. Charact. 2020, 167, 110467. [Google Scholar] [CrossRef]
- Rigby, S.P.; Jahan, H.; Stevens, L.; Uguna, C.; Snape, C.; Macnaughton, B.; Large, D.J.; Fletcher, R.S. Pore structural evolution of shale following thermochemical treatment. Mar. Pet. Geol. 2020, 112, 104058. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Baldwin, C.A.; Sederman, A.J.; Mantle, M.D.; Alexander, P.; Gladden, L.F. Determination and characterization of the structure of a pore space from 3D volume images. J. Colloid Interface Sci. 1996, 181, 79–92. [Google Scholar] [CrossRef]
- Rigby, S.P. Recent Developments in the Structural Characterisation of Disordered, Mesoporous Solids. JMTR 2018, 62, 296–312. [Google Scholar] [CrossRef]
- Pitcher, E.G.; Fletcher, R.S.; Large, D.J.; Rigby, S.P. Pore Structure-Transport Relationships in Bowland Shale. In Processes and Resources in Fine-Grained Sedimentary Rocks; Emmings, J., Ed.; Geological Society of London: London, UK, 2023; in press. [Google Scholar]
- Auroux, A. Calorimetry and Thermal Methods in Catalysis; Springer: Berlin, Germany, 2013. [Google Scholar]
- Woo, H.J.; Monson, P.A. Phase behavior and dynamics of fluids in mesoporous glasses. Phys. Rev. E 2003, 67, 041207. [Google Scholar] [CrossRef] [PubMed]
- Desouza, A.; Monson, P.A. Modeling fluids confined in three-dimensionally ordered mesoporous carbons. Adsorption 2021, 27, 253–264. [Google Scholar] [CrossRef]
- Onoja, M.U.; Williams, J.D.O.; Vosper, H.; Shariatipour, S.M. Effect of sedimentary heterogeneities in the sealing formation on predictive analysis of geological CO2 storage. Int. J. Greenh. Gas Control 2019, 82, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Kvamme, B.; Liu, S. Reactive transport of CO2 in saline aquifers with implicit geomechanical analysis. Energy Procedia 2009, 1, 3267–3274. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Hou, Z.; Bacon, D.H.; Murray, C.J.; White, M.D. Three-dimensional modelling of the reactive transport of CO2 and its impact on geomechanical properties of reservoir rocks and seals. Int. J. Greenh. Gas Control 2016, 46, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Zhang, D. Comprehensive review of caprock-sealing mechanisms for geologic carbon sequestration. Environ. Sci. Technol. 2013, 47, 9. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, H.; Moodie, N.; Esser, R.; Jia, W.; Zheng, L.; Rutqvist, J.; McPherson, B. Chemical-Mechanical Impacts of CO2 Intrusion into Heterogeneous Caprock. Wat. Resour. Res. 2020, 56, e2020WR027193. [Google Scholar] [CrossRef]
- Newell, P.; Ilgen, A.G. Science of Carbon Storage in deep Saline Formations: Process Coupling Across Time and Spatial Scales; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zweigel, P.; Lothe, A.E.; Arts, R.; Hamborg, M. Reservoir Geology of the Storage Units in the Sleipner CO2-Injection Case. Sintef Petroleum Research Report No., 23.4285.00/02/00. Available online: https://www.sintef.no/globalassets/project/ik23430000-sacs/technical-reports/zweigeletal_2000_reservoirreport_text.pdf (accessed on 25 August 2022).
- Agartan, E.; Cihan, A.; Illangasekare, T.H.; Zhou, Q.; Birkholzer, J.T. Mixing and trapping of dissolved CO2 in deep geologic formations with shale layers. Adv. Water Res. 2017, 105, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.L.; Chapman, S.W.; Guilbeault, M.A. Plume persistence caused by back diffusion from thin clay layers in a sand aquifer following TCE source-zone hydraulic isolation. J. Contam. Hydrol. 2008, 102, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Kampman, N.; Busch, A.; Bertier, P.; Snippe, J.; Hangx, S.; Pipich, V.; Di, Z.; Rother, G.; Harrington, J.; Evans, J.; et al. Observational evidence confirms modelling of the long-term integrity of CO2-reservoir caprocks. Nat Commun. 2016, 7, 12268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, V.; McPherson, B.; Priewisch, A.; Moore, J.; Moodie, N. Factors affecting self-sealing of geological faults due to CO2-leakage. Greenh. Gases: Sci. Technol. 2017, 7, 273–294. [Google Scholar] [CrossRef]
- Wangen, M. A 3D model for chimney formation in sedimentary basins. Comput. Geosci. 2020, 137, 104429. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Santamarina, J.C. CO2 breakthrough—Caprock sealing efficiency and integrity for carbon geological storage. Int. J. Greenh. Gas Control 2017, 66, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Alkan, H.; Cinar, Y.; Ülker, E. Impact of Capillary Pressure, Salinity and in situ Conditions on CO2 Injection into Saline Aquifers. Transp. Porous Media 2010, 84, 799–819. [Google Scholar] [CrossRef]
- Rohmer, J.; Seyedi, D. Coupled Large Scale Hydromechanical Modelling for Caprock Failure Risk Assessment of CO2 Storage in Deep Saline Aquifers. Oil Gas Sci. Technol. 2010, 65, 503–517. [Google Scholar] [CrossRef] [Green Version]
- Rutqvist, J.; Birkholzer, J.; Cappa, F.; Tsang, C.F. Estimating maximum sustainable injection pressure during geological sequestration of CO2 using coupled fluid flow and geomechanical fault-slip analysis. Energy Convers. Manag. 2007, 48, 1798–1807. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Gilbert, S.; Nauroy, J.-F.; Brosse, E. 3D geomechanical modelling for CO2 geologic storage in the Dogger carbonates of the Paris Basin. Int. J. Greenh. Gas Control 2009, 3, 288–299. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Bolster, D.; Olivella, S.; Carrera, J. Coupled hydromechanical modeling of CO2 sequestration in deep saline aquifers. Int. J. Greenh. Gas Control 2010, 4, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Streit, J.E.; Hillis, R.R. Estimating fault stability and sustainable fluid pressures for underground storage of CO2 in porous rock. Energy 2004, 29, 1445–1456. [Google Scholar] [CrossRef]
- Rutqvist, J.; Birkholzer, J.T.; Tsang, C.-F. Coupled reservoir–geomechanical analysis of the potential for tensile and shear failure associated with CO2 injection in multilayered reservoir–caprock systems. Int. J. Rock Mech. Min. Sci. 2008, 45, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Hows, A.; Zoback, M.; Gupta, N.; Ramakrishnan, T. Geomechanical aspects of CO2 sequestration in a deep saline reservoir in the Ohio River Valley region. Environ. Geosci. 2006, 13, 85–103. [Google Scholar]
- Rutqvist, J.; Vasco, D.W.; Myer, L. Coupled reservoir-geomechanical analysis of CO2 injection and ground deformations at In Salah, Algeria. Int. J. Greenh. Gas Control 2010, 4, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.P.; Hao, Y.; Foxall, W.; McNab, W. In Salah CO2 storage JIP: Hydromechanical simulations of surface uplift due to CO2 injection at In Salah. Energy Procedia 2011, 4, 3269–3275. [Google Scholar] [CrossRef] [Green Version]
- Bissell, R.C.; Vasco, D.W.; Atbi, M.; Hamdani, M.; Okwelegbe, M.; Goldwater, M.H. A full field simulation of the In Salah gas production and CO2 storage project using a coupled geo-mechanical and thermal flow simulator. Energy Procedia 2011, 4, 3290–3297. [Google Scholar] [CrossRef] [Green Version]
- Zoback, M.D.; Gorelick, M. Earthquake triggering and large-scale geologic storage of carbon dioxide. Proc. Natl. Acad. Sci. USA 2012, 109, 10164–10168. [Google Scholar] [CrossRef]
- Martinez, M.J.; Newell, P.; Bishop, J.E.; Turner, D.Z. Coupled multiphase flow and geomechanics model for analysis of joint reactivation during CO2 sequestration operations. Int. J. Greenh. Gas Control 2013, 17, 148–160. [Google Scholar] [CrossRef]
- Pan, P.-Z.; Rutqvist, J.; Feng, X.-T.; Yan, F. TOUGH–RDCA modeling of multiple fracture interactions in caprock during CO2 injection into a deep brine aquifer. Comput. Geosci. 2014, 65, 24–36. [Google Scholar] [CrossRef]
- Lee, J.; Min, K.-B.; Rutqvist, J. Probabilistic Analysis of Fracture Reactivation Associated with Deep Underground CO2 Injection. Rock Mech. Rock Eng. 2013, 46, 801–820. [Google Scholar] [CrossRef] [Green Version]
- Siriwardane, H.J.; Gondle, R.K.; Bromhal, G.S. Coupled Flow and Deformation Modeling of Carbon Dioxide Migration in the Presence of a Caprock Fracture during Injection. Energy Fuels 2013, 27, 4232–4243. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Winterfeld, P.H.; Xiong, Y.; Wu, Y.-S.; Yao, J. Parallel simulation of fully-coupled thermal-hydro-mechanical processes in CO2 leakage through fluid-driven fracture zones. Int. J. Greenh. Gas Control 2015, 34, 39–51. [Google Scholar] [CrossRef]
- Wangen, M.; Gasda, S.; Bjornara, T. Geomechanical consequences of large-scale fluid storage in the Utsira Formation in the North Sea. Energy Procedia 2016, 97, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.; Hangx, S.J.T.; Marshall, J.D.; Wentinck, H.M. Swelling clay minerals and containment risk assessment for the storage seal of the Peterhead CCS project. Int. J. Greenh. Gas Control 2020, 94, 102924. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.; Yang, C.; et al. Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Bakker, E.; Kaszuba, J.; Den Hartog, S.; Hangx, S. Chemo-mechanical behavior of clay-rich fault gouges affected by CO2 -brine-rock interactions: Original Research Article: Chemo-mechanical behavior of clay-rich fault gouges. Greenh. Gases: Sci. Technol. 2018, 9, 19–36. [Google Scholar] [CrossRef] [Green Version]
- Khan, C.; Ge, L.; Rudolph, V. Reservoir Simulation Study for CO2 Sequestration in Saline Aquifers. Int. J. Appl. Sci. Technol. 2015, 4, 30–45. [Google Scholar]
- Yang, G.; Ma, X.; Feng, T.; Ying, Y.; Yin, S.; Huang, M.; Wang, Y. Geochemical modelling of the evolution of caprock sealing capacity at the Shenhua CCS demonstration project. Minerals 2020, 10, 1009. [Google Scholar] [CrossRef]
- Ma, X.; Yang, G.; Li, X.; Yu, Y.; Dong, J. Geochemical modeling of changes in caprock permeability caused by CO2 –brine–rock interactions under the diffusion mechanism. Oil Gas Sci. Technol. 2019, 74, 83. [Google Scholar] [CrossRef] [Green Version]
- Armitage, P.J.; Worden, R.H.; Faulkner, D.R.; Butcher, A.R.; Espie, A.A. Permeability of the Mercia Mudstone: Suitability as caprock to carbon capture and storage sites. Geofluids 2016, 16, 26–42. [Google Scholar] [CrossRef]
- Green, C.; Ennis-King, J. Effect of Vertical Heterogeneity on Long-Term Migration of CO2 in Saline Formations. Transp. Porous Media 2009, 82, 31–47. [Google Scholar] [CrossRef]
- Kumar, A.; Noh, M.; Pope, G.A.; Sepehrnoori, K.; Bryant, S.; Lake, L.W. Reservoir Simulation of CO2 Storage in Deep Saline Aquifers. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17 April 2004; SPE-89343-MS. [Google Scholar]
- Liu, H.; Hou, Z.; Were, P.; Gou, Y.; Xiong, L.; Sun, X. Modelling CO2-brine-rock interactions in the Upper Paleozoic formations of Ordos Basin used for CO2 sequestration. Environ. Earth Sci. 2014, 73, 2205–2222. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Z.; Zhang, Y.; Jiang, L.; Liu, Y.; Song, Y. Unstable Density Driven Convection of CO2 in Homogeneous and Heterogeneous Porous Media with Implications for Deep Saline Aquifers. Water Resour. Res. 2021, 57, e28132. [Google Scholar]
- Ghanbari, S.; Al-Zaabi, Y.; Pickup, G.E.; Mackay, E.; Gozalpour, F.; Todd, A.C. Simulation of CO2 Storage in Saline Aquifers. Chem. Eng. Res. Des. 2006, 84, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Yong, W.P.; Azahree, A.I.; Ali, S.S.; Jaafar Azuddin, F.; Amin, S. A New Modelling Approach to Simulate CO2 Movement and Containment Coupled with Geochemical Reactions and Geomechanical Effects for an Offshore CO2 Storage in Malaysia. In Proceedings of the SPE Europec Featured at 81st EAGE Conference and Exhibition, London, UK, 3–6 June 2019; D041S008R010. [Google Scholar]
- Zhang, Y.; Xue, Z.; Park, H.; Shi, J.-Q.; Kiyama, T.; LEei, X.; Sun, Y.; Liang, Y. Tracking CO2 Plumes in Clay-Rich Rock by Distributed Fiber Optic Strain Sensing (DFOSS): A Laboratory Demonstration. Water Resour. Res. 2019, 55, 856–867. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.; Peters, C.A.; Pyrak-Nolte, L. Influence of Rock Mineralogy on Reactive Fracture Evolution in Carbonate-Rich Caprocks. Environ. Sci. Technol. 2018, 52, 10144–10152. [Google Scholar] [CrossRef]
- Ojala, I.O. The effect of CO2 on the mechanical properties of reservoir and cap rock. Energy Procedia 2011, 4, 5392–5397. [Google Scholar] [CrossRef] [Green Version]
- Fleury, M.; Pironon, J.; Nindre, L.E.; Bildstein, O.; Berne, P.; Lagneau, V.; Broseta, D.; Pichery, T.; Fillacier, S.; Lescanne, M.; et al. Evaluating sealing efficiency of caprocks for CO2 storage: An overviewof the Geocarbone Integrity program and results. Energy Procedia 2011, 4, 5227–5234. [Google Scholar] [CrossRef]
- Alsayah, A.; Rigby, S.P. Coupled multiphase flow, geochemical, and geomechanical modelling of the impact of shale interlayers on CO2 migration. 2022. submitted for publication. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigby, S.P.; Alsayah, A.; Seely, R. Impact of Exposure to Supercritical Carbon Dioxide on Reservoir Caprocks and Inter-Layers during Sequestration. Energies 2022, 15, 7538. https://doi.org/10.3390/en15207538
Rigby SP, Alsayah A, Seely R. Impact of Exposure to Supercritical Carbon Dioxide on Reservoir Caprocks and Inter-Layers during Sequestration. Energies. 2022; 15(20):7538. https://doi.org/10.3390/en15207538
Chicago/Turabian StyleRigby, Sean P., Ali Alsayah, and Richard Seely. 2022. "Impact of Exposure to Supercritical Carbon Dioxide on Reservoir Caprocks and Inter-Layers during Sequestration" Energies 15, no. 20: 7538. https://doi.org/10.3390/en15207538



