Solar Pyrolysis of Spirulina platensis Assisted by Fresnel Lens Using Hydrocalumite-Type Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Characterization
2.2. Catalyst Synthesis and Characterization
2.3. Experimental Apparatus and Solar Pyrolysis Conditions
2.4. Experimental Design
2.5. Bio-Oil Characterization
2.5.1. Gas Chromatography Mass Spectrometry (GC/MS)
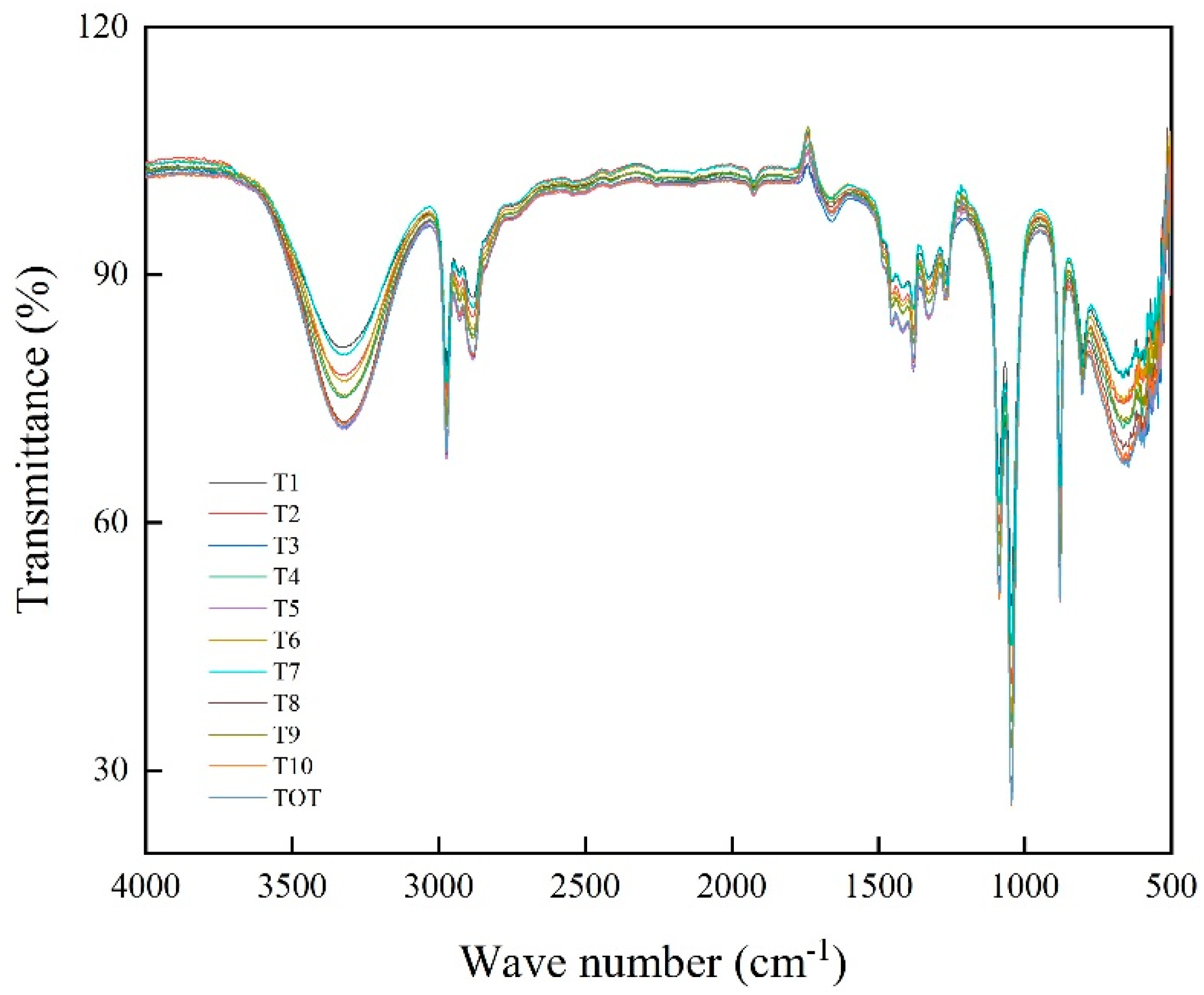
2.5.2. Infrared Spectroscopy Analyses
3. Results
3.1. Biomass Characteristics
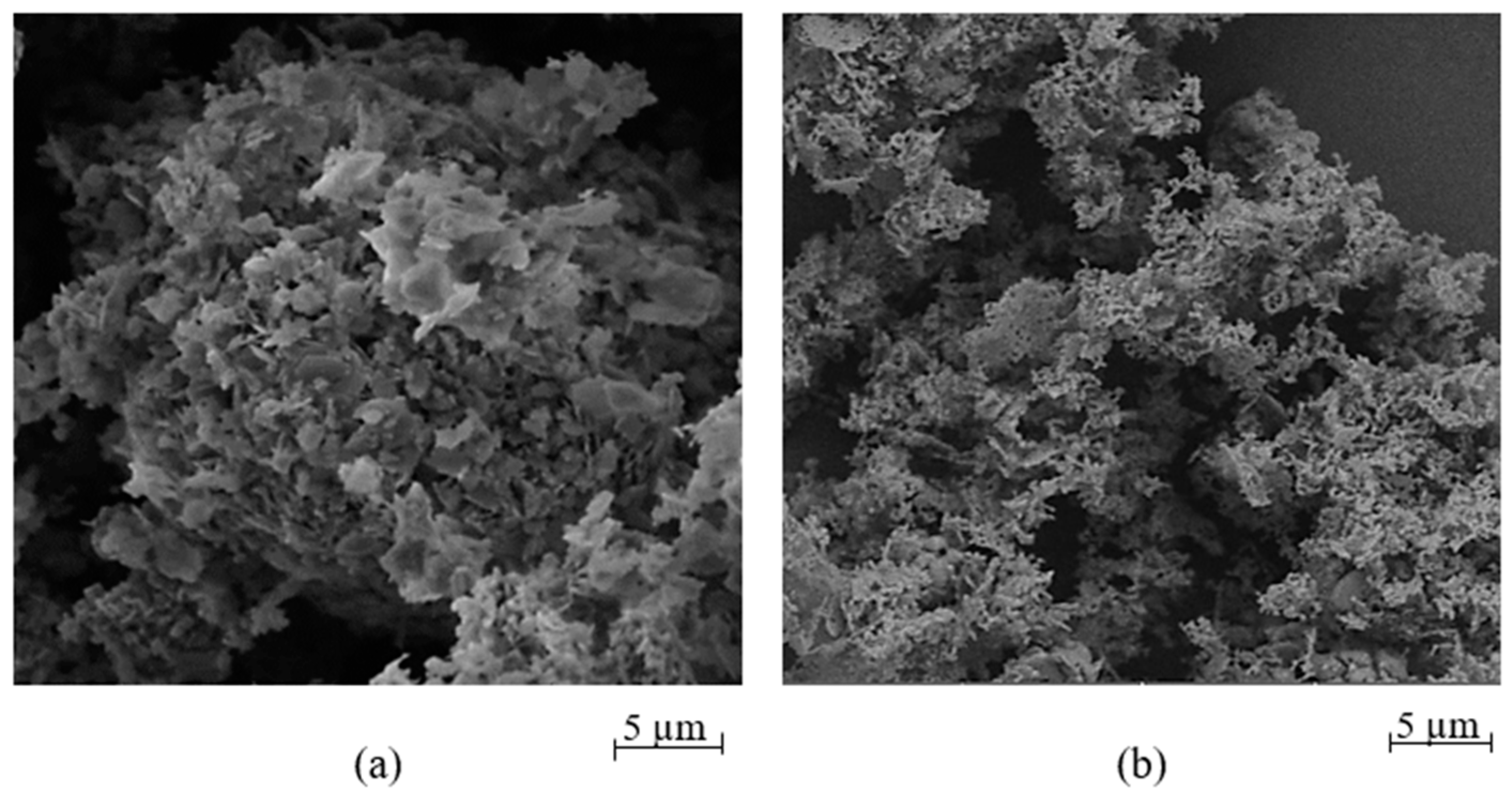
3.2. Catalyst Characteristics
3.3. Product Yields
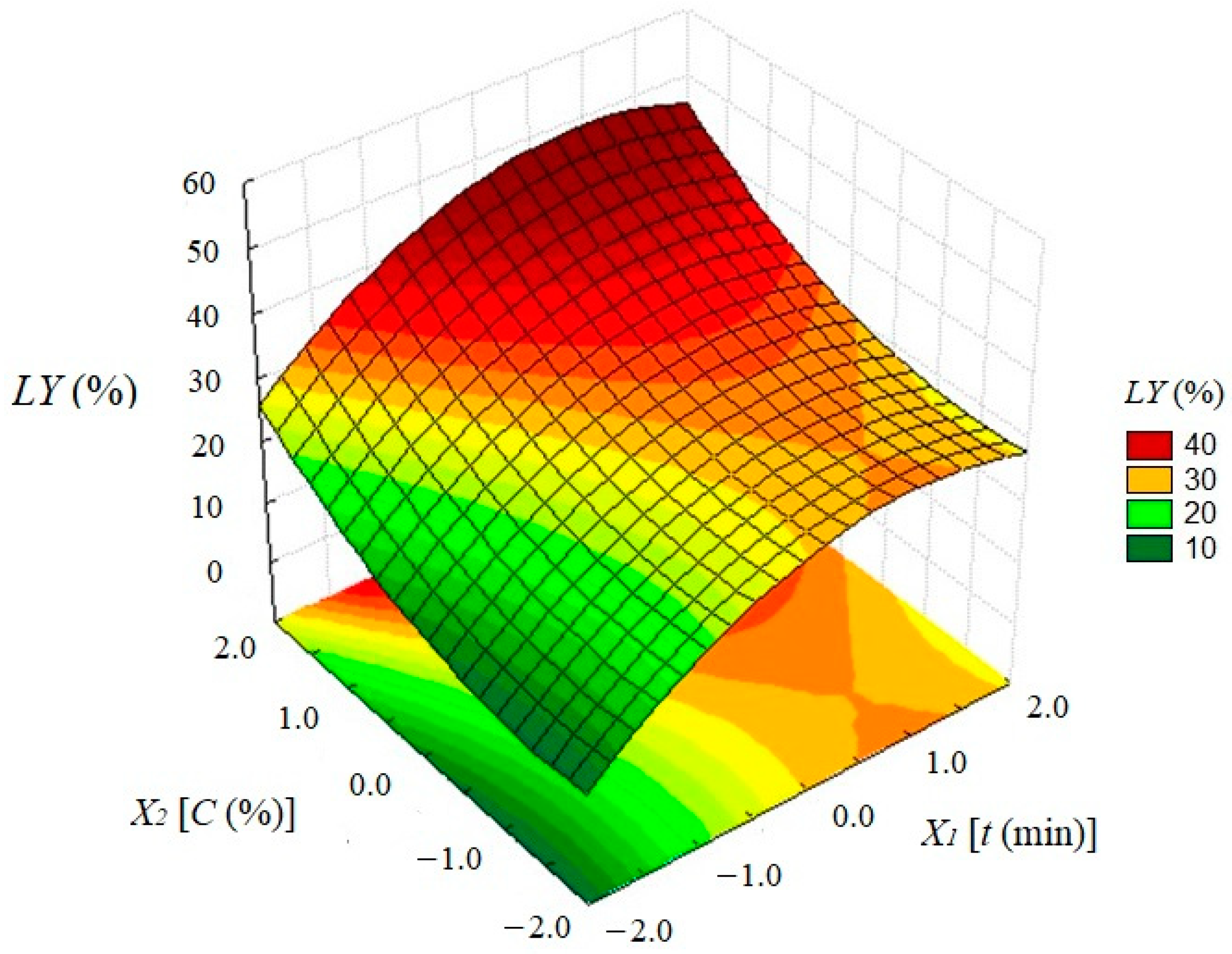
3.4. Effect of a Multivariable System on Bio-Oil Yield
3.5. Liquid Yield Optimization
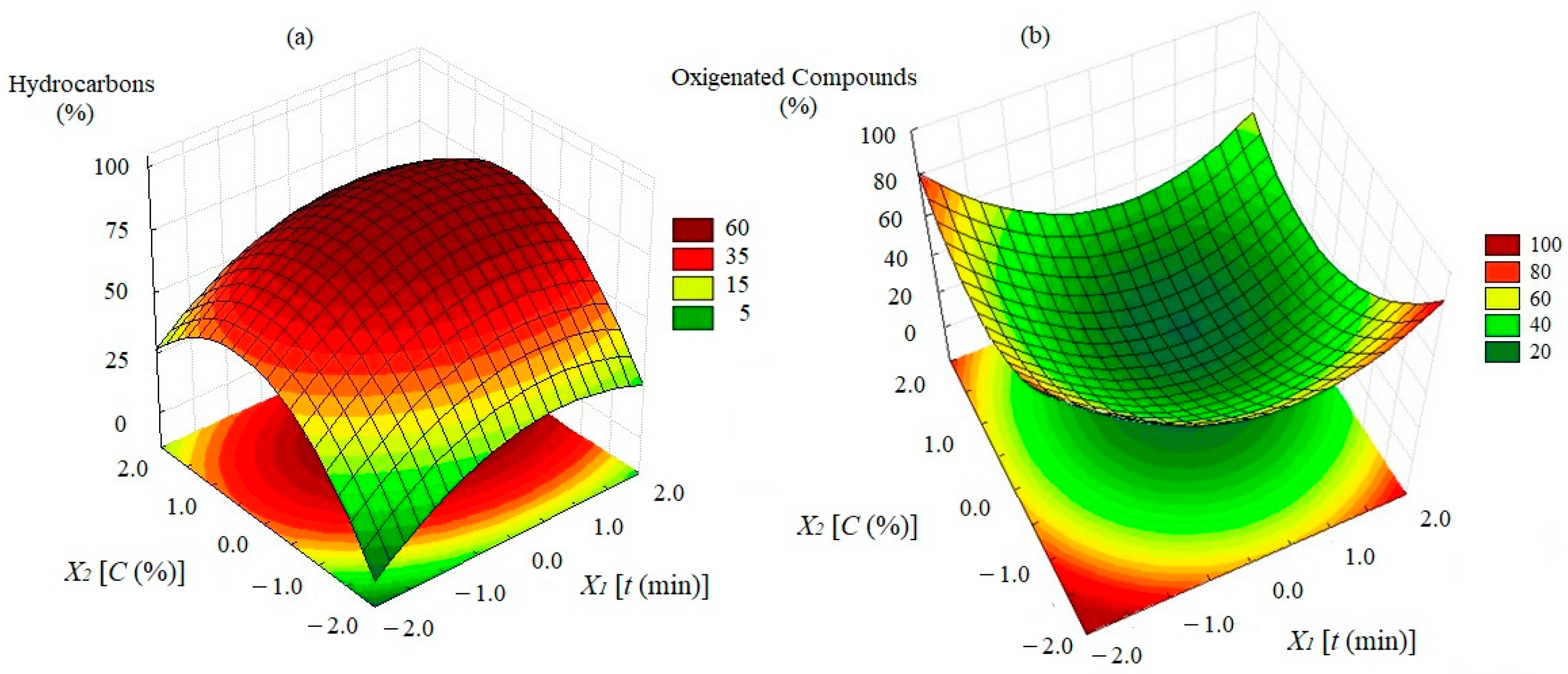
3.6. Bio-Oil Composition
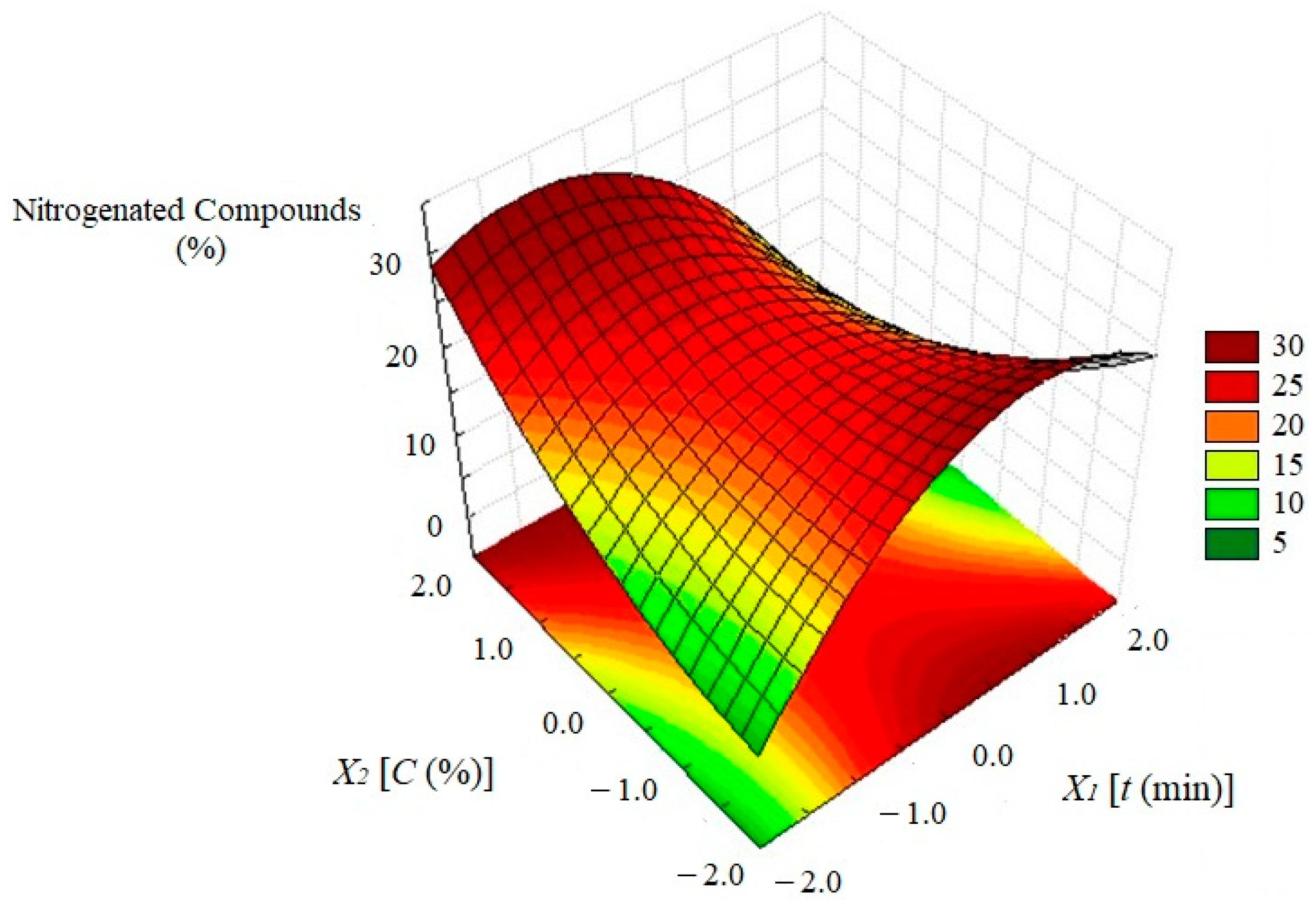
3.7. Effect of the Studied Variables on Bio-Oil Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| ASTM | American Society for Testing and Materials |
| C | percentage of catalyst (%) |
| Dpore | pore diameter (Å) |
| d# | sieve diameter (mm) |
| di | internal diameter of the catalytic reactor (cm) |
| GY | gas yield (%) |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| HHV | higher heating value (MJ/kg) |
References
- Assis, T.C.; Calijuri, M.L.; Assemany, P.P.; Pereira, A.S.A.; Martins, M.A. Using atmospheric emissions as CO2 source in the cultivation of microalgae: Productivity and economic viability. J. Clean. Prod. 2019, 215, 1160–1169. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; Silva, J.C.G.; Domenico, M.D.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting pecan nutshell pyrolysis as a source of bioenergy and bio-based chemicals using multicomponent kinetic modeling, thermodynamic parameters estimation, and Py-GC/MS analysis. Renew. Sustain. Energy Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Santos, M.R.; Sales, R.F.; Silva, A.O.S.; Teixeira, C.M.; Pacheco, J.G.A.; Fréty, R. Flash pyrolysis of myristic acid adsorbed on supported nickel catalysts for biofuel production. J. Therm. Anal. Calorim. 2015, 119, 1875–1885. [Google Scholar] [CrossRef]
- Arias, S.; González, J.F.; Sousa, L.V.; Barbosa, C.B.M.; Silva, A.O.S.; Fréty, R.; Pacheco, J.G.A. Influence of Ni/Al ratio on the fast pyrolysis of myristic acid when adsorbed on unsupported mixed oxides derived from layered double hydroxides. Catal. Today 2021, 381, 181–191. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Andrade, L.A.; Vieira, L.G.M.; Barrozo, M.A.S. Multi-response optimization of bio-oil production from catalytic solar pyrolysis of Spirulina platensis. J. Energy Inst. 2020, 93, 1313–1323. [Google Scholar] [CrossRef]
- Santos, K.G.; Lobato, F.S.; Lira, T.S.; Murata, V.V.; Barrozo, M.A.S. Sensitivity analysis applied to independent parallel reaction model for pyrolysis of bagasse. Chem. Eng. Res. Des. 2012, 90, 1989–1996. [Google Scholar] [CrossRef]
- Mayer, F.M.; Teixeira, C.M.; Pacheco, J.G.A.; Souza, C.T.; Bauer, D.V.; Caramão, E.B.; Espíndola, J.S.; Trierweiler, J.O.; Lopez, O.W.P.; Zini, C.A. Characterization of analytical fast pyrolysis vapors of medium-density fiberboard (MDF) using metal-modified HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 87–95. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, deactivation, and regeneration of heterogeneous catalysts in bio-oil upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Muley, P.D.; Henkel, C.E.; Aguilar, G.; Klasson, K.T.; Boldor, D. Ex situ thermo-catalytic upgrading of biomass pyrolysis vapors using a traveling wave microwave reactor. Appl. Energy 2016, 183, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Andrade, L.A.; Barbosa, J.M.; Barrozo, M.A.S.; Vieira, L.G.M. A comparative study of the behavior of Chlamydomonas reinhardtii and Spirulina platensis in solar catalytic pyrolysis. Int. J. Energy Res. 2020, 44, 5397–5411. [Google Scholar] [CrossRef]
- Andrade, L.A.; Batista, F.R.X.; Lira, T.S.; Barrozo, M.A.S.; Vieira, L.G.M. Characterization and product formation during the catalytic and non-catalytic pyrolysis of the green microalgae Chlamydomonas reinhardtii. Renew. Energy 2018, 119, 731–740. [Google Scholar] [CrossRef]
- Lopez-Salinas, E.; Serrano, M.E.L.; Jacome, M.A.C. Characterization of Synthetic Hydrocalumite-Type [Ca2AI(OH)6]NO3. mH2O: Effect of the Calcination Temperature. J. Porous Mater. 1996, 2, 291–297. [Google Scholar] [CrossRef]
- Rousselot, I.; Taviot-Guého, C.; Leroux, F.; Léone, P.; Palvadeau, P.; Besse, J.P. Insights on the structural chemistry of hydrocalumite and hydrotalcite-like materials: Investigation of the series Ca2M3+(OH)6Cl·2H2O (M3+: Al3+, Ga3+, Fe3+, and Sc3+) by X-ray powder diffraction. J. Solid State Chem. 2002, 167, 137–144. [Google Scholar] [CrossRef]
- Linares, C.F.; Ocanto, F.; Bretto, P.; Monsalve, M. Study of as-synthesized and calcined hydrocalumites as possible antacid agents. Bull. Mater. Sci. 2014, 37, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Campos-Molina, M.J.; Santamaría-Gonźalez, J.; Ḿerida-Robles, J.; Moreno-Tost, R.; Albuquerque, M.C.G.; Bruque-Ǵamez, S. Base catalysts derived from hydrocalumite for the transesterification of sunflower oil. Energy Fuels 2010, 24, 979–984. [Google Scholar] [CrossRef]
- Maree, D.C.; Heydenrych, M. Development of a Mesoporous Silica-Supported Layered Double Hydroxide Catalyst for the Reduction of Oxygenated Compounds in E. grandis Fast Pyrolysis Oils. Catalysts 2021, 11, 1527. [Google Scholar] [CrossRef]
- Zeng, K.; Gauthier, D.; Lu, J.; Flamant, G. Parametric study and process optimization for solar pyrolysis of beech wood. Energy Convers. Manag. 2015, 106, 987–998. [Google Scholar] [CrossRef]
- Zeaiter, J.; Ahmad, M.N.; Rooney, D.; Samneh, B.; Shammas, E. Design of an automated solar concentrator for the pyrolysis of scrap rubber. Energy Convers. Manag. 2015, 101, 118–125. [Google Scholar] [CrossRef]
- Andrade, L.A.; Barrozo, M.A.S.; Vieira, L.G.M. A study on dynamic heating in solar dish concentrators. Renew. Energy 2016, 87, 501–508. [Google Scholar] [CrossRef]
- Andrade, L.A.; Barrozo, M.A.S.; Vieira, L.G.M. Catalytic solar pyrolysis of microalgae Chlamydomonas. Sol. Energy 2018, 173, 928–938. [Google Scholar] [CrossRef]
- Zeng, K.; Gauthier, D.; Soria, J.; Mazza, G.; Flamant, G. Solar pyrolysis of carbonaceous feedstocks: A review. Sol. Energy 2017, 156, 73–92. [Google Scholar] [CrossRef]
- Zeng, K.; Li, R.; Minh, D.P.; Weiss-Hortala, E.; Nzihou, A.; He, X. Solar pyrolysis of heavy metal contaminated biomass for gas fuel production. Energy 2019, 187, 116016. [Google Scholar] [CrossRef]
- Hijazi, A.; Boyadjian, C.; Ahmad, M.N.; Zeaiter, J. Solar pyrolysis of waste rubber tires using photoactive catalysts. Waste Manag. 2018, 77, 10–21. [Google Scholar] [CrossRef]
- Boutin, R.; Munnier, E.; Renaudeau, N.; Girardot, M.; Pinault, M.; Chevalier, S.; Chourpa, I.; Clément-Larosière, B.; Imbert, C.; Boudesocque-Delaye, L. Spirulina platensis sustainable lipid extracts in alginate-based nanocarriers: An algal approach against biofilms. Algal Res. 2019, 37, 160–168. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132235. [Google Scholar] [CrossRef]
- Silva, N.C.; Duarte, C.R.; Barrozo, M.A.S. Analysis of the use of a non-conventional rotary drum for dehydration of microalga Spirulina platensis. Bioprocess Biosyst. Eng. 2020, 43, 1359–1367. [Google Scholar] [CrossRef]
- D’oca, M.G.M.; Viêgas, C.V.; Lemões, J.S.; Miyasaki, E.K.; Morón-Villarreyes, J.A.; Primel, E.G. Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioenergy 2011, 35, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Wu, Q.; Tu, P. Pyrolytic characteristics of heterotrophic Chlorella protothecoides for renewable bio-fuel production. J. Appl. Phycol. 2001, 13, 5–12. [Google Scholar] [CrossRef]
- Jafarian, S.; Tavasoli, A. A comparative study on the quality of bioproducts derived from catalytic pyrolysis of green microalgae Spirulina (Arthrospira) plantensis over transition metals supported on HMS-ZSM5 composite. Int. J. Hydrogen Energy 2018, 43, 19902–19917. [Google Scholar] [CrossRef]
- Barrozo, M.A.S.; Murata, V.V.; Costa, S.M. The drying of soybean seeds in countercurrent and concurrent moving bed dryers. Dry. Technol. 1998, 16, 2033–2047. [Google Scholar] [CrossRef]
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F. Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Joubert, J.E.; Danje, S.; Hugo, T.; Görgens, J.; Knoetze, J.H. Impact of the lignocellulosic material on fast pyrolysis yields and product quality. Bioresour. Technol. 2013, 150, 129–138. [Google Scholar] [CrossRef]
- Norouzi, O.; Tavasoli, A.; Jafarian, S.; Esmailpour, S. Catalytic upgrading of bio-products derived from pyrolysis of red macroalgae Gracilaria gracilis with a promising novel micro/mesoporous catalyst. Bioresour. Technol. 2017, 243, 1–8. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef]
- Bui, H.H.; Tran, K.Q.; Chen, W.H. Pyrolysis of microalgae residues—A Kinetic study. Bioresour. Technol. 2015, 199, 362–366. [Google Scholar] [CrossRef]
- Rossi, R.A.S.; Barbosa, J.M.; Barrozo, M.A.S.; Vieira, L.G.M. Solar assisted catalytic thermochemical processes: Pyrolysis and hydropyrolysis of Chlamydomonas reinhardtii microalgae. Renew. Energy 2021, 170, 669–682. [Google Scholar] [CrossRef]
- Kanda, H.; Hoshino, R.; Murakami, K.; Zheng, Q.; Goto, M. Lipid extraction from microalgae covered with biomineralized cell walls using liquefied dimethyl ether. Fuel 2020, 262, 116590. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, J.; Cao, C.; Cheng, Z.; Zhu, Y.; Wen, W. Comparative study of different algae pyrolysis using photoionization mass spectrometry and gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2021, 155, 105068. [Google Scholar] [CrossRef]
- Wang, X.; Tang, X.; Yang, X. Pyrolysis mechanism of microalgae Nannochloropsis sp. based on model compounds and their interaction. Energy Convers. Manag. 2017, 140, 203–210. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W.; Enayatpour, S.; Tavangarian, F.; Fahami, M. Rapid preparation of nano hexagonal-shaped hydrocalumite via one-pot mechanochemistry method. Appl. Clay Sci. 2017, 136, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Xia, S.; Lu, X.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over calcined Ca-Al hydrocalumite. Cuihua Xuebao/Chinese. J. Catal. 2015, 36, 1759–1765. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F. Influence of acid-base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic glycerol etherification to short-chain polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Vieille, L.; Rousselot, I.; Leroux, F.; Besse, J.P.; Taviot-Guého, C. Hydrocalumite and Its Polymer Derivatives. 1. Reversible Thermal Behavior of Friedel’s Salt: A Direct Observation by Means of High-Temperature in Situ Powder X-ray Diffraction. Chem. Mater. 2003, 15, 4361–4368. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Hydrothermal synthesis of hexagonal and orthorhombic MoO3 nanoparticles. J. Alloys Compd. 2011, 509, 8105–8110. [Google Scholar] [CrossRef]
- Sen, S.K.; Paul, T.C.; Dutta, S.; Hossain, M.N.; Mia, M.N.H. XRD peak profile and optical properties analysis of Ag-doped h-MoO3 nanorods synthesized via hydrothermal method. J. Mater. Sci. Mater. Electron. 2020, 31, 1768–1786. [Google Scholar] [CrossRef]
- Sipos, P.; Pálinkó, I. As-prepared and intercalated layered double hydroxides of the hydrocalumite type as efficient catalysts in various reactions. Catal. Today 2018, 306, 32–41. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Cesteros, Y.; Díaz, F.; Pallarès, J. Fast aging treatment for the synthesis of hydrocalumites using microwaves. Appl. Clay Sci. 2013, 80, 313–319. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-oil from sawdust: Pyrolysis of sawdust in a fixed-bed system. Energy Fuels 2009, 23, 3767–3772. [Google Scholar] [CrossRef]
- Aysu, T.; Ola, O.; Maroto-Valer, M.M.; Sanna, A. Effects of tania based catalysts on in-situ pyrolysis of Pavlova microalgae. Fuel Process Technol. 2017, 166, 291–298. [Google Scholar] [CrossRef]
- Felipe, C.A.S.; Barrozo, M.A.S. Drying of soybean seeds in a concurrent moving bed: Heat and mass transfer and quality analysis. Dry. Technol. 2003, 21, 439–456. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Na, J.G.; Park, Y.K.; Kim, D.I.; Oh, Y.K.; Jeon, S.G.; Kook, J.W. Rapid pyrolysis behavior of oleaginous microalga, Chlorella sp. KR-1 with different triglyceride contents. Renew. Energy 2015, 81, 779–784. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Wang, Y.; Liu, L.; Wang, H.; Deng, S. Studies on the co-pyrolysis characteristics of oil shale and spent oil shale. J. Therm. Anal. Calorim. 2016, 123, 1707–1714. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, F.; Cai, Q.; Zhu, L.; Luo, Z. Steam reforming of acetic acid over coal ash supported Fe and Ni catalysts. Int. J. Hydrogen Energy 2015, 40, 11406–11413. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Fang, S.; Ma, X. Behaviors, product characteristics and kinetics of catalytic co-pyrolysis spirulina and oil shale. Energy Convers. Manag. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El Harfi, K. Valorization of algal waste via pyrolysis in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Srivatsa, S.C.; Batchelor, W.; Bhattacharya, S. A study on growth and pyrolysis characteristics of microalgae using Thermogravimetric Analysis-Infrared Spectroscopy and synchrotron Fourier Transform Infrared Spectroscopy. Bioresour. Technol. 2017, 229, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; Guil-Lopez, R.; Fierro, J.L.G.; Mota, N.; Jiménez, S.; Pizarro, P. Catalytic fast pyrolysis of biomass over Mg-Al mixed oxides derived from hydrotalcite-like precursors: Influence of Mg/Al ratio. J. Anal. Appl. Pyrolysis 2018, 134, 362–370. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C. Aromatic hydrocarbon selectivity as a function of CaO basicity and aging during CaO-catalyzed PET pyrolysis using tandem µ-reactor-GC/MS. Chem. Eng. J. 2018, 332, 169–173. [Google Scholar] [CrossRef]
- Wang, S.; Uzoejinwa, B.B.; Abomohra, A.E.F.; Wang, Q.; He, Z.; Feng, Y. Characterization and pyrolysis behavior of the green microalga Micractinium conductrix grown in lab-scale tubular photobioreactor using Py-GC/MS and TGA/MS. J. Anal. Appl. Pyrolysis 2018, 135, 340–349. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Chattanathan, S.A.; Gupta, R.B. Catalytic pyrolysis of green algae for hydrocarbon production using H +ZSM-5 catalyst. Bioresour. Technol. 2012, 118, 150–157. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Hernández, F.; Navarro, R. HZSM5 and HUSY deactivation during the catalytic pyrolysis of polyethylene. Appl. Catal. A Gen. 2004, 278, 37–43. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-ong, D.; Sricharoenchaikul, V.; Atong, D. Catalytic upgrading pyrolysis vapors of Jatropha waste using metal promoted ZSM-5 catalysts: An analytical PY-GC/MS. Renew. Energy 2014, 65, 70–77. [Google Scholar] [CrossRef]
- Jia, L.Y.; Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Bettahar, M.M. Catalytic fast pyrolysis of biomass: Superior selectivity of hierarchical zeolites to aromatics. Green Chem. 2017, 19, 5442–5459. [Google Scholar] [CrossRef]
- Persson, H.; Duman, I.; Wang, S.; Pettersson, L.J.; Yang, W. Catalytic pyrolysis over transition metal-modified zeolites: A comparative study between catalyst activity and deactivation. J. Anal. Appl. Pyrolysis 2019, 138, 54–61. [Google Scholar] [CrossRef]
- Prado, G.H.C.; Rao, Y.; De Klerk, A. Nitrogen removal from oil: A review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]





| Test | X1 | X2 | t (min) | C (%) |
|---|---|---|---|---|
| 1 | −1.00 | −1.00 | 6.00 | 8.58 |
| 2 | −1.00 | +1.00 | 6.00 | 50.00 |
| 3 | +1.00 | −1.00 | 24.00 | 8.58 |
| 4 | +1.00 | +1.00 | 24.00 | 50.00 |
| 5 | −1.41 | 0.00 | 2.27 | 29.29 |
| 6 | +1.41 | 0.00 | 27.73 | 29.29 |
| 7 | 0.00 | −1.41 | 15.00 | 0.00 |
| 8 | 0.00 | +1.41 | 15.00 | 58.58 |
| 9 | 0.00 | 0.00 | 15.00 | 29.29 |
| 10 | 0.00 | 0.00 | 15.00 | 29.29 |
| Elemental Analysis a (wt%) | Proximate Analysis a | ||
|---|---|---|---|
| C | 43.67 | Volatile matter | 80.09 ± 0.01 |
| H | 6.80 | Ash | 10.43 ± 0.01 |
| N | 9.84 | Fixed carbon | 9.48 |
| S | 1.89 | HHV | 20.86 ± 0.03 MJ/kg |
| O b | 37.8 | ||
| Compositional analysis | (wt%) | ||
| Protein | 55.86 ± 0.61 | ||
| Carbohydrate | 22.33 | ||
| Lipid | 11.38 ± 0.11 | ||
| Sample | SBET [m2.g−1] | Vpore [cm3.g−1] | Dpore [Å] |
|---|---|---|---|
| HC000 | 23 | 0.05 | 42 |
| HC700 | 11 | 0.02 | 17 |
| Test | SY (%) | LY (%) | GY (%) |
|---|---|---|---|
| 1 | 47.62 | 22.48 | 29.90 |
| 2 | 43.45 | 28.49 | 28.06 |
| 3 | 26.14 | 33.48 | 40.38 |
| 4 | 25.22 | 39.17 | 35.60 |
| 5 | 63.90 | 18.50 | 17.60 |
| 6 | 22.75 | 30.90 | 46.35 |
| 7 | 37.45 | 24.48 | 38.07 |
| 8 | 32.75 | 41.56 | 25.69 |
| 9 | 31.20 | 31.37 | 37.43 |
| 10 | 31.88 | 30.55 | 37.57 |
| Optimized Conditions | |||
|---|---|---|---|
| Coded values | Original values | ||
| X1 | +0.93 | Time (min) | 23.37 |
| X2 | +1.41 | Catalyst (%) | 58.58 |
| Calculated yield (%) | Experimental yield (%) | ||
| Solid | 23.08 | Solid | 25.17 |
| Liquid | 42.68 | Liquid | 43.39 |
| Gas | 33.21 | Gas | 31.44 |
| Hydrocarbon | Oxygenated Compound | Nitrogenous Compound |
|---|---|---|
| 1-Dodecene | 1,3-Cyclopentenedione | Acetamide |
| 1-Heptene | 1-Hexadecanol | 1-Cyanoacetyl-piperidine |
| 1-Tridecene | 1-Hydroxy-2-propanone | 3-Methylbutanonitrile |
| 1,7-Octadiene | 2-Furamethanol | 4-Methylpentanitrile |
| 3-Hexadecene | 2-Heptadecanone | 5-Hidroxypentamide |
| Eicosane | 2-Methylpropanal | Benzenepropanenitrile |
| Heneicosane | 2,3-Butenodione | Butanamide |
| Heptadecane | 3-Methoxy-2-methylphenol | Hexadecanamide |
| Pentadecane | Acetic Acid | Hexanamide |
| d-Limonene | Oleic acid | Indole |
| Styrene | Phenol | Pyrrole |
| Ethylbenzene | n-hexadecanoic acid | |
| o-Xyleno | n-octadecanoic acid | |
| Toluene | p-Cresol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.P.B.; Hori, C.E.; Barrozo, M.A.S.; Vieira, L.G.M. Solar Pyrolysis of Spirulina platensis Assisted by Fresnel Lens Using Hydrocalumite-Type Precursors. Energies 2022, 15, 7590. https://doi.org/10.3390/en15207590
Martins MPB, Hori CE, Barrozo MAS, Vieira LGM. Solar Pyrolysis of Spirulina platensis Assisted by Fresnel Lens Using Hydrocalumite-Type Precursors. Energies. 2022; 15(20):7590. https://doi.org/10.3390/en15207590
Chicago/Turabian StyleMartins, Marcus P. B., Carla E. Hori, Marcos A. S. Barrozo, and Luiz G. M. Vieira. 2022. "Solar Pyrolysis of Spirulina platensis Assisted by Fresnel Lens Using Hydrocalumite-Type Precursors" Energies 15, no. 20: 7590. https://doi.org/10.3390/en15207590






