Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries
Abstract
:1. Introduction
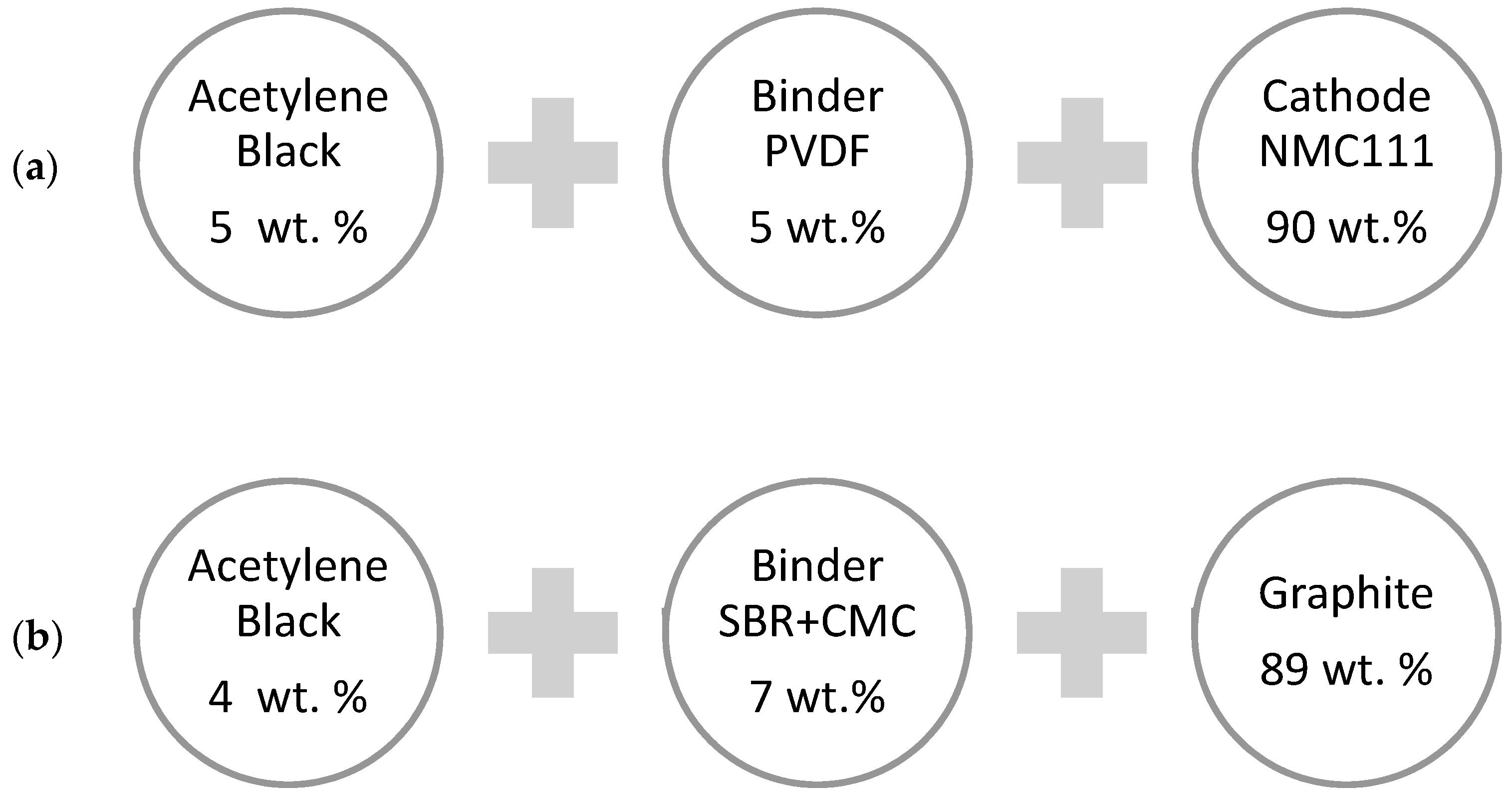
2. Materials and Methods
2.1. Separation of Al and Cu CCs
2.2. Ultrasonic Cleaning and Characterization of Surface and Bulk Impurities
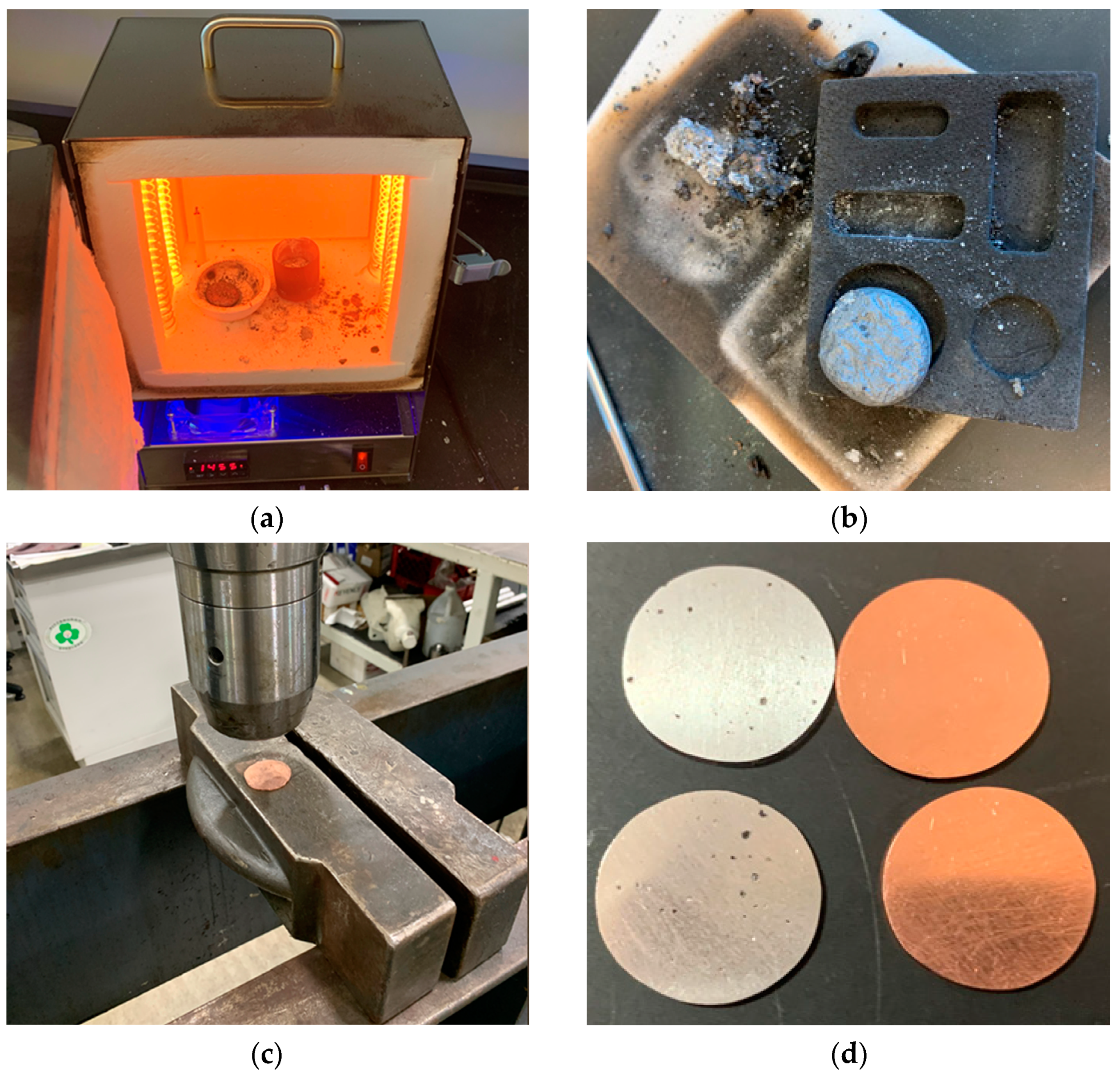
2.3. Melting, Molding, and Characterization of Surface and Bulk Impurities
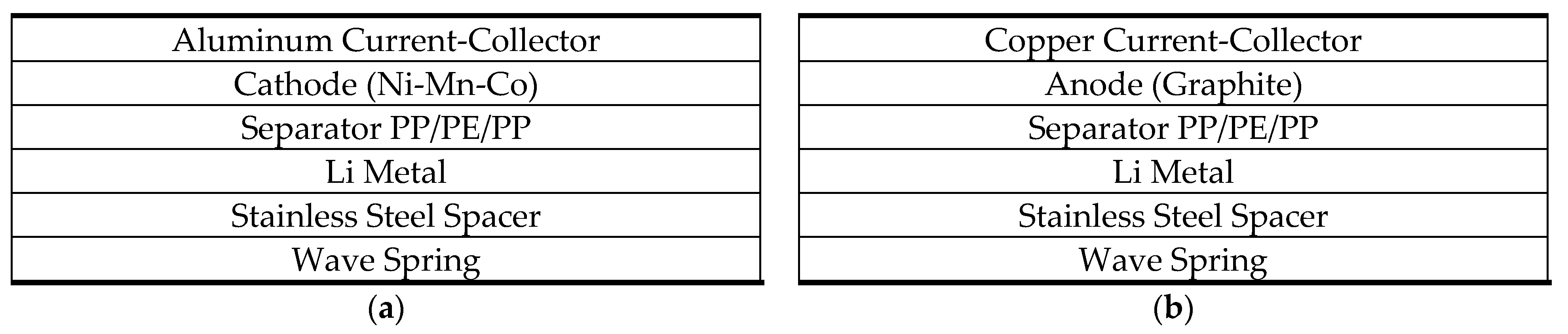
2.4. Half-Cell Construction and Final Characterization of Surface Impurities
3. Results and Discussion
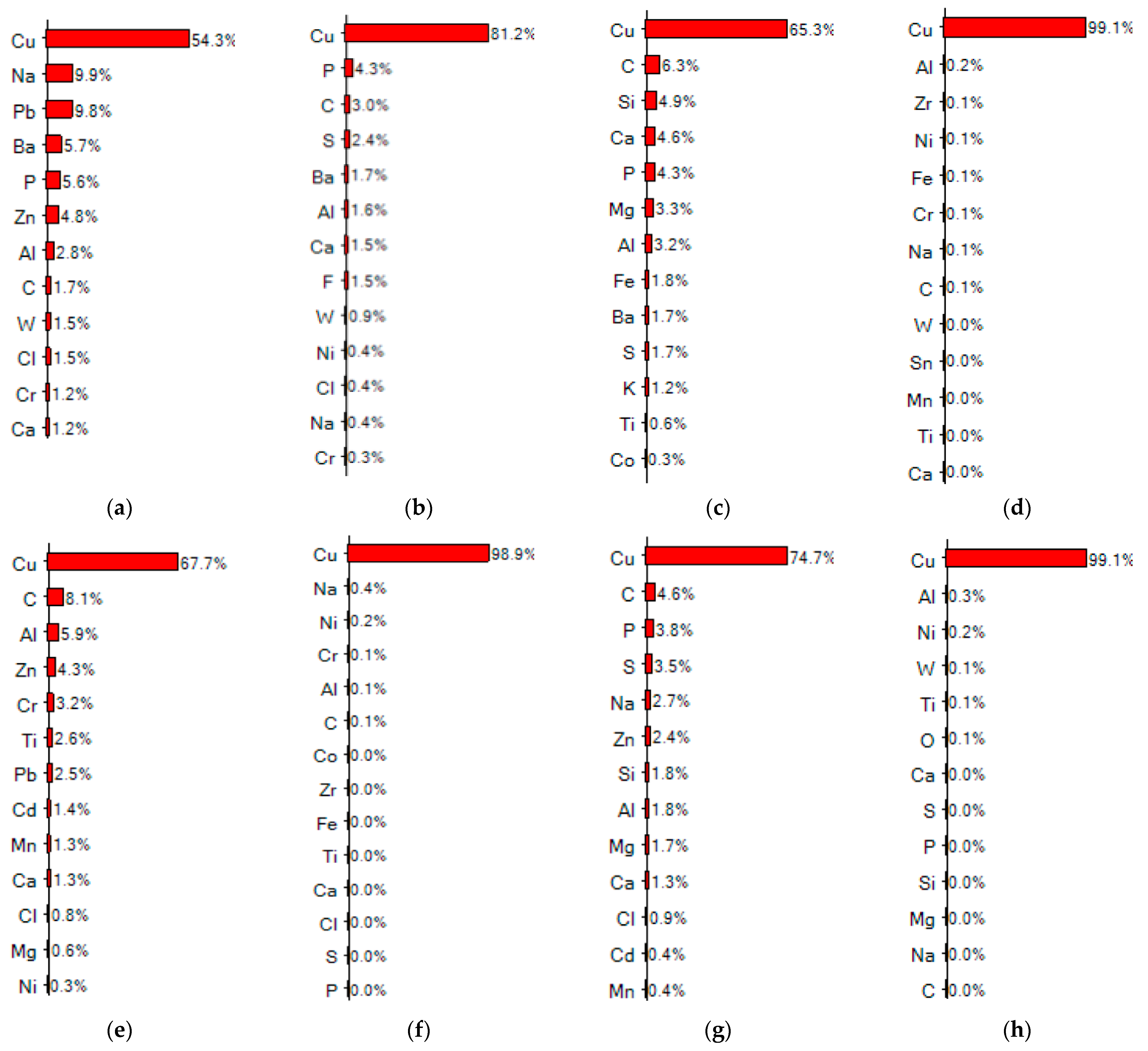
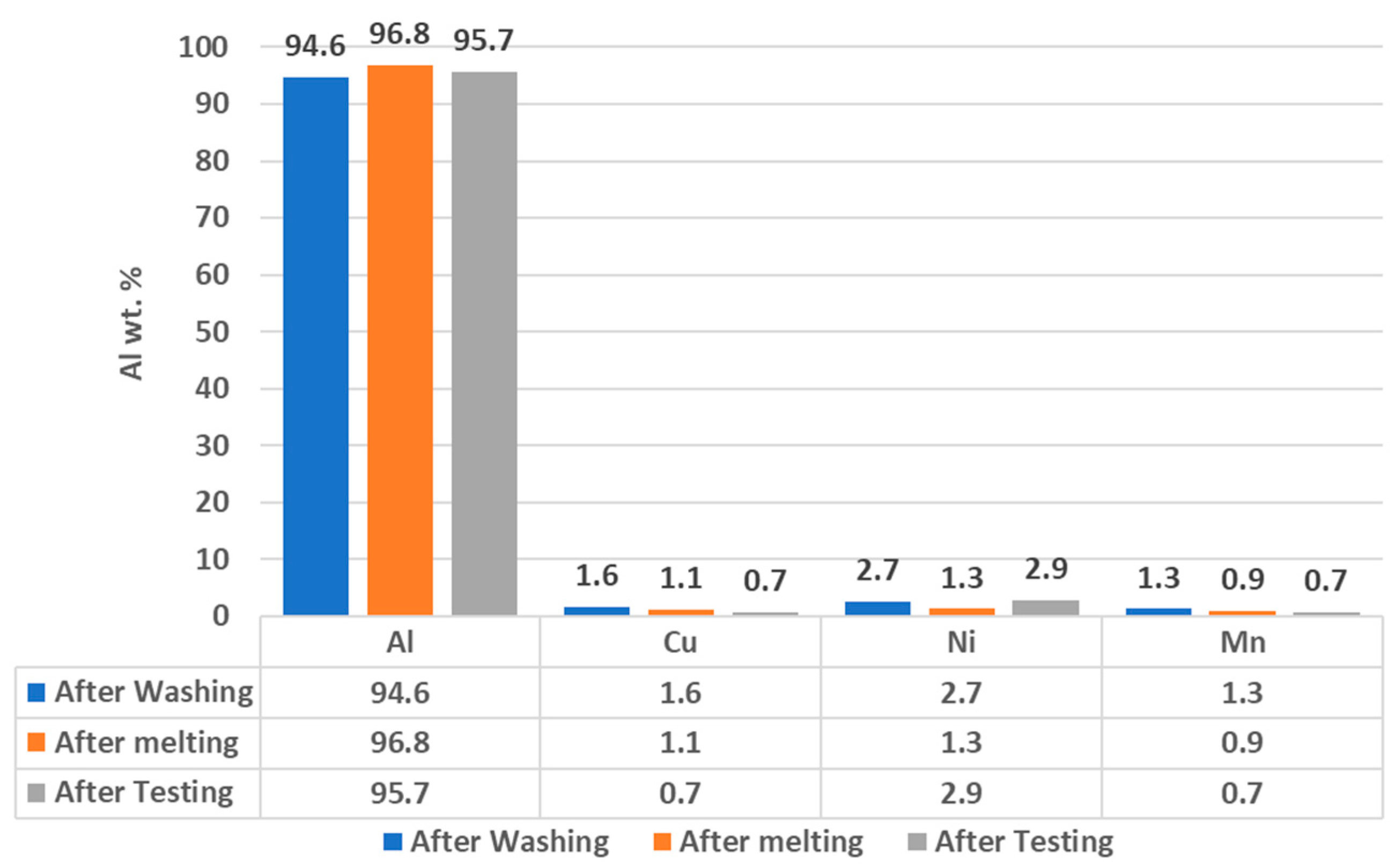
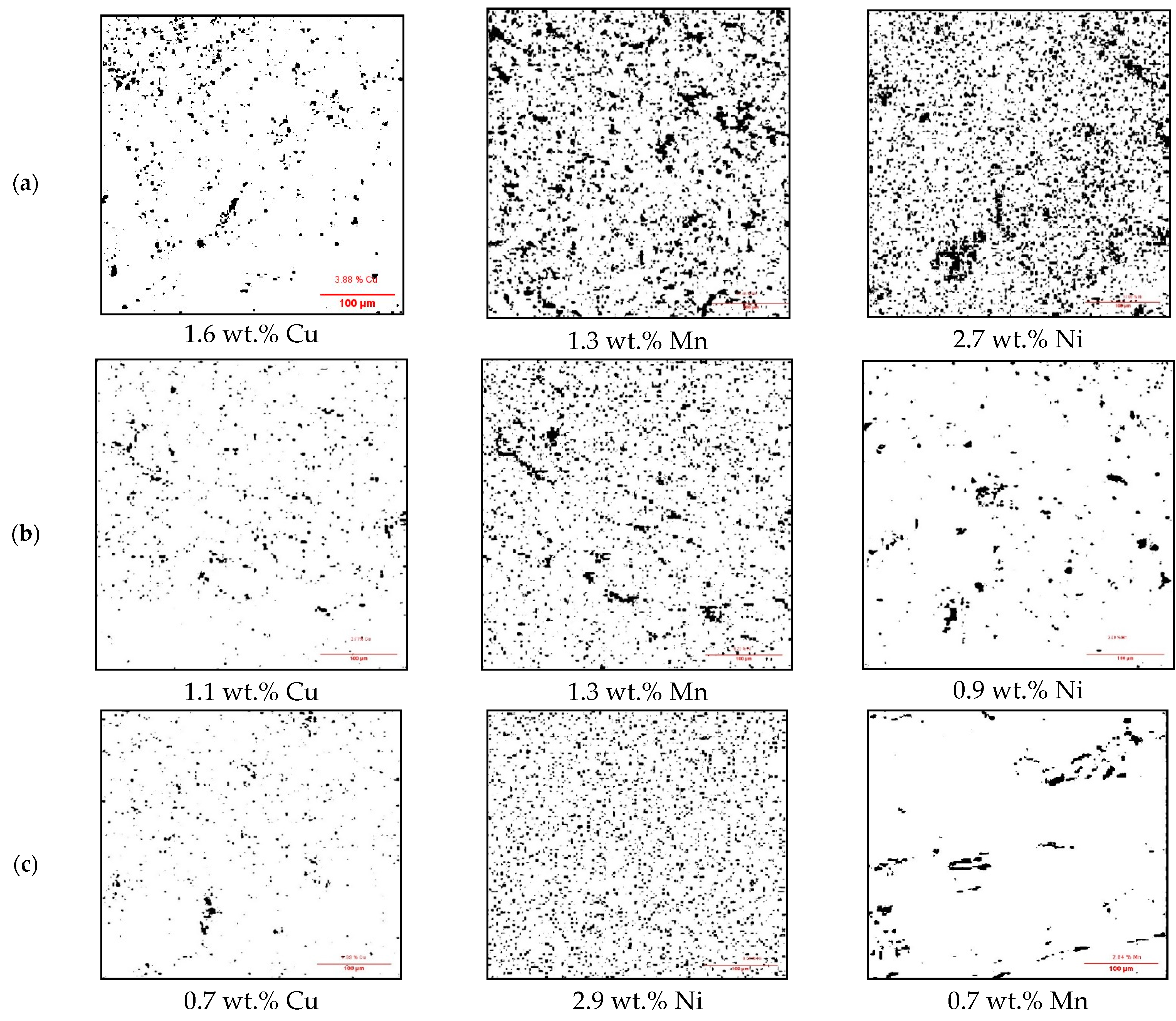
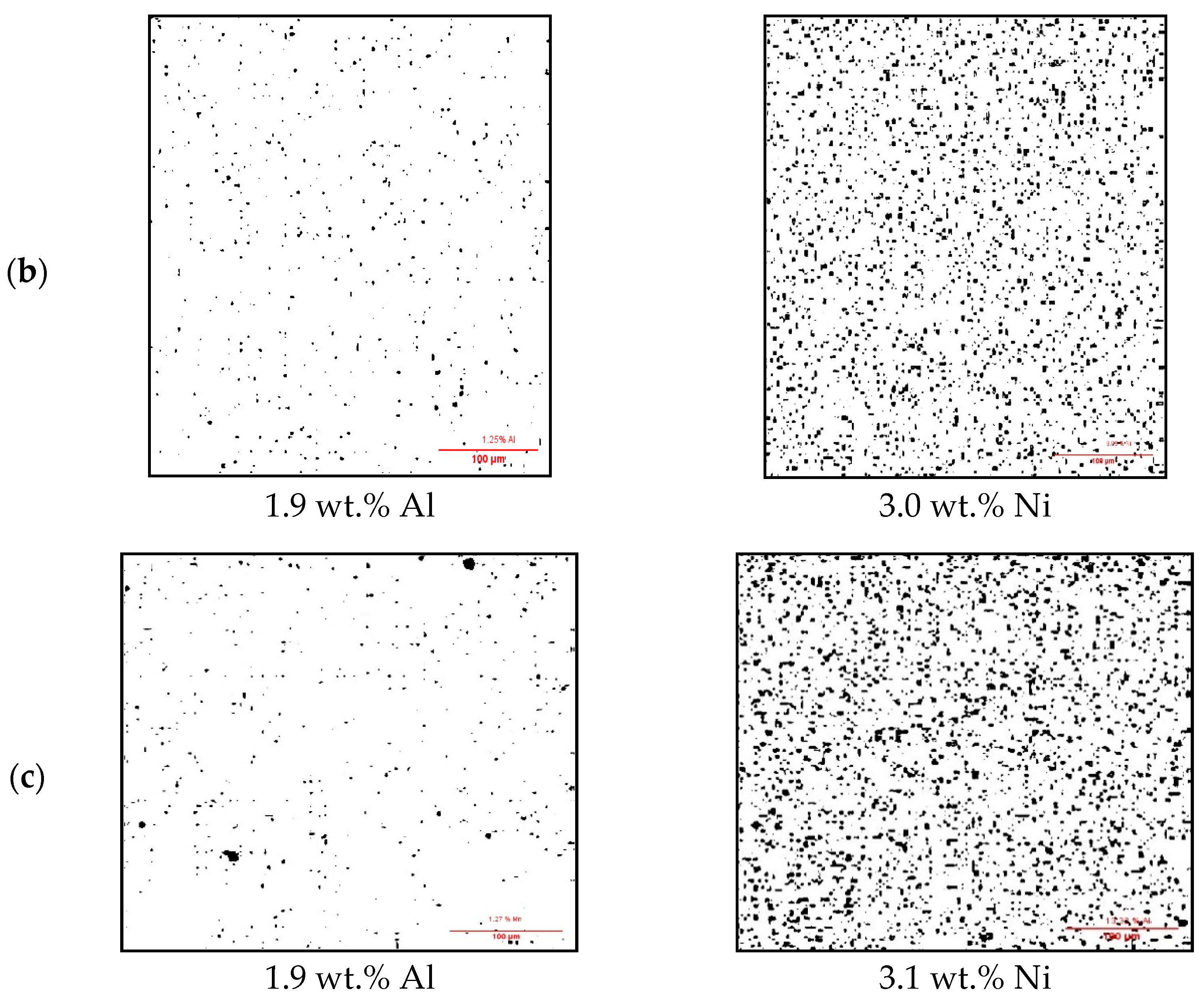
3.1. Surface Impurities of Cleaned CCs by the Ultrasonic Technique
3.2. Surface Impurities of CCs after Melting and Battery Testing
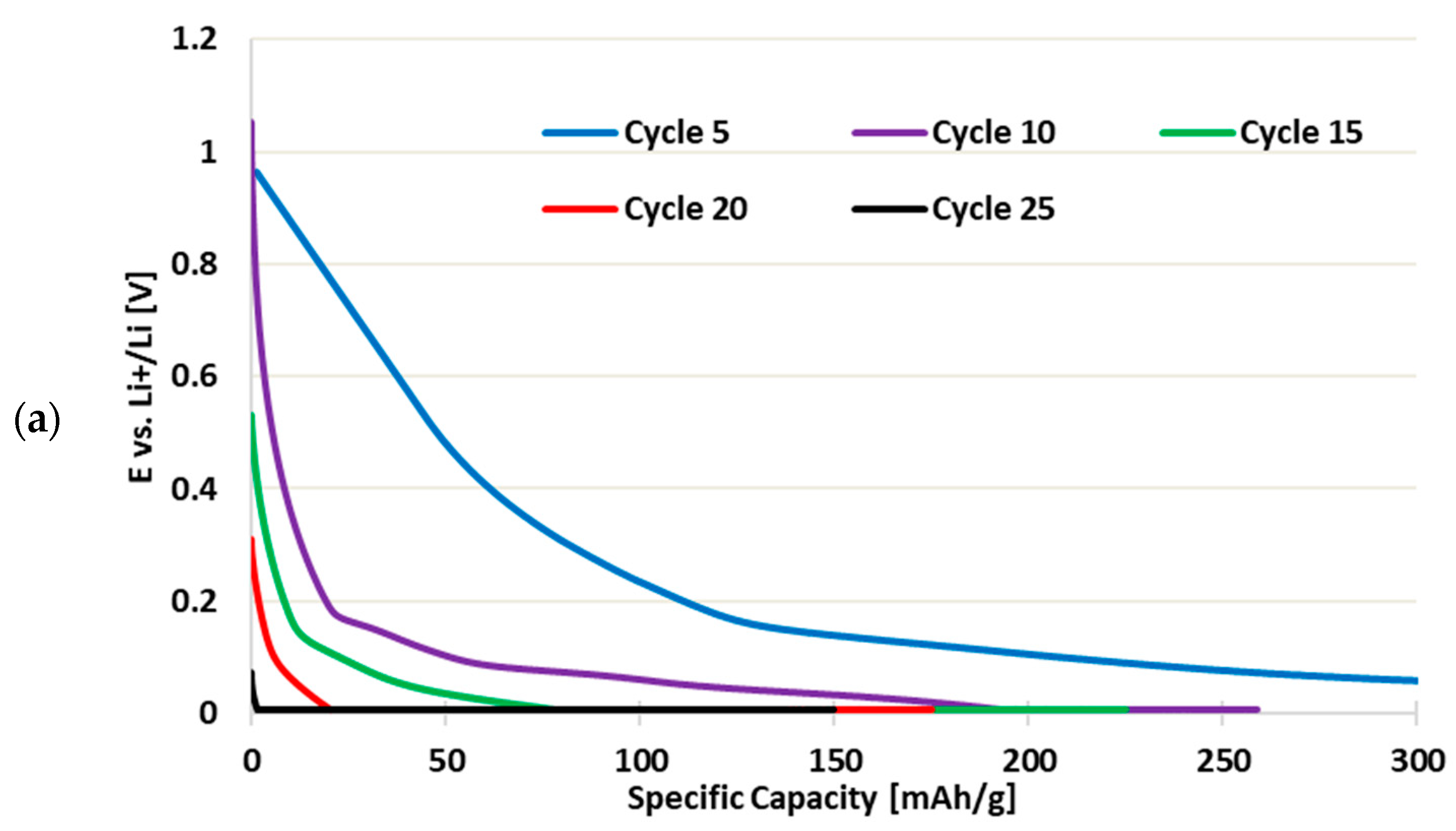
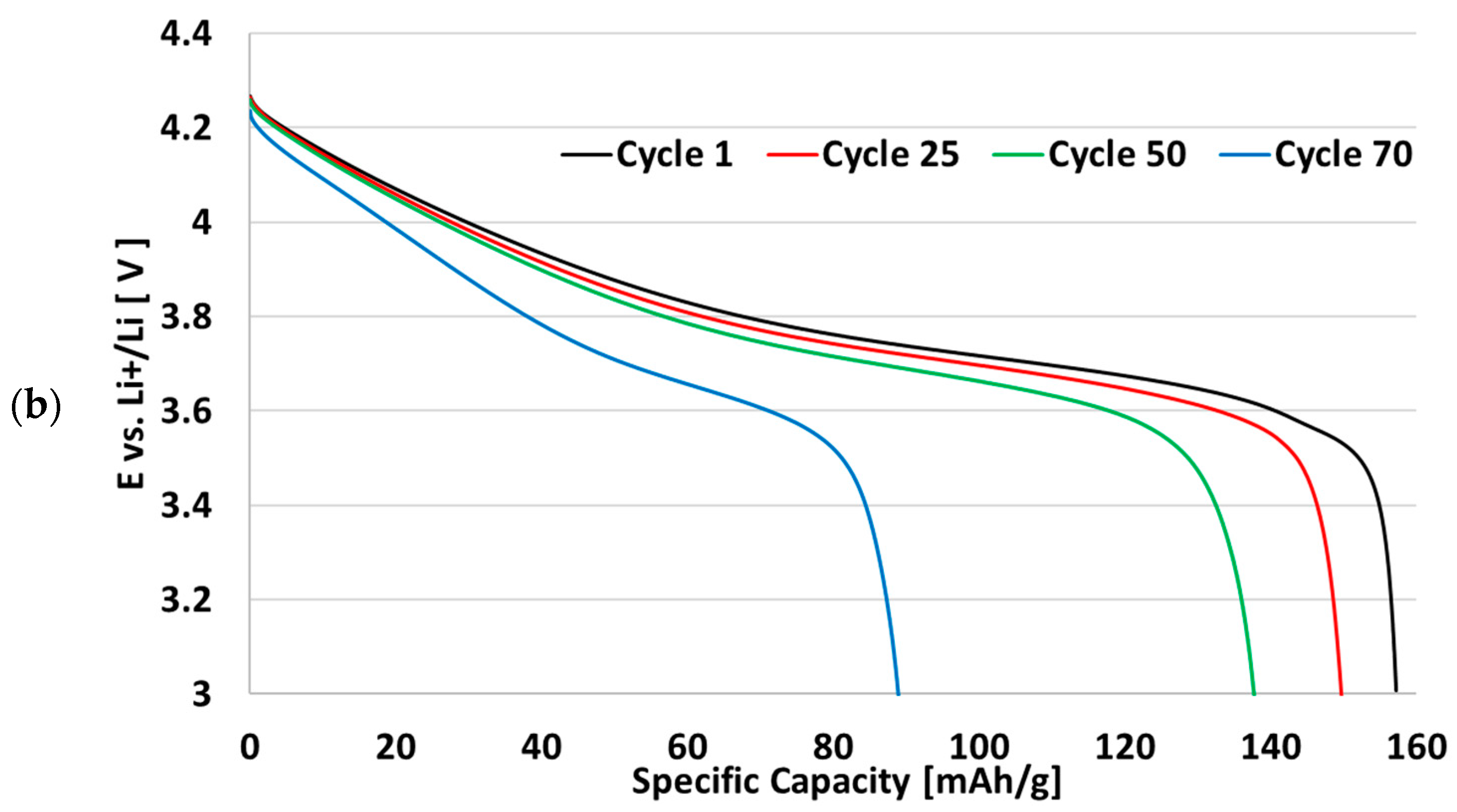
3.3. Cycling Performance of Recycled CCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibra, E.; Connelly, E.; Gorner, M.; Paoli, L. Global EV Outlook 2021. International Energy Agency. Available online: https://iea.blob.core.windows.net/assets/ed5f4484-f556-4110-8c5c-4ede8bcba637/GlobalEVOutlook2021.pdf (accessed on 20 February 2022).
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Kong, L.; Li, C.; Jiang, J.; Pecht, M.G. Li-Ion Battery Fire Hazards and Safety Strategies. Energies 2018, 11, 2191. [Google Scholar] [CrossRef] [Green Version]
- Winslow, K.M.; Laux, T.G.; Townsend, A. Review on the growing concern and potential management strategies of waste lithium-ion batteries Resources. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Chang, T.; You, S.; Yu, B.; Yao, K. A material flow of lithium batteries in Taiwan. J. Hazard. Mater. 2009, 163, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Melchor-Martínez, E.M.; Macias-Garbett, R.; Malacara-Becerra, A.; Iqbal, H.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Environmental impact of emerging contaminants from battery waste: A mini review. Case Stud. Chem. Environ. Eng. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Song, J.; Yan, W.; Cao, H.; Song, Q.; Ding, H.; Lv, Z.; Zhang, Y.; Sun, Z. Material flow analysis on critical raw materials of lithium-ion batteries in China. J. Clean. Prod. 2019, 215, 570–581. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Sommerville, R.; Zhu, P.; Rajaeifar, M.A.; Heidrich, O.; Goodship, V.; Kendrick, E. A qualitative assessment of lithium ion battery recycling processes. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Lander, L.; Cleaver, T.; Rajaeifar, M.A.; Nguyen-Tien, V.; Elliott, R.J.; Heidrich, O.; Kendrick, E.; Edge, J.S.; Offer, G. Financial viability of electric vehicle lithium-ion battery recycling. iScience 2021, 24, 102787. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Huang, Y.; Gu, H.; Deng, C.; Han, X.; Feng, X.; Zheng, Y. Turning waste into wealth: A systematic review on echelon utilization and material recycling of retired lithium-ion batteries. Energy Storage Mater. 2021, 40, 96–123. [Google Scholar] [CrossRef]
- Alhadri, M.; Zakri, W.; Farhad, S. Study on Integration of Retired Lithium-Ion Battery with Photovoltaic for Net-Zero Electricity Residential Homes. J. Sol. Energy Eng. 2022, 145, 21011. [Google Scholar] [CrossRef]
- Alhadri, M.; Zakri, W.; Esmaeeli, R.; Farhad, S. A Study on Degradation of Lithium-Ion Batteries for In Aircraft Applications. In Proceedings of the ASME 2021, International Mechanical Engineering Congress and Exposition (IMECE), Virtual, 1–4 November 2021. [Google Scholar] [CrossRef]
- Ayoola, O.M.; Buldum, A.; Farhad, S.; Ojo, S.A. A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries. Energies 2022, 15, 7288. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Alhadri, M.; Zakri, W.; Aliniagerdroudbari, H.; Esmaeeli, R.; Hashemi, S.R.; Nadkarni, G.; Farhad, S. Design and Comparison of Cooling Plates for a Prismatic Lithium-ion Battery for Electrified Vehicles; SAE International Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Modjtahedi, A.; Amirfazli, A.; Farhad, S. Low catalyst loaded ethanol gas fuel cell sensor. Sens. Actuators B Chem. 2016, 234, 70–79. [Google Scholar] [CrossRef]
- Figgener, J.; Stenzel, P.; Kairies, K.-P.; Linßen, J.; Haberschusz, D.; Wessels, O.; Angenendt, G.; Robinius, M.; Stolten, D.; Sauer, D.U. The development of stationary battery storage systems in Germany—A market review. J. Energy Storage 2020, 29, 101153. [Google Scholar] [CrossRef]
- Fu, R.; Remo, T.W.; Margolis, R.M. 2018 U.S. Utility-Scale Photovoltaics-Plus-Energy Storage System Costs Benchmark; No. NREL/TP-6A20-71714; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2018. [CrossRef]
- Dozein, M.G.; Mancarella, P. Frequency Response Capabilities of Utility-scale Battery Energy Storage Systems, with Application to the August 2018 Separation Event in Australia. In Proceedings of the 2019 9th International Conference on Power and Energy Systems (ICPES), Perth, Australia, 10–12 December 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Gao, Y.; Li, N. State of health estimation of second-life LiFePO4 batteries for energy storage applications. J. Clean. Prod. 2018, 205, 754–762. [Google Scholar] [CrossRef]
- Alkhalidi, A.; Alrousan, T.; Ishbeytah, M.; Abdelkareem, M.A.; Olabi, A. Recommendations for energy storage compartment used in renewable energy project. Int. J. Thermofluids 2022, 15, 100182. [Google Scholar] [CrossRef]
- Bobba, S.; Mathieux, F.; Blengini, G.A. How will second-use of batteries affect stocks and flows in the EU? A model for traction Li-ion batteries. Resour. Conserv. Recycl. 2019, 145, 279–291. [Google Scholar] [CrossRef]
- Abas, A.E.P.; Yong, J.; Mahlia, T.M.I.; Hannan, M.A. Techno-Economic Analysis and Environmental Impact of Electric Vehicle. IEEE Access 2019, 7, 98565–98578. [Google Scholar] [CrossRef]
- Shafique, M.; Luo, X. Environmental life cycle assessment of battery electric vehicles from the current and future energy mix perspective. J. Environ. Manag. 2022, 303, 114050. [Google Scholar] [CrossRef] [PubMed]
- Franzò, S.; Nasca, A. The environmental impact of electric vehicles: A novel life cycle-based evaluation framework and its applications to multi-country scenarios. J. Clean. Prod. 2021, 315, 128005. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total. Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, Y.; Shen, X.; Zhai, Y.; Ji, C.; Ma, X.; Hong, J. Cradle-to-gate life cycle assessment of cobalt sulfate production derived from a nickel–copper–cobalt mine in China. Int. J. Life Cycle Assess. 2021, 26, 1198–1210. [Google Scholar] [CrossRef]
- Choi, H.; Shin, J.; Woo, J. Effect of electricity generation mix on battery electric vehicle adoption and its environmental impact. Energy Policy 2018, 121, 13–24. [Google Scholar] [CrossRef]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Raugei, M.; Steubing, B.; Hartwell, A.; Anderson, P.A.; Heidrich, O. Life cycle assessment of lithium-ion battery recycling using pyrometallurgical technologies. J. Ind. Ecol. 2021, 25, 1560–1571. [Google Scholar] [CrossRef]
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J. Ind. Ecol. 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Kim, S.W.; Cho, K.Y. Current Collectors for Flexible Lithium Ion Batteries: A Review of Materials. J. Electrochem. Sci. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Pathak, R.; Chen, K.; Wu, F.; Mane, A.U.; Bugga, R.V.; Elam, J.W.; Qiao, Q.; Zhou, Y. Advanced strategies for the development of porous carbon as a Li host/current collector for lithium metal batteries. Energy Storage Mater. 2021, 41, 448–465. [Google Scholar] [CrossRef]
- Noelle, D.J.; Wang, M.; Qiao, Y. Improved safety and mechanical characterizations of thick lithium-ion battery electrodes structured with porous metal current collectors. J. Power Sources 2018, 399, 125–132. [Google Scholar] [CrossRef]
- Myung, S.-T.; Hitoshi, Y.; Sun, Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Performance of Cathodes Fabricated from Mixture of Active Materials Obtained from Recycled Lithium-Ion Batteries. Energies 2022, 15, 410. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Heavy liquids for rapid separation of cathode and anode active materials from recycled lithium-ion batteries. Resour. Conserv. Recycl. 2021, 174, 105749. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Separating battery nano/microelectrode active materials with the physical method. In Nanotechnology for Battery Recycling, Remanufacturing, and Reusing; Farhad, S., Gupta, R.K., Yasin, G., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 13; pp. 263–286. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Effects of imperfect separation of cathode active materials in recycling facilities on the performance of remanufactured lithium-ion batteries. In Nanotechnology for Battery Recycling, Remanufacturing, and Reusing; Farhad, S., Gupta, R.K., Yasin, G., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 21; pp. 445–453. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Farhad, S. Regeneration of Cathode Mixture Active Materials Obtained from Recycled Lithium Ion Batteries; SAE International Technical Paper; SAE International: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Esmaeeli, R.; Aliniagerdroudbari, H.; Alhadri, M.; Hashemi, S.R.; Zarrin, H.; Farhad, S. Recycling Lithium-Ion Battery: Mechanical Separation of Mixed Cathode Active Materials. In Proceedings of the ASME 2019, International Mechanical Engineering Congress and Exposition (IMECE), Salt Lake City, UT, USA, 11–14 November 2019. [Google Scholar] [CrossRef]
- Farhad, S.; Gupta, R.K.; Yasin, G.; Nguyen, T.A. Nanotechnology for Battery Recycling, Remanufacturing, and Reusing; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-032391134. [Google Scholar]
- Kay, I.; Farhad, S.; Mahajan, A.; Esmaeeli, R.; Hashemi, S.R. Robotic Disassembly of Electric Vehicles’ Battery Modules for Recycling. Energies 2022, 15, 4856. [Google Scholar] [CrossRef]
- Kay, I.; Esmaeeli, R.; Hashemi, S.R.; Mahajan, A.; Farhad, S. Recycling Li-Ion Batteries: Robotic Disassembly of Electric Vehicle Battery Systems. In Proceedings of the ASME 2019, International Mechanical Engineering Congress and Exposition (IMECE), Salt Lake City, UT, USA, 11–14 November 2019. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatibi, H.; Hassan, E.; Frisone, D.; Amiriyan, M.; Farahati, R.; Farhad, S. Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries. Energies 2022, 15, 9069. https://doi.org/10.3390/en15239069
Khatibi H, Hassan E, Frisone D, Amiriyan M, Farahati R, Farhad S. Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries. Energies. 2022; 15(23):9069. https://doi.org/10.3390/en15239069
Chicago/Turabian StyleKhatibi, Hamid, Eman Hassan, Dominic Frisone, Mahdi Amiriyan, Rashid Farahati, and Siamak Farhad. 2022. "Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries" Energies 15, no. 23: 9069. https://doi.org/10.3390/en15239069




