Review on the Experimental Characterization of Fracture in Active Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Mechanics in LIBs and Factors Affecting Crack Propagation
3. Experimental Characterization of Fracture Mechanics in the Electrodes Active Materials
3.1. Experimental Techniques
- Scanning electron microscopy (SEM) is the most common technique used to observe the electrode microstructure, e.g., particle morphology and fractures, with a precision up to 10 nm. An electron source is collimated into a beam focused on the sample through a set of lenses. The electrons interact with the matter of the sample and some of them are emitted back. The signal from these electrons is used to create the image [26,27].
- Energy-dispersive X-ray spectroscopy (EDX) has the same working principle as SEM, but it is used to identify the type of elements present in the sample by evaluating the energy of the X-rays emitted by the collision of the electrons beam with the sample [28,29]. The materials present in the samples are identified according to the corresponding peaks in the EDX spectrum. EDX is used to detect the variation in material composition during battery aging, and to identify the solid electrolyte interface (SEI) and the products of other undesired reactions.
- Transmission electron microscopy (TEM) is a technique in which a high energy beam of electrons is transmitted through the sample to create a high resolution image and to perform chemical analysis. The sample has to be very thin (less than 150 nm) in order to have a sufficient amount of transmitted electrons. After the interaction with the sample, the following two types of electrons exist: unscattered (no interaction with the sample) and scattered (they change their trajectory due to the interaction with the sample). In the imaging mode, the objective aperture is inserted in the back focal plane of the objective lens. Then, the signal is magnified and projected onto a phosphor screen or charge-coupled camera to obtain the image. Dark areas of the image correspond to low electron transmission through the sample, while bright areas corresponds to high electron transmission. In the diffraction mode, the electrons from the specimen pass through the electromagnetic objective lens, which focuses all the electrons scattered with the same direction into a single point in the image plane. This allows reconstructing the atomic structure (amorphous, polycrystalline or crystalline) of the material [30,31]. TEM is particularly helpful to study two-phase materials, anomalies in the crystallographic structures, as well as side reactions occurring at the electrode/electrolyte interface.
- In-situ TEM and SEM are innovative instruments that can perform a TEM and SEM analysis while the sample is subjected to an external excitation [32,33]. In the LIBs field, electrodes are observed while electrochemically cycled, enabling the characterization of the crystalline structure, the morphology changes of the active material and the crack evolution in real-time.
- Focused Ion Beam (FIB) is generally used for sample preparation rather than imaging. An ion source (generally gallium ions) is used for cutting the sample with high precision, due to the high energy of the ions. In LIBs research, FIB is used to create cross-sections of active material particles, which can later be observed with SEM, or to prepare lamellas to be analyzed with TEM [34].
- Transmission X-ray microscopy (TXM) is a non-destructively way to provide morphological, chemical and structural information of electrode materials up to 10 nm. X-rays can be scattered, absorbed, and re-emitted based on the interaction with the electrons in the matter. The morphological characteristic of the sample is determined by the intensity, energy, and directions of the transmitted X-rays. Furthermore, the chemical composition of the sample can be identified by operating in the X-ray absorption spectroscopy (XAS) mode, whereby the sample is scanned at different energy levels; when the energy of the emitted X-rays equals the core electron binding energy of the element in the sample, the interaction between X-ray photons and the core electrons is increased, and the element composition is identified. X-rays are characterized by high penetration (up to 10 m) and they are more suitable for inspecting bulk material, compared to SEM and TEM [35].This technique is used to study electrode microstructures, chemical and charge distributions, the mechanisms and dynamics of lithium-ions transportation, and chemo-mechanical degradation [36].
- X-ray diffraction analysis (XRD) is used to determine the structural composition of the material, as well as the change in the crystalline structure. The information on the material structure is extrapolated by detecting the intensities and the scattering angles of the X-rays diffracted by the material [37].
- X-ray photoelectron spectroscopy (XPS) is based on the photoelectronic effect. The sample surface is excited with X-rays causing photoelectrons to be emitted from the surface of the sample. The energy of the emitted photoelectrons is measured by an electron energy analyzer. Then, the elemental composition, chemical state, and electronic state of the elements within the material are determined from the binding energy and the intensity of the photoelectron peak. In contrast to the EDX analysis, which has a typical analysis depth of 1–3 m, XPS has a typical analysis depth of less than 5 nm; thus, it can be used to obtain the compositional analysis of ultra-thin layers and thin sample features [38,39].
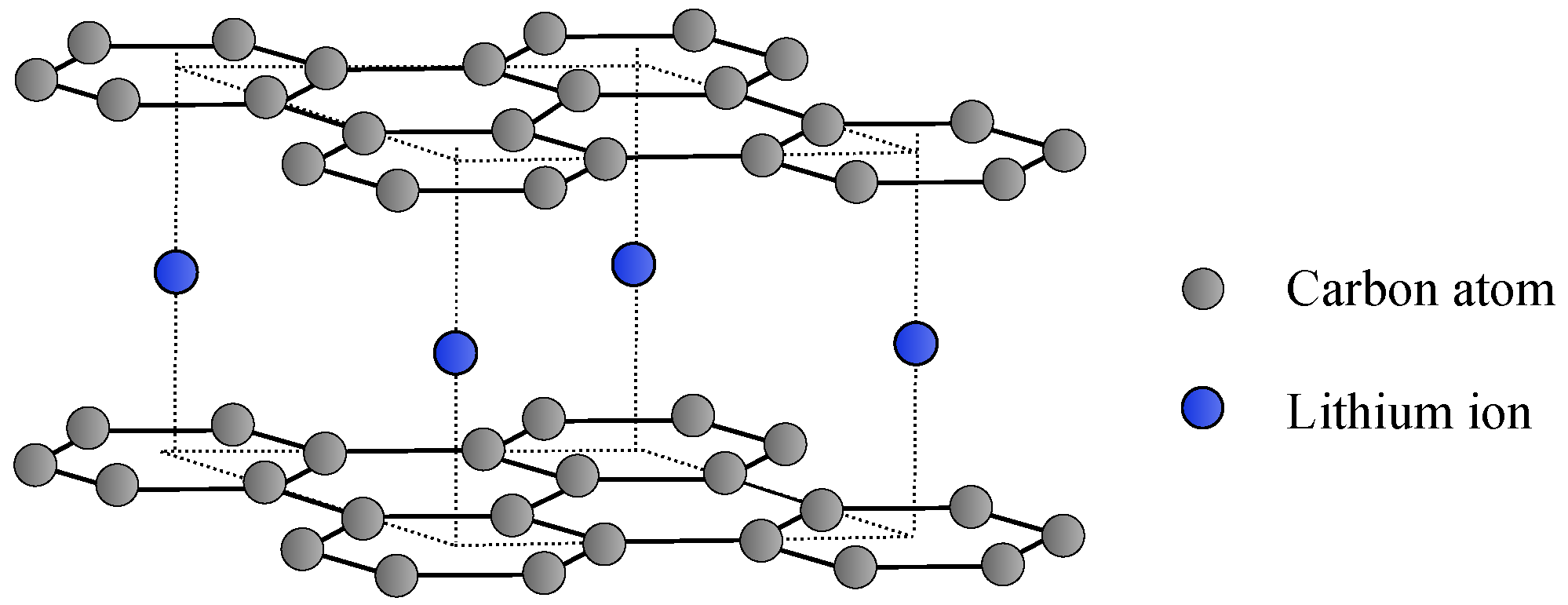
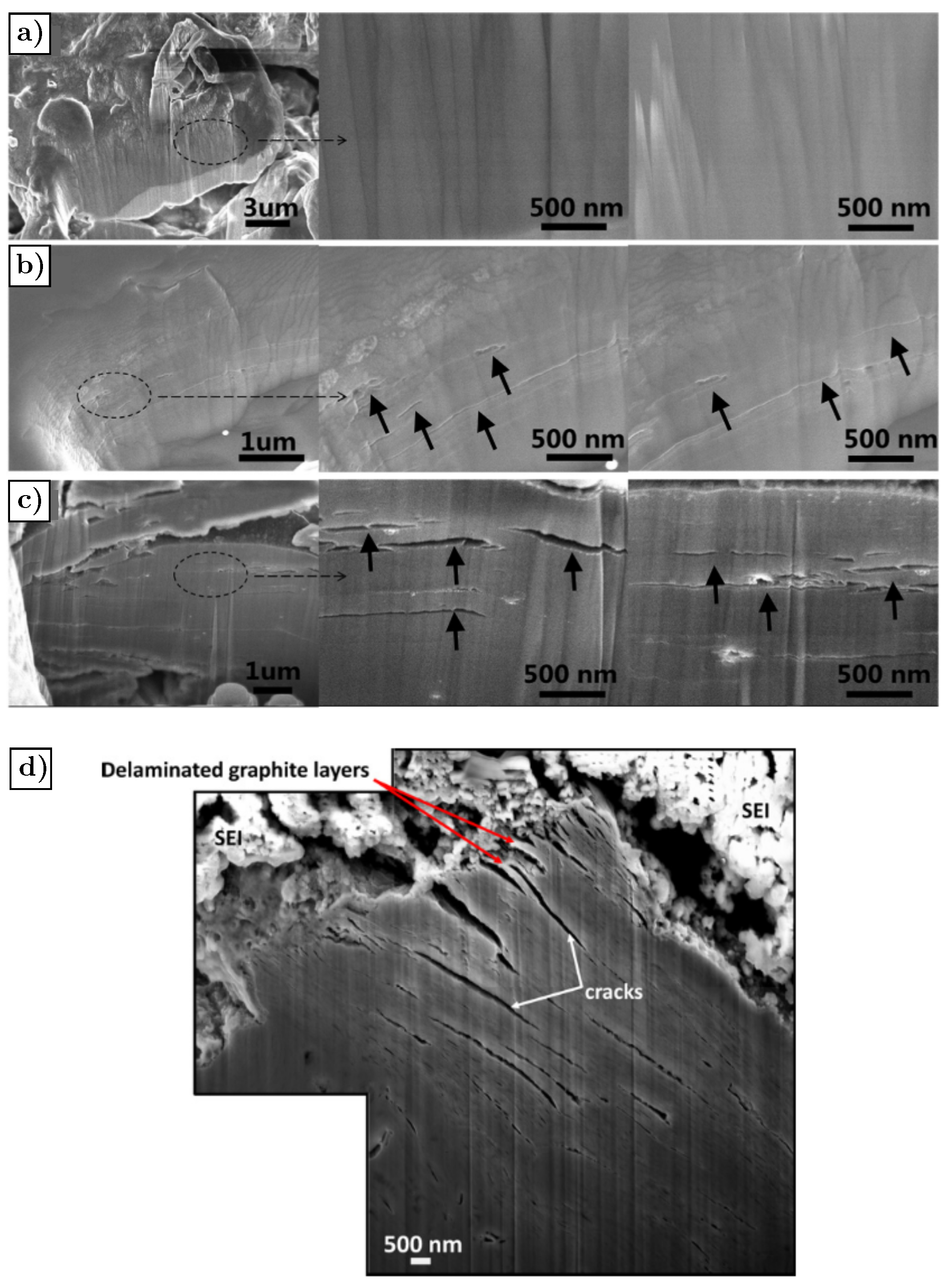
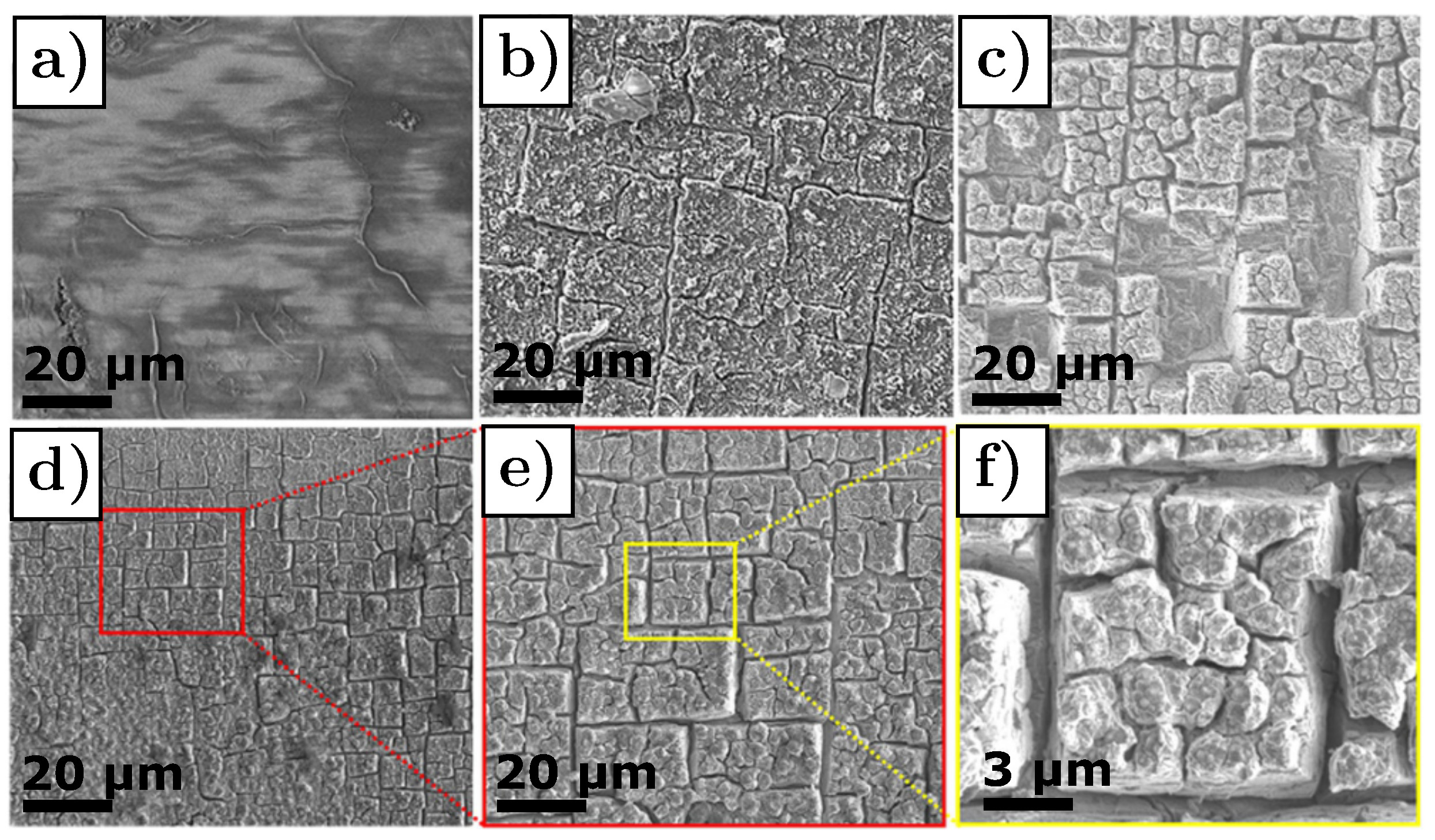
3.2. Graphite—Li
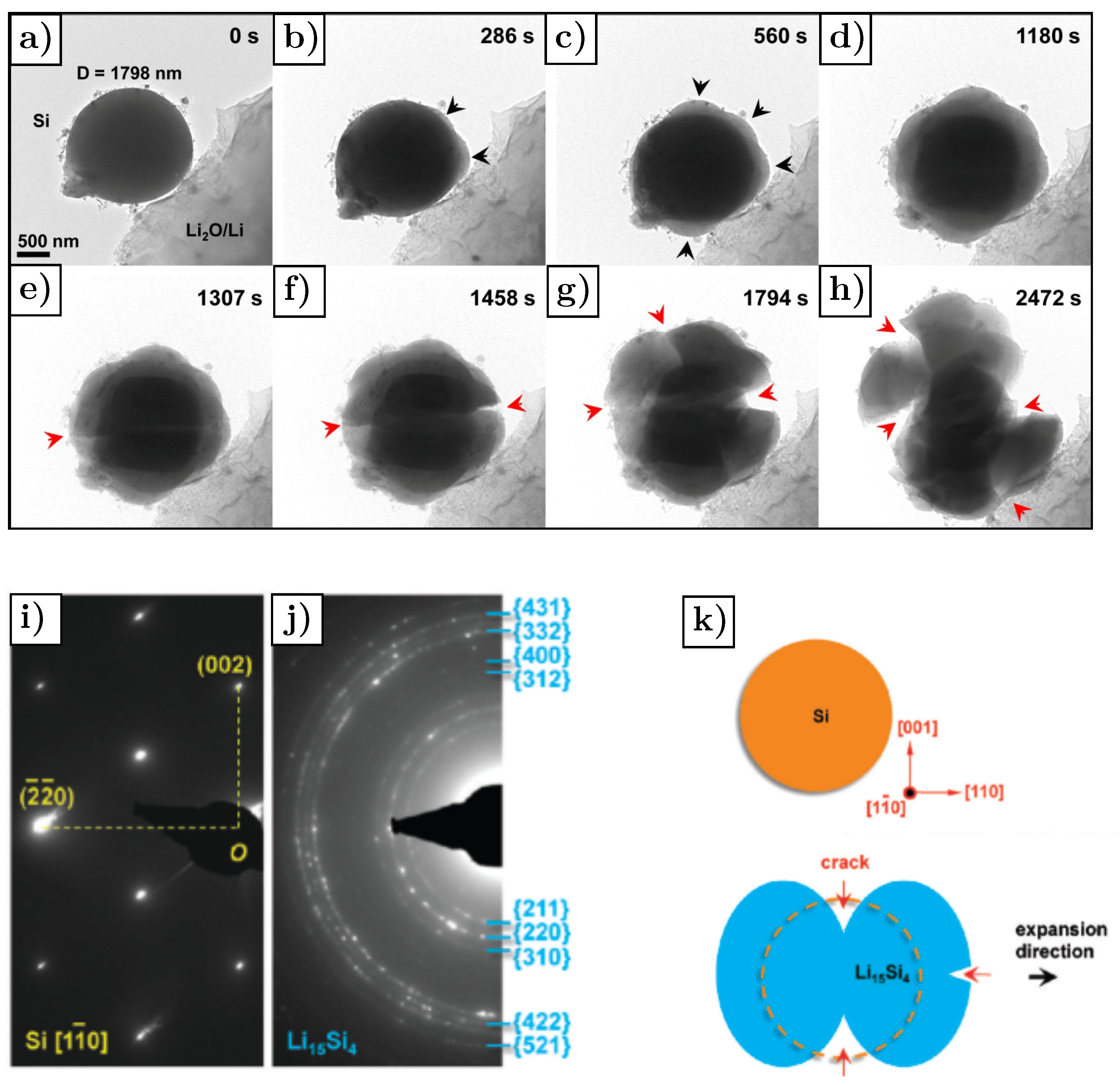
3.3. Silicon
3.4. Silicon Monoxide-SiO
3.5. Lihium Titanium Oxide-LTO
3.6. Lithium Manganese Oxide–
- The acid attack at the particle surface causes the dissolution of the electrode in the electrolyte, consuming the electrode material [85].
- Stress corrosion cracks along the [111] direction are observed in the 4 V plateau [83,86], enhanced by the acid generated by electrolyte decomposition, and the crack density along this direction is observed to increase with cycling both with SEM and TEM [84,86,87,88,89]. Furthermore, the opening of new cracks allows the electrolyte to wet new surfaces, accelerating the dissolution [84].
- The instability of the delithiated spinel structure in organic electrolyte solvent at highly charged states (e.g., oxygen loss).
- Dynamic and non-equilibrium conditions establish during discharge at a high current, making some crystals more lithiated than others, especially close to the separator. This makes those areas fall within the region which are then subjected to Jahn-Teller transformation and to the detrimental effects explained earlier.
3.7. Lithium Iron Phosphate–
3.8. Lithium Nickel Manganese Cobalt Oxide-
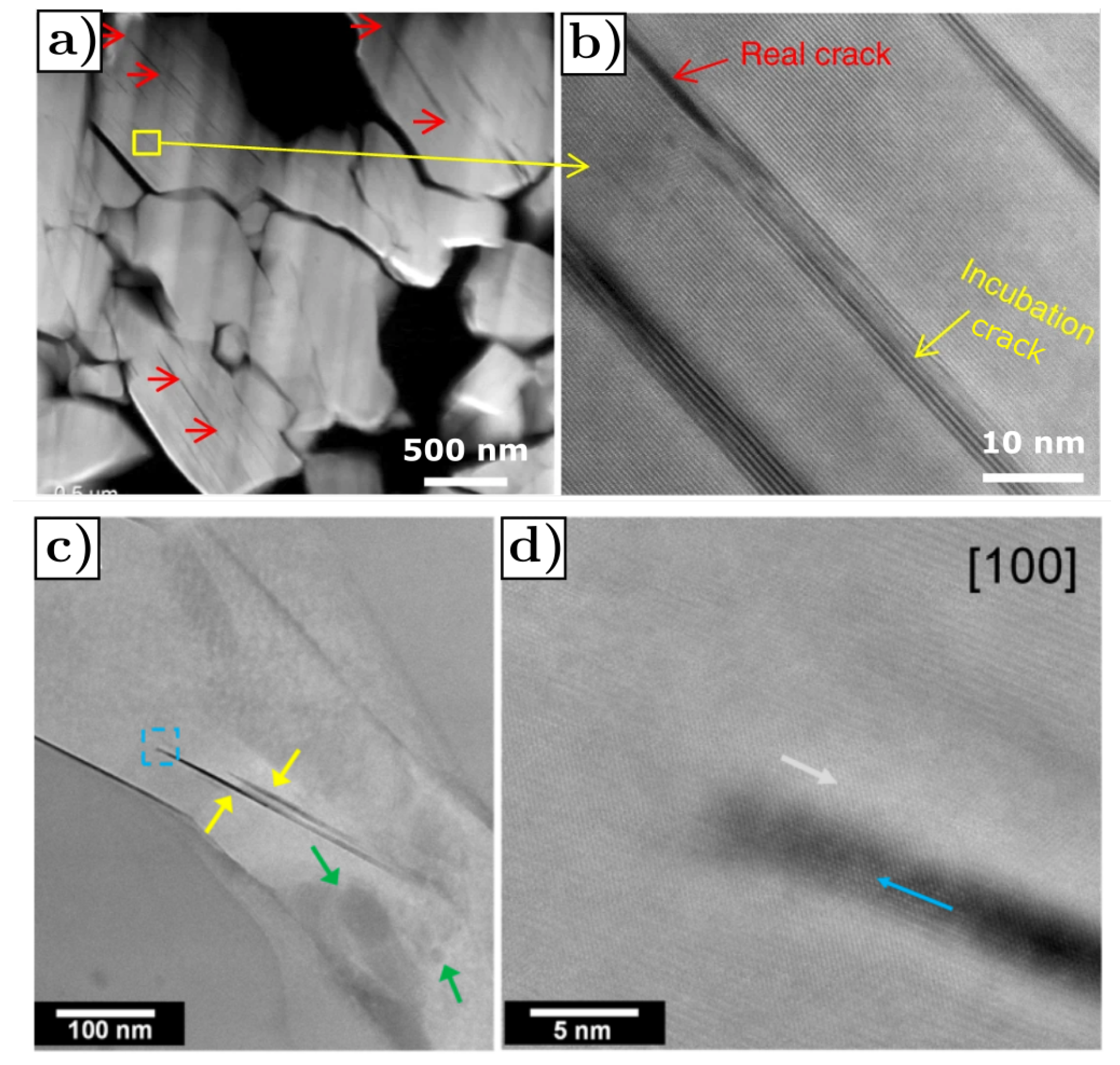
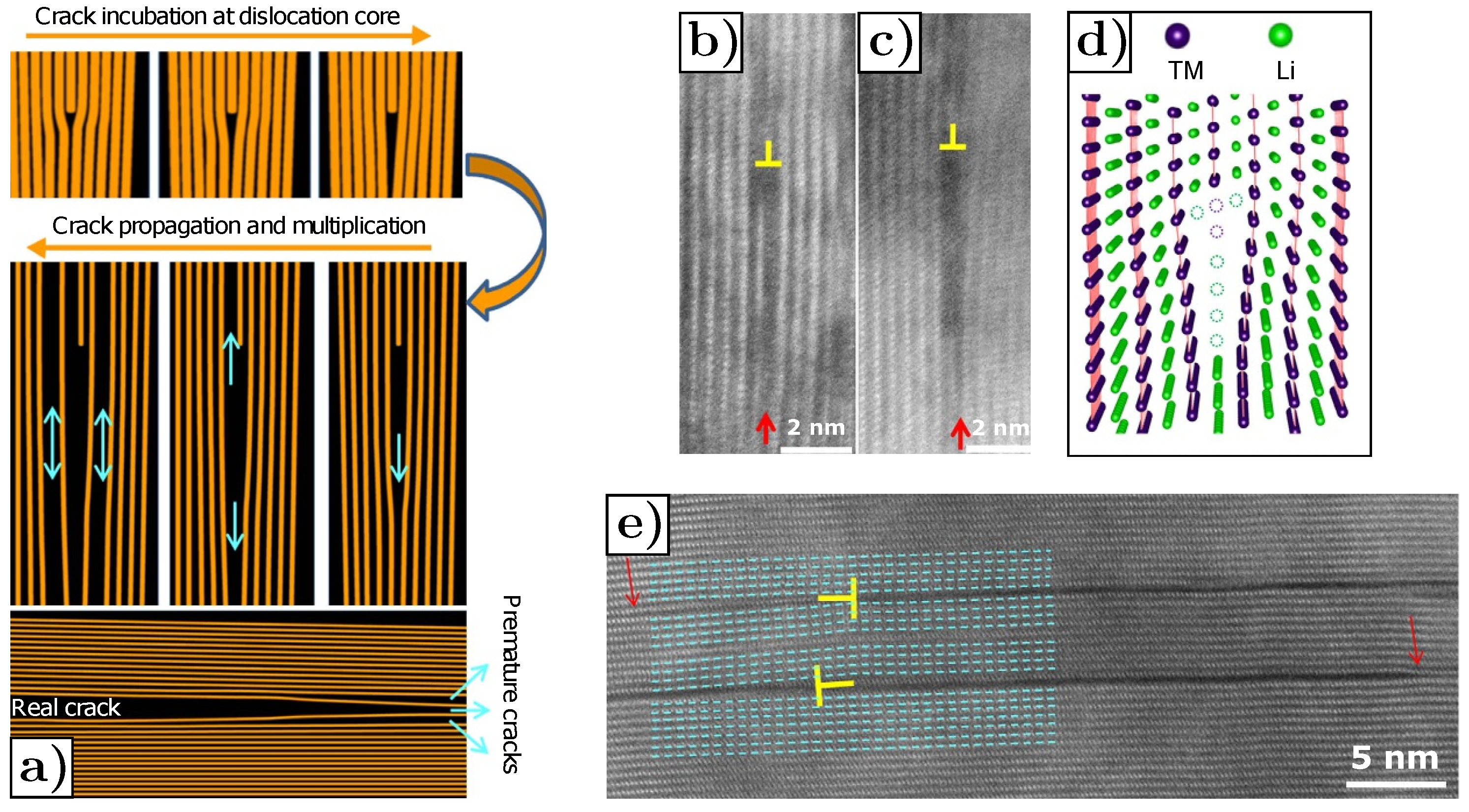
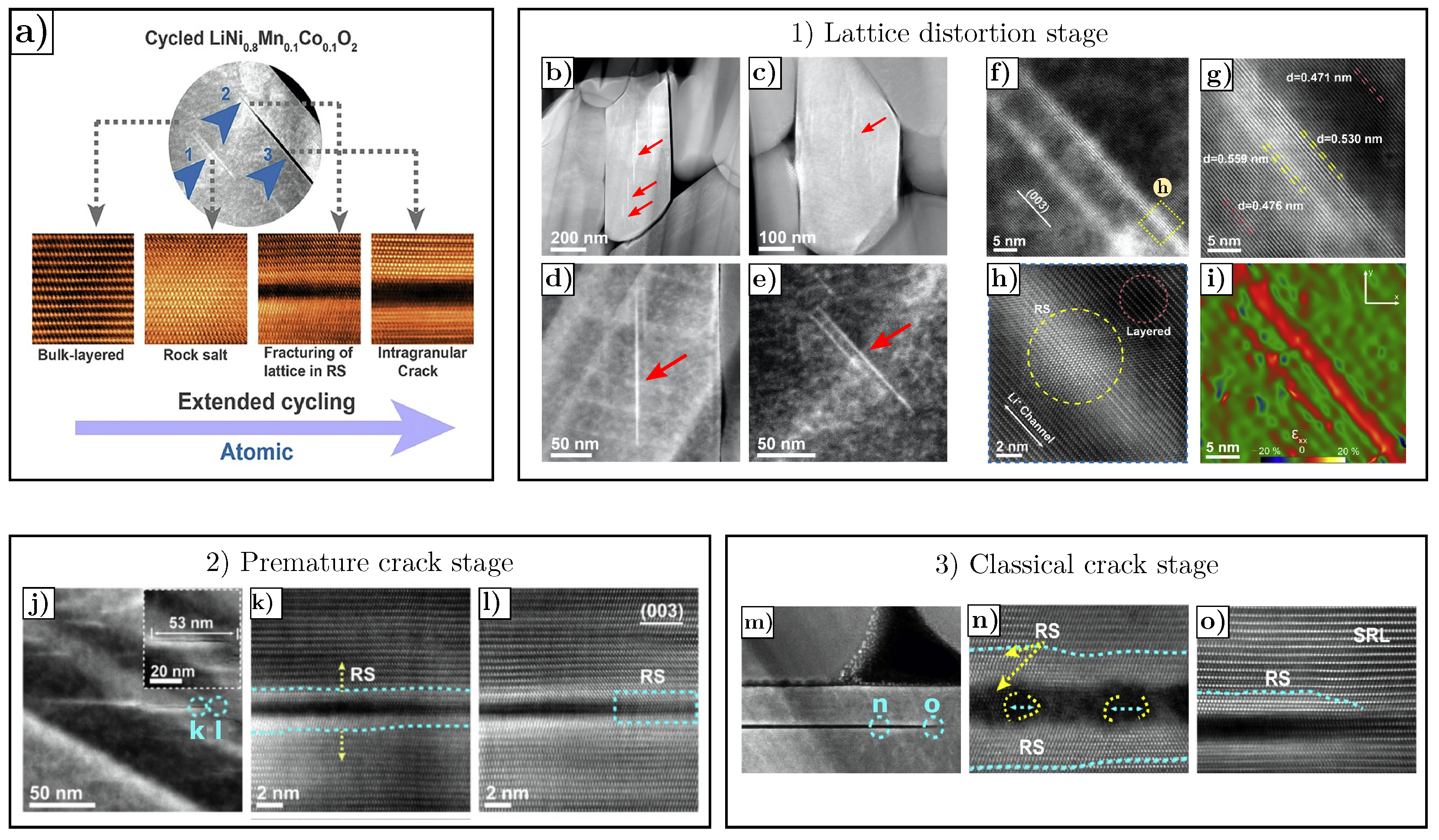
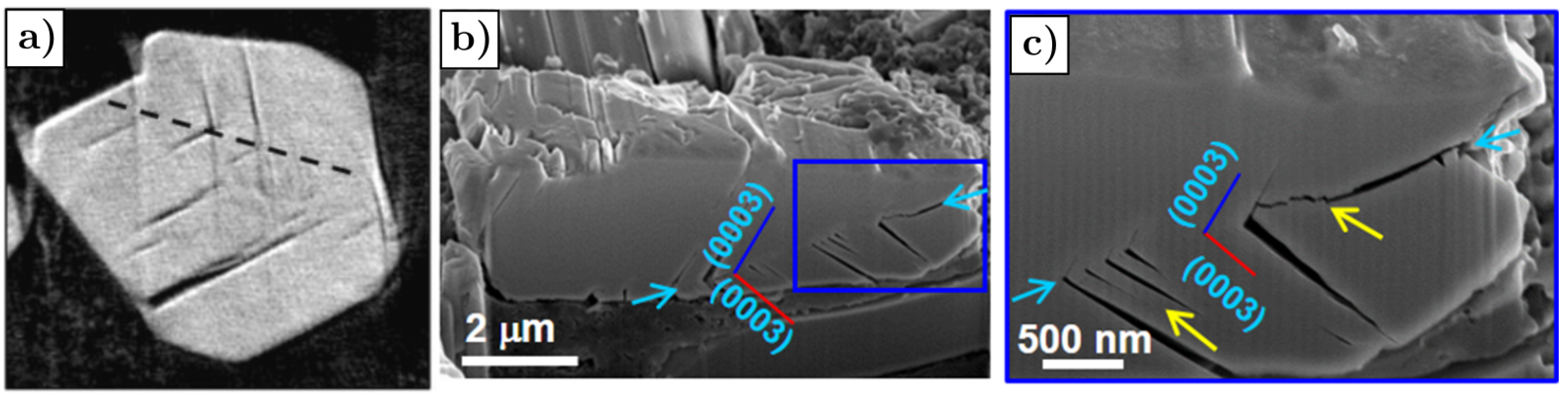
- Cracks are predominantly parallel to (003) planes in the layered structure, as shown in Figure 20a,b, where the red arrows mark the cracks.
- The rock-salt phase appears on the crack surface, as shown in Figure 20c,d where the gray arrows mark the direction parallel to the (003) planes, yellow arrows mark the cracks and blue arrows mark the rock-salt phase at the crack periphery. The presence of the rock-salt phase near the crack is recognizable because it appears brighter compared to the layered NMC structure.
- Cracks start to nucleate at the core region of secondary particles and develop towards outer surfaces upon cycling.
- Cracks propagate along primary particles boundaries.
- Inactive layer forms on the primary particle surface.
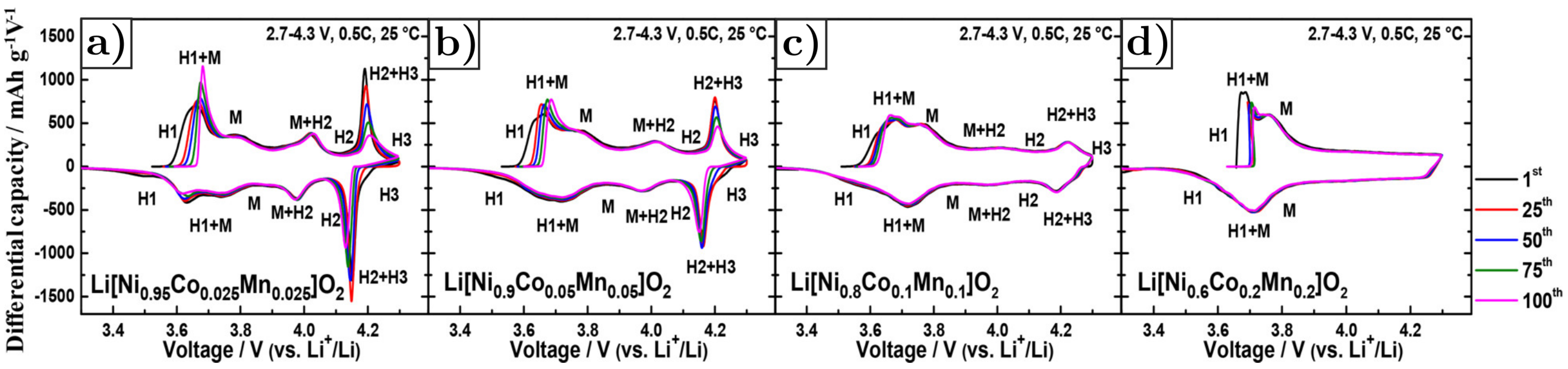
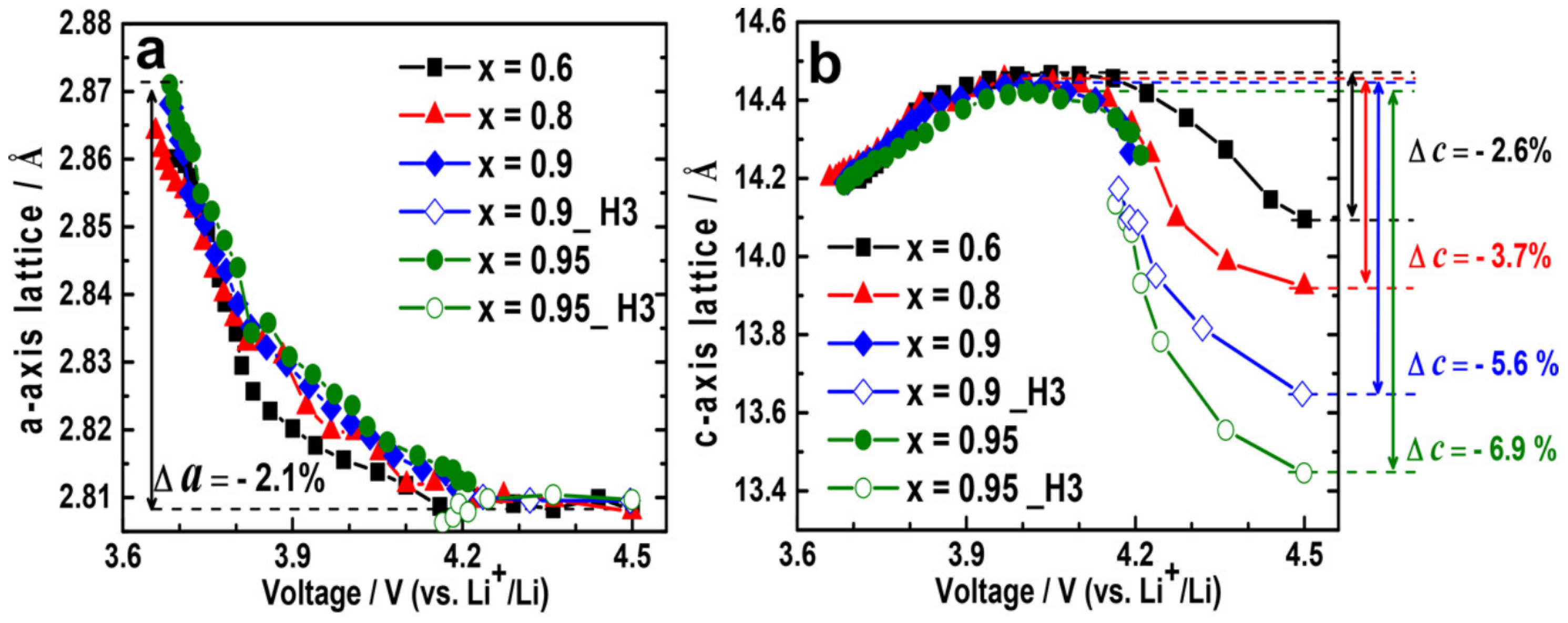
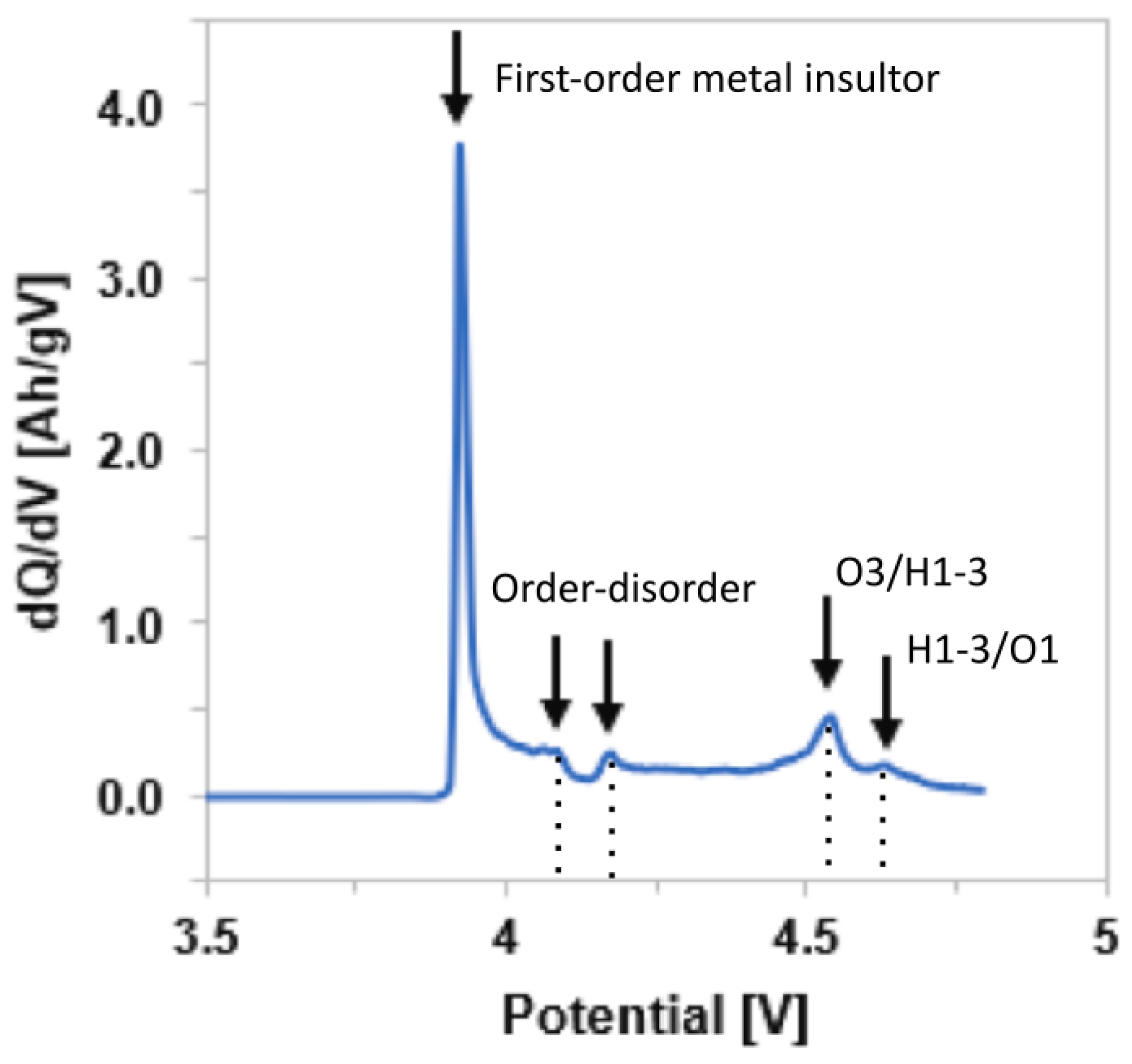
- Phase transitions upon charging and discharging processes. Figure 24a–d shows the differential capacity curves of NMC, as a function of the Ni content and the number of cycles. Considering the charging process, the original layered structure (H1) first turns into the monoclinic phase (M), and then into two other hexagonal phases (H2 and H3) at the end of the charge. The H2–H3 phase transition becomes less pronounced while decreasing the Ni content [106].Phase transitions induce anisotropic lattice parameters changes, as depicted in Figure 25a,b. Indeed, the lattice parameter a keeps the same trend both during charge and discharge, as it decreases during charge and increases during discharge. On the contrary, the variation of the lattice parameter c is significantly different; it first slowly increases during charge and then suddenly decreases due to the H2–H3 phase transition until the end of charging. The process is reversed in discharge, as the c parameter suddenly increases when the transition between H3 and H2 phases occurs, and then decreases until the end of discharge. The large and anisotropic steep change of the lattice parameters caused by the phase transition H2–H3 induces intergranular cracking along grain boundaries. In addition, the higher the nickel content, the greater the c lattice parameter variation, as the H2–H3 phase transition becomes more pronounced. Consequently, larger c-parameter variations cause greater stress, and increase the propagation of intergranular cracks in NMC secondary particles.
- Oxygen release induces phase transformation from the layered structure to the spinel and/or rock-salt structure. The phase transition and the random orientation of primary particles causes anisotropic volume change, thus strain mismatch and the rise of shear stress at the primary particles boundary. The stress and strain fields lead to intergranular cracking nucleation and propagation along the primary particles boundaries, which are the weakest regions within NMC secondary particles [113].
3.9. Lithium Nickel Aluminum Cobalt Oxide-
- Removing primary particles or reducing their size to reduce the strain arising along grain boundaries.
- Selecting the voltage range which minimizes the anisotropic change in lattice parameters.
- Coating secondary particles to mechanically mitigate lattice parameters change.
3.10. Lithium Cobalt Oxide-LCO
3.11. Lithium-Rich Layered Oxides-LLOs
3.12. Concluding Remarks
4. Fracture Influence on Battery Damage
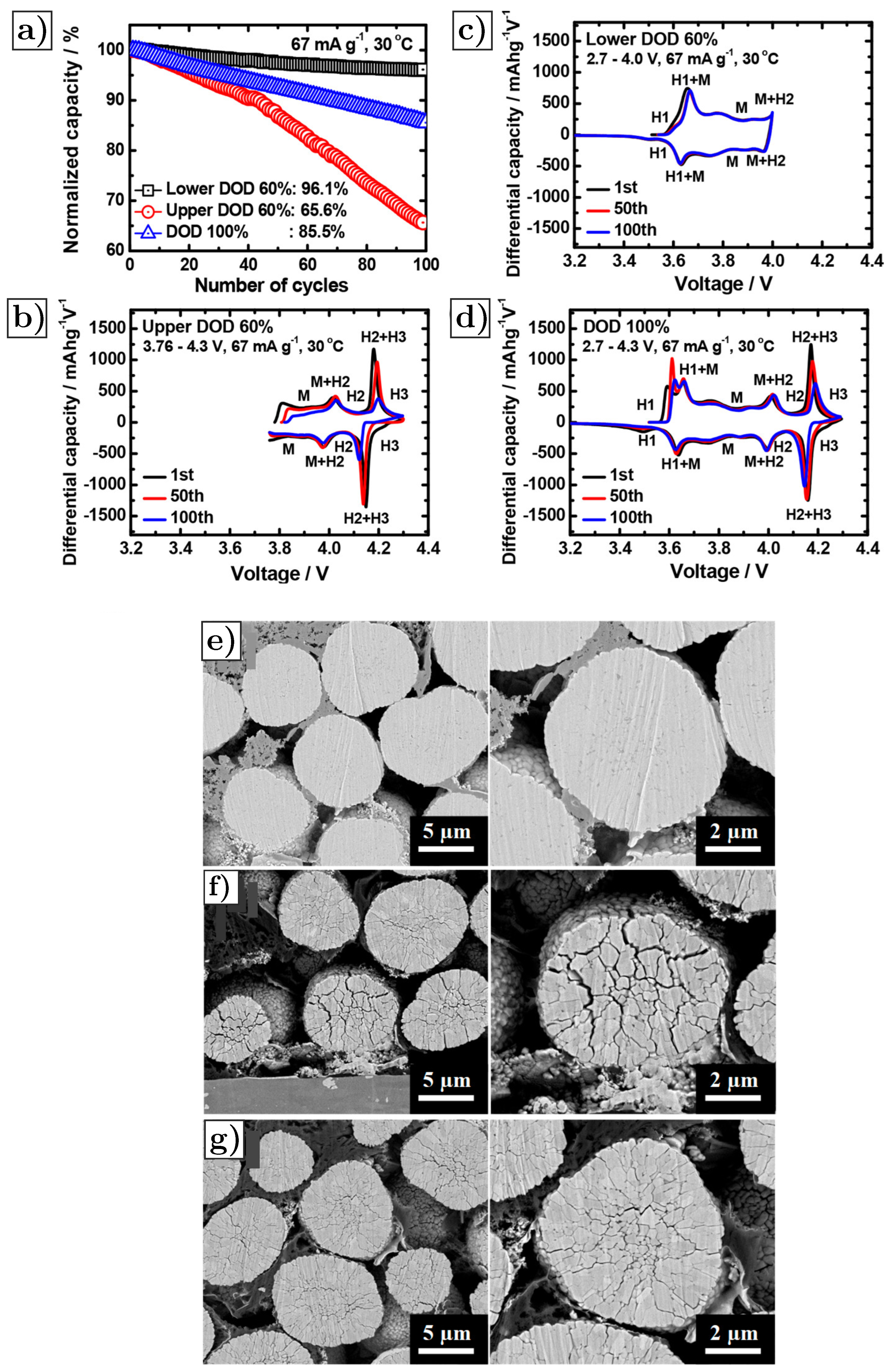
4.1. Crack Growth as a Function of Number of Cycles and Battery Operation
4.2. Real-Time Crack Growth Monitoring
4.3. Correlation between Fracture Mechanics and LIBs Performance Decay
5. Conclusions
- An in-depth description of crack growth as a function of the number of cycles to obtain the empiric coefficients used for crack growth laws, such as Paris’ law.
- Measurements of the toughness of active materials, since this parameter is assumed for most of the common active materials. It is important to point out that although some active materials such as silicon and graphite have been widely studied over the years in the field of classic fracture mechanics, their mechanical properties are strongly influenced by the electrochemical reactions occurring in LIBs; thus, proper experimental measurements are required.
- The relation between performance decay (mainly capacity drop and resistance rise) and the increase in fracture density.
- Quantify how fracture behavior is affected by battery operation with the aim of finding operative strategies to mitigate fractures.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABF | Annular bright-field |
| DIS | Diffused induced stress |
| DOD | Depth of discharge |
| DMC | Dimethyl carbonate |
| EC | Ethylene carbonate |
| EDP | Electron diffraction pattern |
| EDX | Energy-dispersive X-ray spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| ELSA | Element scan analysis |
| FEM | Finite element model |
| FF TMX | Full field transmission X-ray microscope |
| FIB | Focused Ion Beam |
| FMSEM | Field emission scanning electron microscope |
| GPA | Geometric phase analysis |
| HAADF | High-angle annular dark-field |
| HRTEM | High-resolution transmission electron microscope |
| LCO | Lithium cobalt oxide |
| LFP | Lithium iron phosphate |
| LIB | Lithium-ion battery |
| LLO | Li-rich layered oxide |
| LMO | Lithium manganese oxide |
| LTO | Lithium titanate oxide |
| MOS | Multi-beam Optical Sensor |
| NMC | Nickel manganese cobalt oxide |
| NPD | Neutron powder diffraction |
| Pair distribution function | |
| PVDF | Polyvinylidene fluoride |
| SAED | Selected area electron diffraction |
| SEI | Solid electrolyte interface |
| SEM | Scanning electron microscope |
| SOC | State of charge |
| STEM | Scanning transmission electron microscope |
| STMX | Scanning transmission X-ray microscope |
| TEM | Transmission electron microscope |
| TM | Transition metal |
| TXM | Transmission X-rat microscopy |
| XANES | X-ray absorption near edge spectroscope |
| XAS | X-ray absorption spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction analysis |
References
- Duehnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward green battery cells: Perspective on materials and technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 2100771. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Mocera, F.; Somà, A.; Clerici, D. Study of aging mechanisms in lithium-ion batteries for working vehicle applications. In Proceedings of the 2020 Fifteenth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 10–12 September 2020; pp. 1–8. [Google Scholar]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. IScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. A mathematical model of stress generation and fracture in lithium manganese oxide. J. Electrochem. Soc. 2006, 153, A1019. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Somà, A. Analytical solution for coupled diffusion induced stress model for lithium-ion battery. Energies 2020, 13, 1717. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Somà, A. Shape influence of active material micro-structure on diffusion and contact stress in lithium-ion batteries. Energies 2020, 14, 134. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F. Micro-scale modeling of Lithium-ion battery. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1038, 012007. [Google Scholar]
- Li, P.; Zhao, Y.; Shen, Y.; Bo, S.H. Fracture behavior in battery materials. J. Phys. Energy 2020, 2, 022002. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Pistorio, F. Analysis of fracture behaviour in active materials for lithium ion batteries. IOP Conf. Ser.: Mater. Sci. Eng. 2022, 1214, 012018. [Google Scholar]
- Clerici, D.; Mocera, F.; Somà, A. Experimental Characterization of Lithium-Ion Cell Strain Using Laser Sensors. Energies 2021, 14, 6281. [Google Scholar] [CrossRef]
- Clerici, D.; Mocera, F.; Somà, A. Electrochemical–mechanical multi-scale model and validation with thickness change measurements in prismatic lithium-ion batteries. J. Power Sources 2022, 542, 231735. [Google Scholar] [CrossRef]
- Lu, B.; Ning, C.; Shi, D.; Zhao, Y.; Zhang, J. Review on electrode-level fracture in lithium-ion batteries. Chin. Phys. B 2020, 29, 026201. [Google Scholar] [CrossRef]
- Wu, J.; Fenech, M.; Webster, R.F.; Tilley, R.D.; Sharma, N. Electron microscopy and its role in advanced lithium-ion battery research. Sustain. Energy Fuels 2019, 3, 1623–1646. [Google Scholar] [CrossRef]
- Yuan, Y.; Amine, K.; Lu, J.; Shahbazian-Yassar, R. Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 2017, 8, 15806. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sastry, A.M.; Shyy, W. Intercalation-induced stress and heat generation within single lithium-ion battery cathode particles. J. Electrochem. Soc. 2008, 155, A542. [Google Scholar] [CrossRef]
- Jangid, M.K.; Mukhopadhyay, A. Real-time monitoring of stress development during electrochemical cycling of electrode materials for Li-ion batteries: Overview and perspectives. J. Mater. Chem. A 2019, 7, 23679–23726. [Google Scholar] [CrossRef]
- Cheng, X.; Pecht, M. In situ stress measurement techniques on li-ion battery electrodes: A review. Energies 2017, 10, 591. [Google Scholar] [CrossRef]
- Zhu, M.; Park, J.; Sastry, A.M. Fracture analysis of the cathode in Li-ion batteries: A simulation study. J. Electrochem. Soc. 2012, 159, A492. [Google Scholar] [CrossRef]
- Liu, D.; Shadike, Z.; Lin, R.; Qian, K.; Li, H.; Li, K.; Wang, S.; Yu, Q.; Liu, M.; Ganapathy, S.; et al. Review of recent development of in situ/operando characterization techniques for lithium battery research. Adv. Mater. 2019, 31, 1806620. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, N.; Ross, F.M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 2011, 6, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J. Practical Scanning Electron Microscopy: Electron and Ion Microprobe Analysis; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Chu, M.W.; Liou, S.; Chang, C.P.; Choa, F.S.; Chen, C. Emergent chemical mapping at atomic-column resolution by energy-dispersive X-ray spectroscopy in an aberration-corrected electron microscope. Phys. Rev. Lett. 2010, 104, 196101. [Google Scholar] [CrossRef]
- Allen, L.J.; D’Alfonso, A.J.; Freitag, B.; Klenov, D.O. Chemical mapping at atomic resolution using energy-dispersive x-ray spectroscopy. MRS Bull. 2012, 37, 47–52. [Google Scholar] [CrossRef]
- Reimer, L. Transmission Electron Microscopy: Physics of Image Formation and Microanalysis; Springer: Berlin/Heidelberg, Germany, 2013; Volume 36. [Google Scholar]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H., Jr. Conventional transmission electron microscopy. Mol. Biol. Cell 2014, 25, 319–323. [Google Scholar] [CrossRef]
- Podor, R.; Ravaux, J.; Brau, H.P. In situ experiments in the scanning electron microscope chamber. In Scanning Electron Microscopy; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Liu, X.H.; Wang, J.W.; Huang, S.; Fan, F.; Huang, X.; Liu, Y.; Krylyuk, S.; Yoo, J.; Dayeh, S.A.; Davydov, A.V.; et al. In situ atomic-scale imaging of electrochemical lithiation in silicon. Nat. Nanotechnol. 2012, 7, 749–756. [Google Scholar] [CrossRef]
- Bassim, N.; Scott, K.; Giannuzzi, L.A. Recent advances in focused ion beam technology and applications. MRS Bull. 2014, 39, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Guttmann, P.; Bittencourt, C.; Rehbein, S.; Umek, P.; Ke, X.; Van Tendeloo, G.; Ewels, C.P.; Schneider, G. Nanoscale spectroscopy with polarized X-rays by NEXAFS-TXM. Nat. Photonics 2012, 6, 25–29. [Google Scholar] [CrossRef]
- Spence, S.; Lee, W.K.; Lin, F.; Xiao, X. Transmission X-ray Microscopy and Its Applications in Battery Material Research—A Short Review. Nanotechnology 2021, 32, 442003. [Google Scholar] [CrossRef]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-ray photoelectron spectroscopic characterization of chemically modified electrodes used as chemical sensors and biosensors: A review. Materials 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]
- Bondarchuk, O.; LaGrow, A.P.; Kvasha, A.; Thieu, T.; Ayerbe, E.; Urdampilleta, I. On the X-ray photoelectron spectroscopy analysis of LiNixMnyCozO2 material and electrodes. Appl. Surf. Sci. 2021, 535, 147699. [Google Scholar] [CrossRef]
- Lin, N.; Jia, Z.; Wang, Z.; Zhao, H.; Ai, G.; Song, X.; Bai, Y.; Battaglia, V.; Sun, C.; Qiao, J.; et al. Understanding the crack formation of graphite particles in cycled commercial lithium-ion batteries by focused ion beam-scanning electron microscopy. J. Power Sources 2017, 365, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wang, Y.; Xie, Y.; He, L.; Chen, J.; Wu, K.; Xu, R.; Gao, Y. On the stress characteristics of graphite anode in commercial pouch lithium-ion battery. J. Power Sources 2013, 232, 29–33. [Google Scholar] [CrossRef]
- Takahashi, K.; Srinivasan, V. Examination of graphite particle cracking as a failure mode in lithium-ion batteries: A model-experimental study. J. Electrochem. Soc. 2015, 162, A635. [Google Scholar] [CrossRef]
- Harris, S.J.; Deshpande, R.D.; Qi, Y.; Dutta, I.; Cheng, Y.T. Mesopores inside electrode particles can change the Li-ion transport mechanism and diffusion-induced stress. J. Mater. Res. 2010, 25, 1433–1440. [Google Scholar] [CrossRef] [Green Version]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Riahi, A.R.; Alpas, A.T. A transmission electron microscopy study of crack formation and propagation in electrochemically cycled graphite electrode in lithium-ion cells. J. Power Sources 2011, 196, 8719–8727. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, F.; Liu, C.; Tan, J.; Cheng, H.M. New insight into the solid electrolyte interphase with use of a focused ion beam. J. Phys. Chem. B 2005, 109, 22205–22211. [Google Scholar] [CrossRef]
- Sun, G.; Bhattacharya, S.; Alpas, A. Cyclic strain-induced crack growth in graphite during electrochemical testing in propylene carbonate-based Li-ion battery electrolytes. J. Mater. Sci. 2018, 53, 1297–1309. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Chew, H.B.; Hou, B.; Wang, X.; Xia, S. Cracking mechanisms in lithiated silicon thin film electrodes. Int. J. Solids Struct. 2014, 51, 4176–4187. [Google Scholar] [CrossRef] [Green Version]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-dependent fracture of Si nanowire battery anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, H.; Zhong, L.; Huang, S.; Karki, K.; Zhang, L.Q.; Liu, Y.; Kushima, A.; Liang, W.T.; Wang, J.W.; et al. Anisotropic swelling and fracture of silicon nanowires during lithiation. Nano Lett. 2011, 11, 3312–3318. [Google Scholar] [CrossRef]
- Lee, S.W.; McDowell, M.T.; Choi, J.W.; Cui, Y. Anomalous shape changes of silicon nanopillars by electrochemical lithiation. Nano Lett. 2011, 11, 3034–3039. [Google Scholar] [CrossRef]
- Lee, S.W.; McDowell, M.T.; Berla, L.A.; Nix, W.D.; Cui, Y. Fracture of crystalline silicon nanopillars during electrochemical lithium insertion. Proc. Natl. Acad. Sci. USA 2012, 109, 4080–4085. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Song, Z.; Ross, P.N.; Somorjai, G.A.; Ritchie, R.O.; Komvopoulos, K. Failure mechanisms of single-crystal silicon electrodes in lithium-ion batteries. Nat. Commun. 2016, 7, 11886. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, V.A.; Chon, M.J.; Shimshak, M.; Srinivasan, V.; Guduru, P.R. In situ measurements of stress evolution in silicon thin films during electrochemical lithiation and delithiation. J. Power Sources 2010, 195, 5062–5066. [Google Scholar] [CrossRef]
- Li, J.; Dozier, A.K.; Li, Y.; Yang, F.; Cheng, Y.T. Crack pattern formation in thin film lithium-ion battery electrodes. J. Electrochem. Soc. 2011, 158, A689. [Google Scholar] [CrossRef]
- Liu, X.H.; Huang, J.Y. In situ TEM electrochemistry of anode materials in lithium ion batteries. Energy Environ. Sci. 2011, 4, 3844–3860. [Google Scholar] [CrossRef]
- Pharr, M.; Suo, Z.; Vlassak, J.J. Measurements of the fracture energy of lithiated silicon electrodes of Li-ion batteries. Nano Lett. 2013, 13, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Min, B.I.; Wang, Y.; Hayashida, R.; Tanaka, M.; Watanabe, T. Preparation of carbon-coated silicon nanoparticles with different hydrocarbon gases in induction thermal plasma. J. Phys. Chem. C 2021, 125, 15551–15559. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Wetjen, M.; Solchenbach, S.; Pritzl, D.; Hou, J.; Tileli, V.; Gasteiger, H.A. Morphological changes of silicon nanoparticles and the influence of cutoff potentials in silicon-graphite electrodes. J. Electrochem. Soc. 2018, 165, A1503. [Google Scholar] [CrossRef] [Green Version]
- Casino, S.; Beuse, T.; Küpers, V.; Boerner, M.; Gallasch, T.; Winter, M.; Niehoff, P. Quantification of aging mechanisms of carbon-coated and uncoated silicon thin film anodes in lithium metal and lithium ion cells. J. Energy Storage 2021, 41, 102812. [Google Scholar] [CrossRef]
- Li, W.; Cao, K.; Wang, H.; Liu, J.; Zhou, L.; Yao, H. Carbon coating may expedite the fracture of carbon-coated silicon core–shell nanoparticles during lithiation. Nanoscale 2016, 8, 5254–5259. [Google Scholar] [CrossRef]
- Xia, Y.; Hirai, Y.; Tsuchiya, T. Fracture behavior of single-crystal silicon microstructure coated with stepwise bias-graded aC: H film. Surf. Coatings Technol. 2021, 405, 126559. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Li, X.; Hu, S.; Zhang, X.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; Liu, J.; et al. In situ TEM study of lithiation behavior of silicon nanoparticles attached to and embedded in a carbon matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Cao, K.; Abouali, S.; Garakani, M.A.; Huang, J.; Huang, J.Q.; Heidari, E.K.; Wang, H.; Kim, J.K. Study of lithiation mechanisms of high performance carbon-coated Si anodes by in-situ microscopy. Energy Storage Mater. 2016, 3, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Sandu, G.; Brassart, L.; Gohy, J.F.; Pardoen, T.; Melinte, S.; Vlad, A. Surface coating mediated swelling and fracture of silicon nanowires during lithiation. ACS Nano 2014, 8, 9427–9436. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, H.; Yan, P.; Travis, J.J.; Lee, Y.; Liu, N.; Molina Piper, D.; Lee, S.H.; Zhao, P.; George, S.M.; et al. Surface-coating regulated lithiation kinetics and degradation in silicon nanowires for lithium ion battery. ACS Nano 2015, 9, 5559–5566. [Google Scholar] [CrossRef] [PubMed]
- Michan, A.L.; Divitini, G.; Pell, A.J.; Leskes, M.; Ducati, C.; Grey, C.P. Solid electrolyte interphase growth and capacity loss in silicon electrodes. J. Am. Chem. Soc. 2016, 138, 7918–7931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.; Lee, M.J.; Oh, S.M.; Kim, J.J. Fading mechanisms of carbon-coated and disproportionated Si/SiOx negative electrode (Si/SiOx/C) in Li-ion secondary batteries: Dynamics and component analysis by TEM. Electrochim. Acta 2012, 85, 369–376. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, X.; Liu, Y.; Zhu, L. Crack-free silicon monoxide as anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 57141–57145. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, B.; Lu, H.; Chu, G.; Liu, J.; Guo, Y.G.; Yu, X.; Luo, F.; Ren, Y.; et al. Size effect on the growth and pulverization behavior of Si nanodomains in SiO anode. Nano Energy 2020, 78, 105101. [Google Scholar] [CrossRef]
- Hovington, P.; Dontigny, M.; Guerfi, A.; Trottier, J.; Lagacé, M.; Mauger, A.; Julien, C.; Zaghib, K. In situ Scanning electron microscope study and microstructural evolution of nano silicon anode for high energy Li-ion batteries. J. Power Sources 2014, 248, 457–464. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Choi, H.; Song, S.W. Roles of oxygen and interfacial stabilization in enhancing the cycling ability of silicon oxide anodes for rechargeable lithium batteries. J. Electrochem. Soc. 2013, 160, A906. [Google Scholar] [CrossRef]
- Takezawa, H.; Ito, S.; Yoshizawa, H.; Abe, T. Structural stabilization on SiOx film anode with large areal capacity for enhanced cyclability in lithium-ion batteries. J. Power Sources 2016, 324, 45–51. [Google Scholar] [CrossRef]
- Meng, X.; Huo, H.; Cui, Z.; Guo, X.; Dong, S. Influences of oxygen content on the electrochemical performance of a-SiOx thin-film anodes. Electrochim. Acta 2018, 283, 183–189. [Google Scholar] [CrossRef]
- Haruta, M.; Doi, T.; Inaba, M. Oxygen-content dependence of cycle performance and morphology changes in amorphous-SiOx thin-film negative electrodes for lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A258. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Schilcher, C.; Meyer, C.; Kwade, A. Structural and electrochemical properties of calendered lithium manganese oxide cathodes. Energy Technol. 2016, 4, 1604–1610. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Ukumori, N. Intragranular Fracture Mechanism of Highly Crystalline Lithium Manganese Oxide during Lithium Insertion/Extraction Reactions. ACS Appl. Energy Mater. 2021, 4, 8142–8149. [Google Scholar] [CrossRef]
- McGrogan, F.P.; Raja, S.N.; Chiang, Y.M.; Van Vliet, K.J. Electrochemomechanical fatigue: Decoupling mechanisms of fracture-induced performance degradation in LiXMn2O4. J. Electrochem. Soc. 2018, 165, A2458. [Google Scholar] [CrossRef] [Green Version]
- Thackeray, M.M.; Shao-Horn, Y.; Kahaian, A.J.; Kepler, K.D.; Skinner, E.; Vaughey, J.T.; Hackney, S.A. Structural fatigue in spinel electrodes in high voltage (4 V) Li/LixMn2O4 cells. Electrochem. Solid-State Lett. 1998, 1, 7. [Google Scholar] [CrossRef]
- Warburton, R.E.; Castro, F.C.; Deshpande, S.; Madsen, K.E.; Bassett, K.L.; Dos Reis, R.; Gewirth, A.A.; Dravid, V.P.; Greeley, J. Oriented LiMn2O4 particle fracture from delithiation-driven surface stress. ACS Appl. Mater. Interfaces 2020, 12, 49182–49191. [Google Scholar] [CrossRef]
- Hao, X.; Lin, X.; Lu, W.; Bartlett, B.M. Oxygen vacancies lead to loss of domain order, particle fracture, and rapid capacity fade in lithium manganospinel (LiMn2O4) batteries. ACS Appl. Mater. Interfaces 2014, 6, 10849–10857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, K.; Chen-Wiegart, Y.c.K.; Wang, J.; Kung, H.H.; Wang, J.; Barnett, S.A.; Faber, K.T. X-ray nanotomography analysis of the microstructural evolution of LiMn2O4 electrodes. J. Power Sources 2017, 360, 460–469. [Google Scholar] [CrossRef]
- Yu, Y.S.; Kim, C.; Liu, Y.; Van der Ven, A.; Meng, Y.S.; Kostecki, R.; Cabana, J. Nonequilibrium pathways during electrochemical phase transformations in single crystals revealed by dynamic chemical imaging at nanoscale resolution. Adv. Energy Mater. 2015, 5, 1402040. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Z.; Wang, G.; Ren, H.; Pan, M.; Xiao, L.; Wu, K.; Zhao, L.; Yang, J.; Wu, Q.; et al. Dual-doping to suppress cracking in spinel LiMn2O4: A joint theoretical and experimental study. Phys. Chem. Chem. Phys. 2016, 18, 6893–6900. [Google Scholar] [CrossRef] [PubMed]
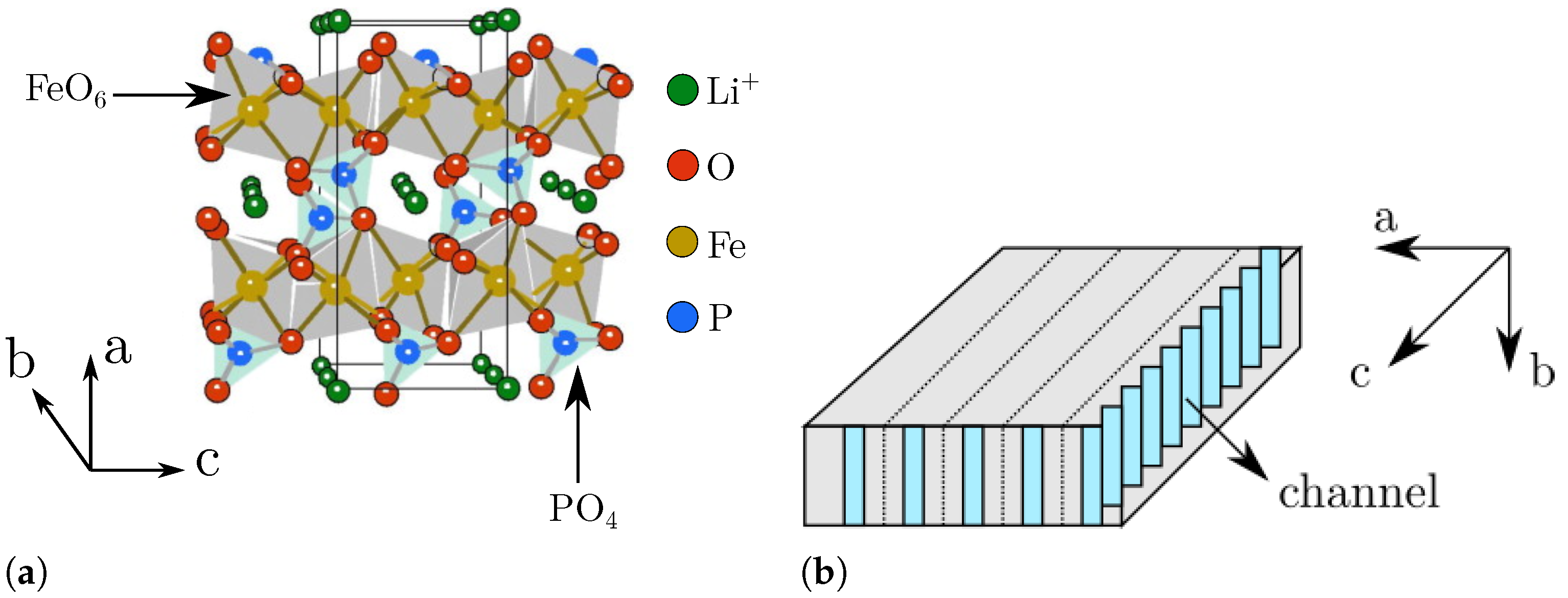
- Morgan, D.; Van der Ven, A.; Ceder, G. Li conductivity in li x mpo 4 (m = mn, fe, co, ni) olivine materials. Electrochem. Solid-State Lett. 2003, 7, A30. [Google Scholar] [CrossRef]
- Ramana, C.; Mauger, A.; Gendron, F.; Julien, C.; Zaghib, K. Study of the Li-insertion/extraction process in LiFePO4/FePO4. J. Power Sources 2009, 187, 555–564. [Google Scholar] [CrossRef]
- Love, C.T.; Korovina, A.; Patridge, C.J.; Swider-Lyons, K.E.; Twigg, M.E.; Ramaker, D.E. Review of LiFePO4 phase transition mechanisms and new observations from x-ray absorption spectroscopy. J. Electrochem. Soc. 2013, 160, A3153. [Google Scholar] [CrossRef]
- Malik, R.; Abdellahi, A.; Ceder, G. A critical review of the Li insertion mechanisms in LiFePO4 electrodes. J. Electrochem. Soc. 2013, 160, A3179. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Richardson, T.J. Electron microscopy study of the LiFePO4 to FePO4 phase transition. Electrochem. -Solid-State Lett. 2006, 9, A295. [Google Scholar] [CrossRef]
- Boesenberg, U.; Meirer, F.; Liu, Y.; Shukla, A.K.; Dell’Anna, R.; Tyliszczak, T.; Chen, G.; Andrews, J.C.; Richardson, T.J.; Kostecki, R.; et al. Mesoscale phase distribution in single particles of LiFePO4 following lithium deintercalation. Chem. Mater. 2013, 25, 1664–1672. [Google Scholar] [CrossRef]
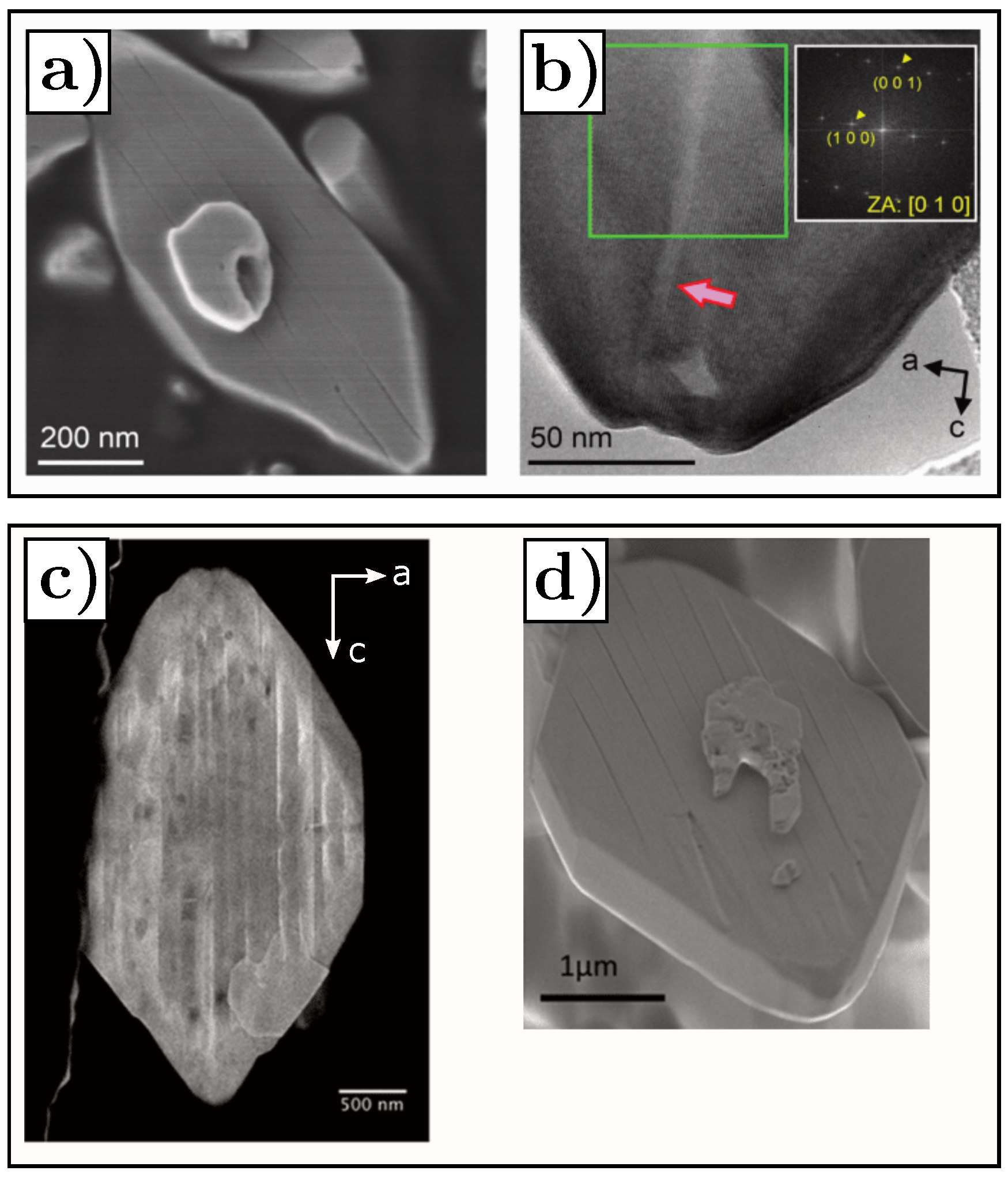
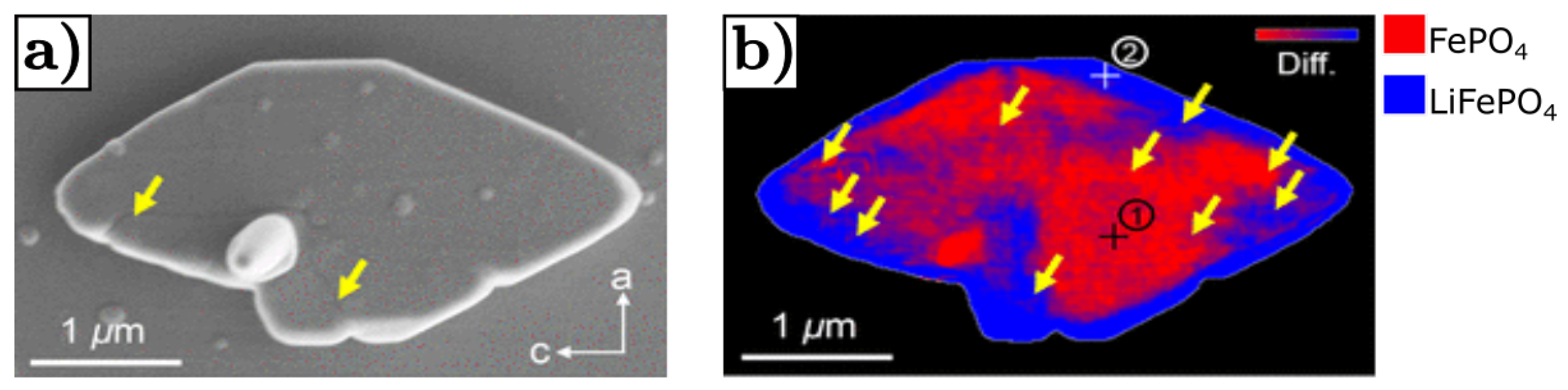
- Yu, Y.S.; Kim, C.; Shapiro, D.A.; Farmand, M.; Qian, D.; Tyliszczak, T.; Kilcoyne, A.D.; Celestre, R.; Marchesini, S.; Joseph, J.; et al. Dependence on crystal size of the nanoscale chemical phase distribution and fracture in LixFePO4. Nano Lett. 2015, 15, 4282–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrisch, H.; Wilcox, J.; Doeff, M. TEM study of fracturing in spherical and plate-like LiFePO4 particles. Electrochem. Solid-State Lett. 2008, 11, A25. [Google Scholar] [CrossRef]
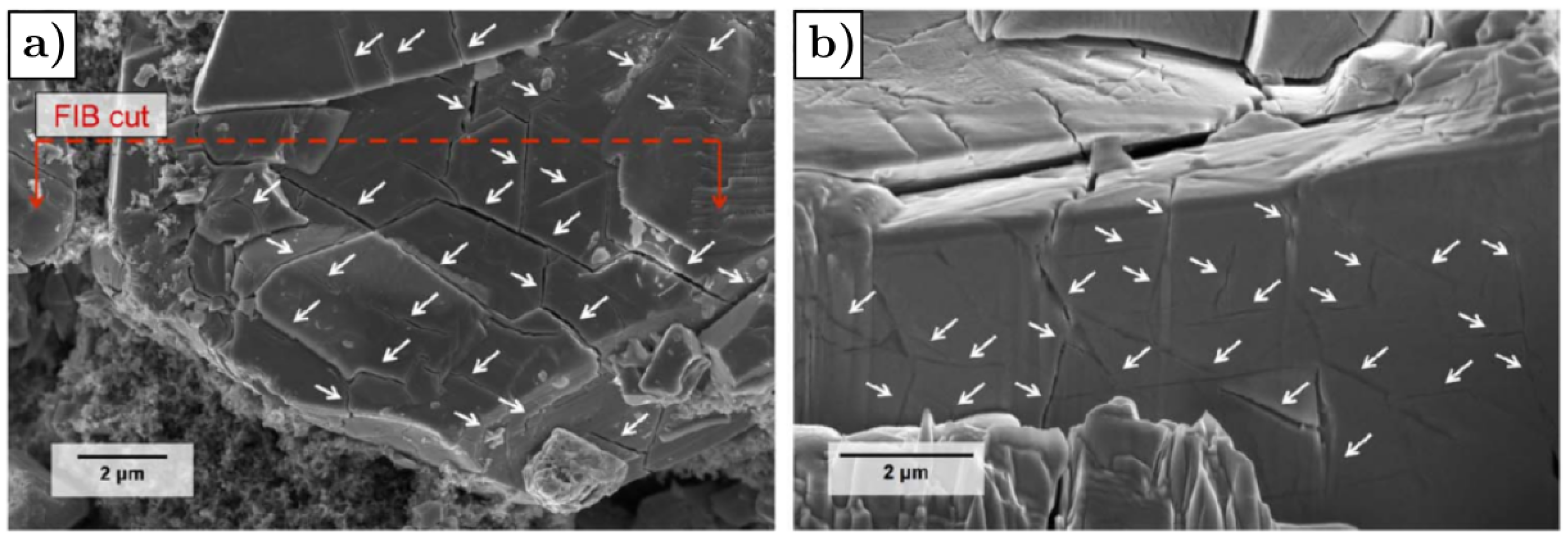
- Fu, J.; Wang, K.; Liu, D.; Zhang, Z.; Sui, M.; Yan, P. b-Axis Phase Boundary Movement Induced (020) Plane Cracking in LiFePO4. ACS Appl. Mater. Interfaces 2020, 12, 39245–39251. [Google Scholar] [CrossRef]
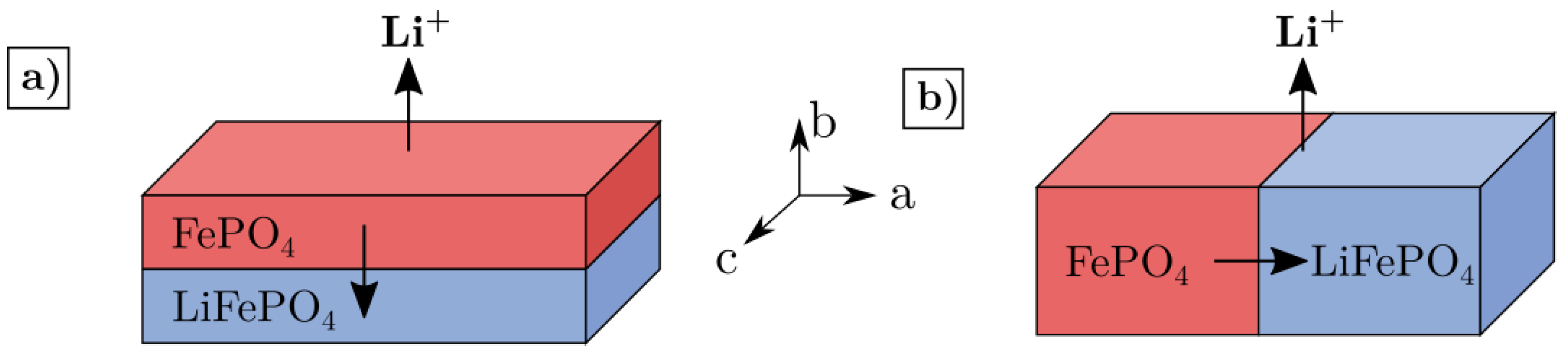
- Brunetti, G.; Robert, D.; Bayle-Guillemaud, P.; Rouviere, J.; Rauch, E.; Martin, J.; Colin, J.; Bertin, F.; Cayron, C. Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction. Chem. Mater. 2011, 23, 4515–4524. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Wang, Z.; Chen, L. Cracking causing cyclic instability of LiFePO4 cathode material. J. Power Sources 2005, 140, 125–128. [Google Scholar] [CrossRef]
- Shapiro, D.A.; Yu, Y.S.; Tyliszczak, T.; Cabana, J.; Celestre, R.; Chao, W.; Kaznatcheev, K.; Kilcoyne, A.; Maia, F.; Marchesini, S.; et al. Chemical composition mapping with nanometre resolution by soft X-ray microscopy. Nat. Photonics 2014, 8, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.; Wu, M.Y.; Ramanujapuram, A.; Benson, J.; Lee, J.T.; Magasinski, A.; Nitta, N.; Huang, C.; Yushin, G. Enhancing cycle stability of lithium iron phosphate in aqueous electrolytes by increasing electrolyte molarity. Adv. Energy Mater. 2016, 6, 1501805. [Google Scholar] [CrossRef]
- Malik, M.; Chan, K.H.; Azimi, G. Review on the synthesis of LiNixMnyCo1-x-yO2 (NMC) cathodes for lithium-ion batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierarchical Structuring of NMC111-Cathode Materials in Lithium-Ion Batteries: An In-Depth Study on the Influence of Primary and Secondary Particle Sizes on Electrochemical Performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich NMC cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Xu, Z.; Rahman, M.M.; Mu, L.; Liu, Y.; Lin, F. Chemomechanical behaviors of layered cathode materials in alkali metal ion batteries. J. Mater. Chem. A 2018, 6, 21859–21884. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.G.; Wang, C.M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, P.; Zheng, J.; Chen, T.; Luo, L.; Jiang, Y.; Wang, K.; Sui, M.; Zhang, J.G.; Zhang, S.; Wang, C. Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat. Commun. 2018, 9, 2437. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Pokle, A.; Schweidler, S.; Beyer, A.; Bianchini, M.; Walther, F.; Mazilkin, A.; Hartmann, P.; Brezesinski, T.; Janek, J.; et al. The Role of Intragranular Nanopores in Capacity Fade of Nickel-Rich Layered Li (Ni1–x–yCoxMny)O2 Cathode Materials. ACS Nano 2019, 13, 10694–10704. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Zhou, J.; Meng, J.; Huang, W.; Chen, T.; Gao, Q.; Wei, X.; Zeng, Y.; Li, J.; et al. Ni–Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode. Nano Energy 2020, 76, 105021. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Zhang, J.; Engelhard, M.H.; Zhu, Z.; Polzin, B.J.; Trask, S.; Xiao, J.; Wang, C.; Zhang, J. Suppressed oxygen extraction and degradation of LiNixMnyCozO2 cathodes at high charge cut-off voltages. Nano Res. 2017, 10, 4221–4231. [Google Scholar] [CrossRef]
- Mu, L.; Lin, R.; Xu, R.; Han, L.; Xia, S.; Sokaras, D.; Steiner, J.D.; Weng, T.C.; Nordlund, D.; Doeff, M.M.; et al. Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Lett. 2018, 18, 3241–3249. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Ma, J.; Hu, E.; Yu, X.; Gu, L.; Nam, K.W.; Chen, L.; Wang, Z.; Yang, X.Q. Tuning charge–discharge induced unit cell breathing in layer-structured cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 5381. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.H.; Manthiram, A. Impact of microcrack generation and surface degradation on a nickel-rich layered Li [Ni0.9Co0.05Mn0.05]O2 cathode for lithium-ion batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Xia, S.; Mu, L.; Xu, Z.; Wang, J.; Wei, C.; Liu, L.; Pianetta, P.; Zhao, K.; Yu, X.; Lin, F.; et al. Chemomechanical interplay of layered cathode materials undergoing fast charging in lithium batteries. Nano Energy 2018, 53, 753–762. [Google Scholar] [CrossRef]
- Gent, W.E.; Li, Y.; Ahn, S.; Lim, J.; Liu, Y.; Wise, A.M.; Gopal, C.B.; Mueller, D.N.; Davis, R.; Weker, J.N.; et al. Persistent State-of-Charge Heterogeneity in Relaxed, Partially Charged Li1-xNi1/3Co1/3Mn1/3O2 Secondary Particles. Adv. Mater. 2016, 28, 6631–6638. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic lattice strain and mechanical degradation of high-and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Hu, E.; Chae, S.; Jia, H.; Liu, T.; Wu, B.; Bi, Y.; Amine, K.; Wang, C.; et al. Mesoscale-architecture-based crack evolution dictating cycling stability of advanced lithium ion batteries. Nano Energy 2021, 79, 105420. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Huang, Z.; Yang, C.; Zuo, Z.; Zhou, H. Understanding the accumulated cycle capacity fade caused by the secondary particle fracture of LiNi1-x-yCoxMnyO2 cathode for lithium ion batteries. J. Solid State Electrochem. 2017, 21, 673–682. [Google Scholar] [CrossRef]
- Kim, Y. Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: Morphology and performance as a cathode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2329–2333. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Xu, R.; Sun, H.; de Vasconcelos, L.S.; Zhao, K. Mechanical and structural degradation of LiNixMnyCozO2 cathode in Li-ion batteries: An experimental study. J. Electrochem. Soc. 2017, 164, A3333. [Google Scholar] [CrossRef]
- De Vasconcelos, L.; Sharma, N.; Xu, R.; Zhao, K. In-situ nanoindentation measurement of local mechanical behavior of a Li-ion battery cathode in liquid electrolyte. Exp. Mech. 2019, 59, 337–347. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Stoll, M.; Henriksen, G.; Vissers, D.; Amine, K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sources 2004, 128, 278–285. [Google Scholar] [CrossRef]
- Jo, M.; Noh, M.; Oh, P.; Kim, Y.; Cho, J. A new high power LiNi0.81Co0.1Al0.09O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1301583. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1-x-yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Liu, H.; Wolfman, M.; Karki, K.; Yu, Y.S.; Stach, E.A.; Cabana, J.; Chapman, K.W.; Chupas, P.J. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes. Nano Lett. 2017, 17, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.l.; Zhou, X.a.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.b.; Zhang, N.s.; Li, S.y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Park, K.J.; Hwang, J.Y.; Ryu, H.H.; Maglia, F.; Kim, S.J.; Lamp, P.; Yoon, C.S.; Sun, Y.K. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Choi, M.J.; Maglia, F.; Kim, S.J.; Kim, K.H.; Yoon, C.S.; Sun, Y.K. High-capacity concentration gradient Li[Ni0.865Co0.120Al0.015]O2 cathode for lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1703612. [Google Scholar] [CrossRef]
- Besli, M.M.; Shukla, A.K.; Wei, C.; Metzger, M.; Alvarado, J.; Boell, J.; Nordlund, D.; Schneider, G.; Hellstrom, S.; Johnston, C.; et al. Thermally-driven mesopore formation and oxygen release in delithiated NCA cathode particles. J. Mater. Chem. A 2019, 7, 12593–12603. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, K.; Jones, P.; Wiseman, P.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Jiang, Y.; Yan, P.; Yu, M.; Li, J.; Jiao, H.; Zhou, B.; Sui, M. Atomistic mechanism of cracking degradation at twin boundary of LiCoO2. Nano Energy 2020, 78, 105364. [Google Scholar] [CrossRef]
- Hitt, A.; Wang, F.; Li, Z.; Ge, M.; Zhang, Y.; Savsatli, Y.; Xiao, X.; Lee, W.K.; Stephens, R.; Tang, M. Nanotomographic observation and statistical analysis of overcharging induced cracks in LiCoO2 single crystalline particles. Energy Storage Mater. 2022, 52, 320–328. [Google Scholar] [CrossRef]
- Marianetti, C.A.; Kotliar, G.; Ceder, G. A first-order Mott transition in LixCoO2. Nat. Mater. 2004, 3, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.N.; Dahn, J. Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091. [Google Scholar] [CrossRef]
- Van der Ven, A.; Aydinol, M.; Ceder, G.; Kresse, G.; Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 1998, 58, 2975. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Z.; Dahn, J. Staging phase transitions in LixCoO2. J. Electrochem. Soc. 2002, 149, A1604. [Google Scholar] [CrossRef]
- Ohnishi, T.; Mitsuishi, K.; Takada, K. In Situ X-ray Diffraction of LiCoO2 in Thin-Film Batteries under High-Voltage Charging. ACS Appl. Energy Mater. 2021, 4, 14372–14379. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, C.; Yan, P.; Sui, M. Origins of capacity and voltage fading of LiCoO2 upon high voltage cycling. J. Mater. Chem. A 2019, 7, 20824–20831. [Google Scholar] [CrossRef]
- Amatucci, G.; Tarascon, J.; Klein, L. CoO2, the end member of the LixCoO2 solid solution. J. Electrochem. Soc. 1996, 143, 1114. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Xiang, L. Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies. Particuology 2022, 61, 1–10. [Google Scholar] [CrossRef]
- Chen, C.J.; Pang, W.K.; Mori, T.; Peterson, V.K.; Sharma, N.; Lee, P.H.; Wu, S.h.; Wang, C.C.; Song, Y.F.; Liu, R.S. The Origin of Capacity Fade in the Li2MnO3·LiMO2 (M= Li, Ni, Co, Mn) Microsphere Positive Electrode: An Operando Neutron Diffraction and Transmission X-ray Microscopy Study. J. Am. Chem. Soc. 2016, 138, 8824–8833. [Google Scholar] [CrossRef]
- Cha, H.; Kim, J.; Lee, H.; Kim, N.; Hwang, J.; Sung, J.; Yoon, M.; Kim, K.; Cho, J. Boosting reaction homogeneity in high-energy lithium-ion battery cathode materials. Adv. Mater. 2020, 32, 2003040. [Google Scholar] [CrossRef]
- Wang, L.; Dai, A.; Xu, W.; Lee, S.; Cha, W.; Harder, R.; Liu, T.; Ren, Y.; Yin, G.; Zuo, P.; et al. Structural distortion induced by manganese activation in a lithium-rich layered cathode. J. Am. Chem. Soc. 2020, 142, 14966–14973. [Google Scholar] [CrossRef]
- Song, B.; Liu, Z.; Lai, M.O.; Lu, L. Structural evolution and the capacity fade mechanism upon long-term cycling in Li-rich cathode material. Phys. Chem. Chem. Phys. 2012, 14, 12875–12883. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface Modification of Li-Rich Mn-Based Layered Oxide Cathodes: Challenges, Materials, Methods, and Characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Kim, U.H.; Kuo, L.Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Quaternary layered Ni-rich NCMA cathode for lithium-ion batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, J.; Zhang, W. Capacity Decay Mechanism of the LCO+ NMC532/Graphite cells combined with post-mortem technique. Energies 2017, 10, 1147. [Google Scholar] [CrossRef]
- Schweidler, S.; de Biasi, L.; Garcia, G.; Mazilkin, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Investigation into mechanical degradation and fatigue of high-Ni NCM cathode material: A long-term cycling study of full cells. ACS Appl. Energy Mater. 2019, 2, 7375–7384. [Google Scholar] [CrossRef]
- Chen, D.; Kramer, D.; Mönig, R. Chemomechanical fatigue of LiMn1.95Al0.05O4 electrodes for lithium-ion batteries. Electrochim. Acta 2018, 259, 939–948. [Google Scholar] [CrossRef]
- Wu, H.; Qin, C.; Wang, K.; Han, X.; Sui, M.; Yan, P. Revealing two distinctive intergranular cracking mechanisms of Ni-rich layered cathode by cross-sectional scanning electron microscopy. J. Power Sources 2021, 503, 230066. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Cao, T.; Wu, R.; Wang, M.; Liu, H.; Liu, X.; Lu, J.; Zhang, Y. Real-time observation of chemomechanical breakdown in a layered nickel-rich oxide cathode realized by in situ scanning electron microscopy. ACS Energy Lett. 2021, 6, 1703–1710. [Google Scholar] [CrossRef]
- Ali, Y.; Iqbal, N.; Lee, S. Role of SEI layer growth in fracture probability in lithium-ion battery electrodes. Int. J. Energy Res. 2021, 45, 5293–5308. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.Y.; Park, K.J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Didier, C.; Pang, W.K.; Guo, Z.; Schmid, S.; Peterson, V.K. Phase Evolution and Intermittent Disorder in Electrochemically Lithiated Graphite Determined Using in Operando Neutron Diffraction. Chem. Mater. 2020, 32, 2518–2531. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Schweidler, S.; de Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume changes of graphite anodes revisited: A combined operando X-ray diffraction and in situ pressure analysis study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of lithium-graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 1993, 140, 2490. [Google Scholar] [CrossRef]
















| Particle Morphology | Particle Size | Method | Technique | Fracture Observation | Ref. |
|---|---|---|---|---|---|
| Platelet | Chemical delithiation | SEM, TEM, HRTEM | Parallel to plane | [95] | |
| Almost Spherical | Electrochemical cycling | XRD, TEM, FMSEM | Parallel to plane | [101] | |
| Almost Spherical | Electrochemical cycling | TEM | Parallel to and planes | [98] | |
| Platelet | Chemical delithiation | TEM | Parallel to plane | [98] | |
| Almost Spherical | Electrochemical cycling | SEM, TEM | Parallel to plane | [99] | |
| Platelet | Chemical delithiation | SEM, TEM, HRTEM | Superficial and internal crack along c-axis | [97] | |
| Platelet | Chemical delithiation | SEM, TEM, HRTEM | Superficial and internal crack along c-axis | [97] | |
| Platelet | Chemical and Electrochemical delithiation | SEM, TEM HRTEM | No crack | [97] | |
| Platelet | Chemical delithiation | SEM, TEM STEM, STMX FF TMX, XANES | Cracks along c-axis mostly localized in the centre of the particle | [96] |
| Active Material | Fracture Index | Fracture Mechanism | Side Reaction | Lacks to Cover |
|---|---|---|---|---|
| Graphite | 2 | Superficial exfoliation and internal cracks | SEI formation and growth on the crack surfaces | Few detailed characterizations of fracture mechanisms |
| Silicon | 3 | Surface cracks in thin film, internal and superficial crack in nano-wires and nano-particles | SEI formation and growth on the crack surfaces | Development of new structures with different composition mitigating volume change |
| 2 | Cracks due to volume changes | SEI formation and growth on the crack surfaces | Determination of the optimal composition and particle size to mitigate volume change | |
| LMO | 2 | Intragranular cracks at the phase boundary due to Jahn–Teller transformation | dissolution | Few studies on the correlation between fracture and capacity decay |
| LFP | 1 | Cracks at the phase boundary interface | Fe-deficient amorphous layer on crack surfaces | Few studies on side reactions at crack surfaces, correlation between fracture and capacity decay, and controversial results about the correlation between fracture and phase transformation |
| NMC | 2 | Intragranular and intergranular fracture | Rock-salt formation on crack surfaces | Not clear crack initiation and growth mechanism and few studies on the correlation between fracture and capacity decay |
| NCA | 2 | Intergranular and intragranular fracture | Rock-salt formation on crack surfaces | Not clear effects influencing intergranular fracture and few studies on intragranular cracking |
| LCO | 1 | Cleavage cracks and cracks at the twin boundary due to phase transition | Not observed | Few characterization of fracture mechanisms, side-reaction at crack surfaces and correlation between fracture and capacity decay |
| LLO | 2 | Intergranular cracking | Not observed | Not clear effects influencing intergranular fracture and poor characterization of fracture mechanisms, side-reaction at crack surfaces and correlation between fracture and capacity decay |
| Material | Crystal Structure Deformation | Crack Morphology | Crack Growth with Cycling | Effects of Fracture on Electrochemical Performance |
|---|---|---|---|---|
| Graphite | [160,161,162,163] | [40,43,45,46] | [42,44] | [46] |
| Silicon | - | [49,50,54,55,57] | [51,55,57] | [71] |
| SiO | - | [72,73,74,75] | - | - |
| LMO | [83,85,86,87,89] | [83,86,89] | [84,87,88,155] | [84,88] |
| LFP | [93,94,95,96,97,98,99,100] | [95,96,97,99,101] | [98,99,101] | [99,101,103] |
| NMC | [106,108,109,110,111,112,113,114,115] | [108,109,110,111,113,115] | [106,108,110,111,115,152,153,154,156,157,159] | [106,112,115,121,159] |
| NCA | [132] | [132,134] | [129,130,133] | [132] |
| LCO | [136] | [136,137] | [136] | - |
| LLO | [146] | [146] | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistorio, F.; Clerici, D.; Mocera, F.; Somà, A. Review on the Experimental Characterization of Fracture in Active Material for Lithium-Ion Batteries. Energies 2022, 15, 9168. https://doi.org/10.3390/en15239168
Pistorio F, Clerici D, Mocera F, Somà A. Review on the Experimental Characterization of Fracture in Active Material for Lithium-Ion Batteries. Energies. 2022; 15(23):9168. https://doi.org/10.3390/en15239168
Chicago/Turabian StylePistorio, Francesca, Davide Clerici, Francesco Mocera, and Aurelio Somà. 2022. "Review on the Experimental Characterization of Fracture in Active Material for Lithium-Ion Batteries" Energies 15, no. 23: 9168. https://doi.org/10.3390/en15239168








