Polarization Voltage Characterization of Lithium-Ion Batteries Based on a Lumped Diffusion Model and Joint Parameter Estimation Algorithm
Abstract
:1. Introduction
- (i.)
- In the cell design phase, improvements are made for the internal composition of the cell. Zheng et al. pointed out that polarization leads to uneven distribution of electrode active material, which causes performance decline for lithium-ion batteries [6]. The separator is a key component inside a lithium-ion battery cell. Feng et al. redeveloped the separator material to mitigate the polarization phenomenon during battery operation, which enhanced the power performance and cycling stability of the battery [8]. Kim et al. improved the anode materials for lithium-ion batteries to mitigate the polarization phenomenon of lithium-ion batteries during operation [9]. In [10], Shi et al. prepared an electrical conductive graphene nanosheet with hybrid lithium titanate nanoparticles dispersed on it as an anode, which shortened the ion transport path, greatly improving the ion and electron transport efficiency at the particle/electrolyte coupling interface, and remarkably reducing the charge transfer impedance, and improving the battery cycling performance under large rate conditions. The DRT (Distribution of Relaxation Times) method has been used to analyze the impedance spectrum of lithium-ion batteries in the frequency domain to precisely characterize various types of polarization, and used to develop new cathode materials [11]. Song et al. developed a ferroelectric polyvinylidene difluoride (PVDF) polymer as a binder material and demonstrated by the galvanostatic intermittent titration technique (GITT) measurement and in situ galvanostatic electrochemical impedance spectroscopy (GS-EIS) analysis that this new material can significantly reduce the lithium-ion diffusion impendence as a binder inside cells, compared to the paraelectric PVDF binder material, thereby improving the battery performance under large rate conditions. This provided new inspiration for the design of high-performance lithium-ion batteries [12].
- (ii.)
- Polarization has been considered an influential factor in numerous studies related to the thermal management system of lithium-ion batteries. In [13], the respective proportion of ohmic internal resistance and polarization internal resistance under different discharge rate conditions were explored to summarize the contribution of polarization in the accumulation of internal battery temperature at the early stage of thermal runaway, which provides a theoretical basis for safer battery design. As discussed in [14,15], the heat of polarization is the main component of the internal heat production of the cell and consists of four parts: ohmic heat, polarization heat, reaction heat, and side-reaction heat. Taheri P, Mansouri A et al. developed a two-dimensional analytical model of the lithium-ion battery, and a concentration-independent polarization voltage was derived to explore the application for battery thermal management through the thermal coupling model [16]. Goonetilleke D. et al. found that increasing the ambient temperature increased the reaction rate inside the battery and reduced polarization inside battery cells [17]. In [18], the optimal alternating current (AC) frequency was determined based on the Thevenin-thermal coupling model in the frequency domain, and an internal heating strategy based on a constant polarization voltage at low ambient temperatures was developed, which achieved a good balance between cell aging and heating efficiency.
- (iii.)
- State of Charge and State of Health (SOH) are two very important state parameters in BMS, and the influence of polarization on estimation accuracy cannot be ignored. Li et al. pointed out that polarization resistance contributes significantly to the battery capacity decay, so the accurate characterization of polarization is important for developing the estimation method of SOH [19]. Marino C. et al. quantified the electrode polarization resistance and established a functional relationship with the external current excitation at different cycle rates to estimate the aging state of the battery to determine battery failure [20]. SOH decays with increasing cycle number, and Xia and Chen et al. defined the concept of Degree of Polarization (DOP) to correct SOH estimation results to improve the accuracy of SOC estimation [21].
- (iv.)
- In battery charging technology, polarization is also considered a controlled variable. Zhang et al. used the voltage drop from polarization as a controlled variable in the charging process to make a balance between charging time and temperature rise and combined it with a Genetic Algorithm (GA) to find the optimal charging current trajectory. Verification experiments of battery aging proved that this charging method has similar capacity retention to the 0.5C Constant current Constant voltage (CC-CV) charging method, but with improved charging efficiency [22,23].
2. Battery Modeling and Joint Algorithm Scheme
2.1. Battery Model Description
2.2. Joint Parameter Estimation Algorithm Design
3. Experiment Verification
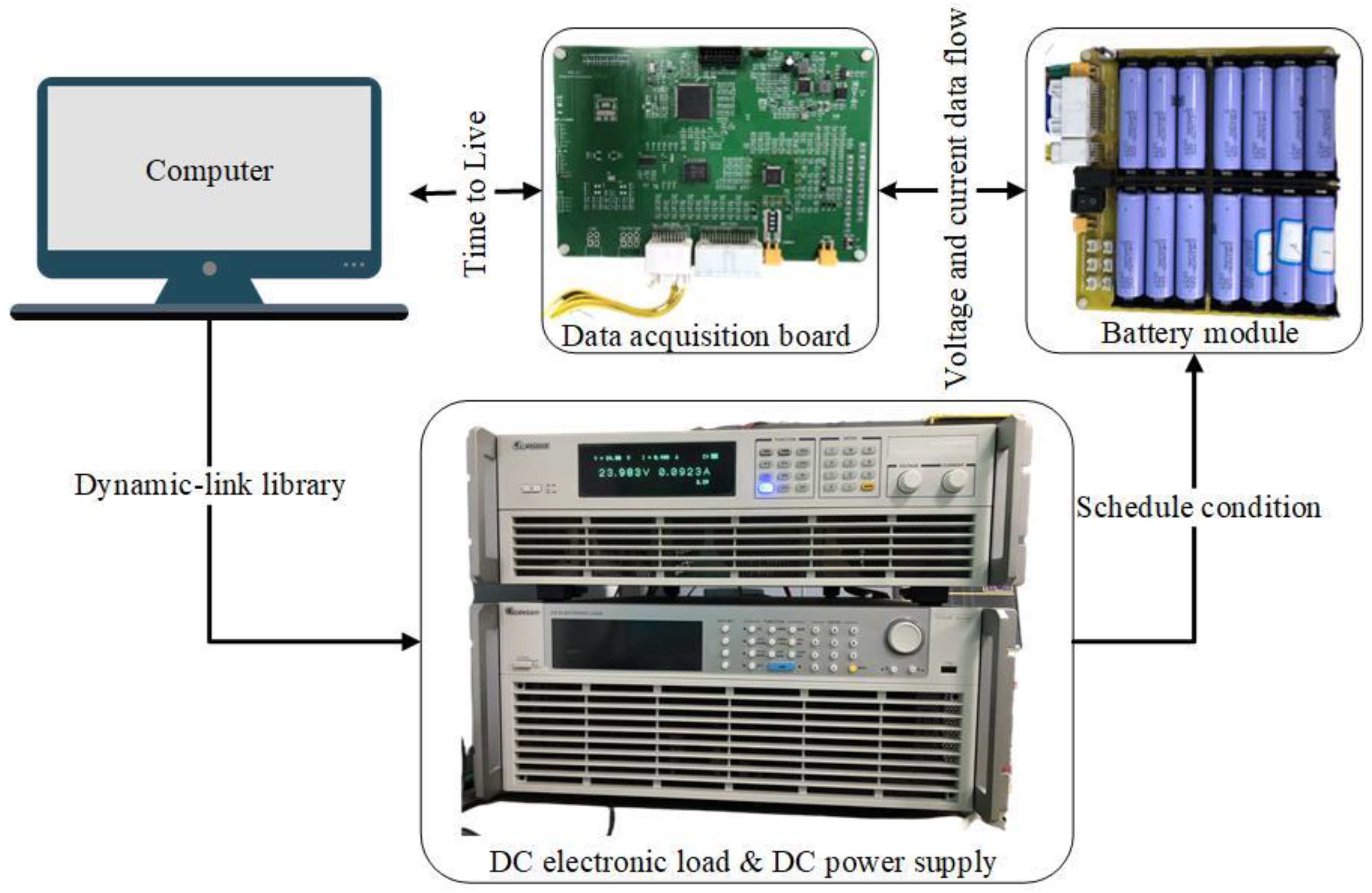
3.1. Introduction of the Experiment Bench
3.2. Experiment Configuration
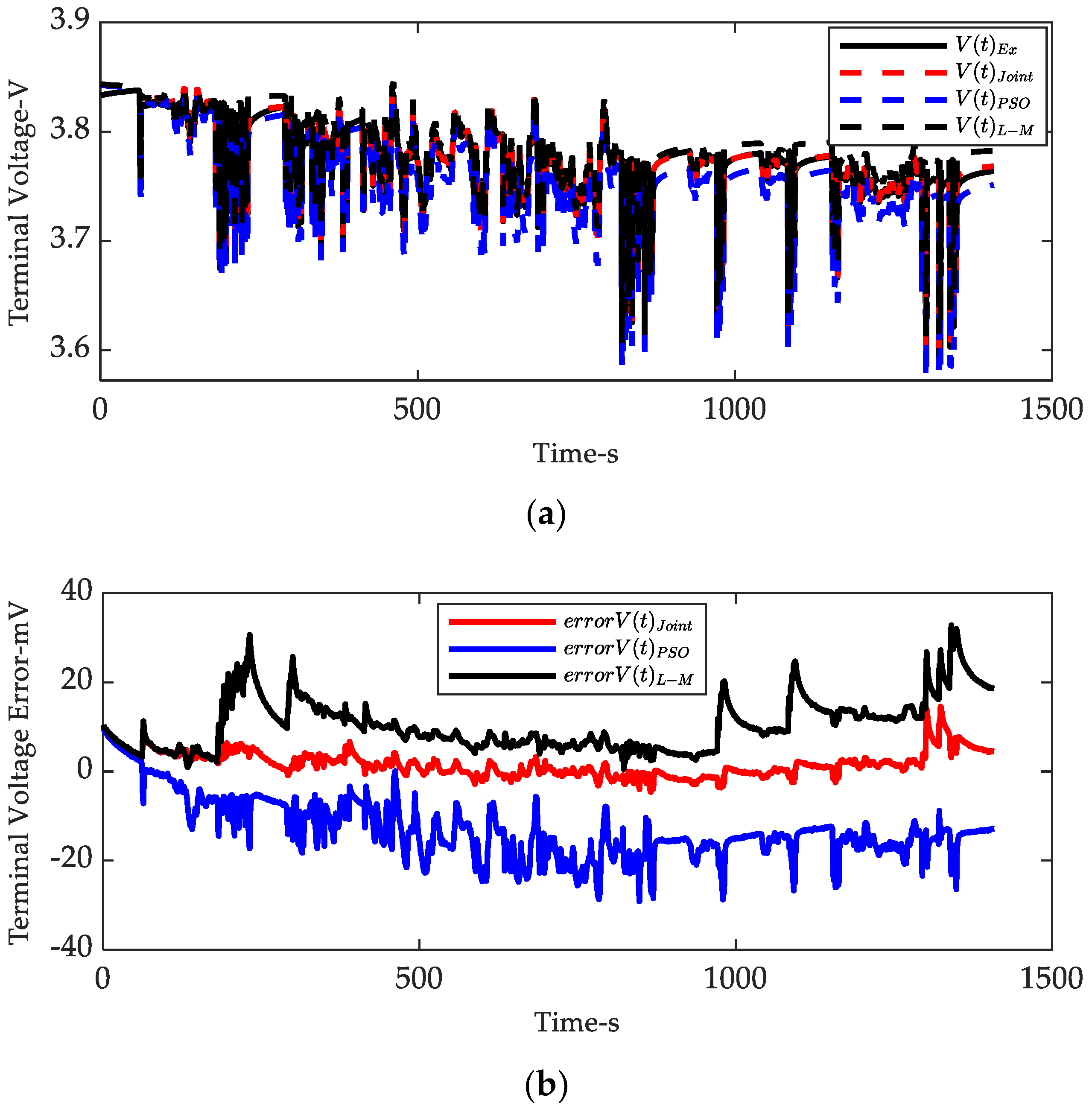
3.3. Offline Verification of Proposed Method
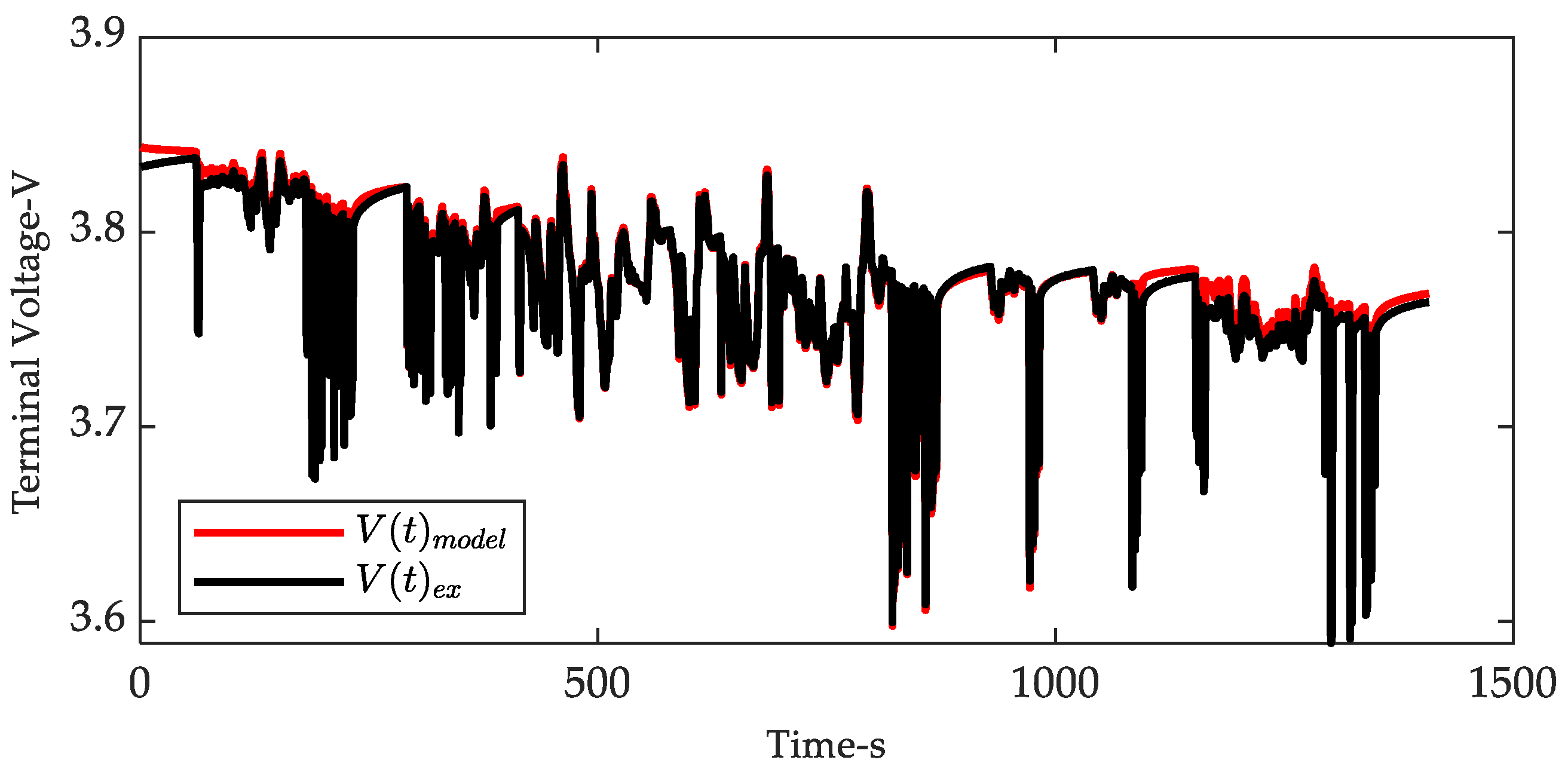
3.4. Online Polarization Voltage Characterization Using a Hardware Platform
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Acronyms | |
| AC | Alternating current |
| BMS | Battery management system |
| CC-CV | Constant current Constant voltage |
| CCV | Closed-circuit voltage |
| DC | Direct current |
| DOP | Degree of Polarization |
| DRT | Distribution of Relaxation Times |
| GA | Genetic Algorithm |
| GITT | Galvanostatic intermittent titration technique |
| GS-EIS | Galvanostatic electrochemical impedance spectroscopy |
| LDM | Lumped diffusion model |
| L-M method | Levenberg-Marquardt method |
| Pseudo-2D | Pseudo 2 dimensional |
| PSO | Particle swarm optimization |
| PVDF | Ferroelectric polyvinylidene difluoride |
| QOCV | Quasi-open-circuit voltage |
| SOC | State of Charge |
| SOH | State of Health |
| SPM | Single particle model |
| Symbols | |
| Coefficients of polynomial describing SOC-OCV curve | |
| Local learning factor | |
| Global learning factor | |
| Randomly picked dimension among three dimensions | |
| Open-circuit voltage | |
| Battery terminal voltage | |
| Voltage error | |
| F | Faraday constant |
| Fitness function of PSO algorithm | |
| G | Maximum iteration number |
| , global optimum particle | |
| Applied current taken at 1C rate | |
| Applied current under discrete time domain | |
| Instantaneous current | |
| Local SOC in electrode particle | |
| Dimensionless charge exchange current | |
| Jacobian matrix | |
| Learning factor gain | |
| Maximum iteration number | |
| Current iteration number | |
| Lower velocity bound | |
| Lower position bound | |
| Identity matrix | |
| Dimension number of the particle | |
| , history optimum for the pth particle | |
| P | Population size |
| Battery capacity | |
| R | Molar gas constant |
| Ohmic resistance | |
| Random numbers uniformly distributed in (0,1) | |
| Average SOC of electrode particle | |
| Surface SOC of electrode particle | |
| SOC at initial time point | |
| Search space | |
| T | Reference temperature |
| Initial time | |
| Current time | |
| Upper velocity bound | |
| Upper position bound | |
| Model output voltage | |
| Real world terminal voltage | |
| , velocity vector for the pth particle | |
| , position vector for the pth particle | |
| Dimensionless space variable in particle size scale | |
| Greek symbols | |
| Charge transfer coefficient | |
| Tolerance | |
| Activation polarization overpotential | |
| Concentration polarization overpotential | |
| Ohmic polarization overpotential | |
| Parameter vector in the L-M method | |
| Diffusion time constant | |
| Maximum inertia weight | |
| Minimum inertia weight | |
| Inertia weight | |
| Subscripts/Superscripts | |
| Positive electrode | |
| Negative electrode | |
| The pth particle | |
| Iteration number | |
References
- Bizeray, A.M.; Kim, J.-H.; Duncan, S.R.; Howey, D.A. Identifiability and Parameter Estimation of the Single Particle Lithium-Ion Battery Model. IEEE Trans. Control Syst. Technol. 2019, 27, 1862–1877. [Google Scholar] [CrossRef] [Green Version]
- Xiaosong, H.; Li, S.; Peng, H. A Comparative Study of Equivalent Circuit Models for Li-Ion Batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar]
- Junfu, L.; Wang, D.; Pecht, M. An Electrochemical Model for High C-Rate Conditions in Lithium-Ion Batteries. J. Power Sources 2019, 436, 226885. [Google Scholar]
- Shunli, W.; Stroe, D.; Fernandez, C.; Yu, C.; Zou, C.; Li, X. A Novel Energy Management Strategy for the Ternary Lithium Batteries Based on the Dynamic Equivalent Circuit Modeling and Differential Kalman Filtering under Time-Varying Conditions. J. Power Sources 2020, 450, 227652. [Google Scholar]
- Andreas, N.; Zavalis, T.G.; Elger, R.; Behm, M.; Lindbergh, G. Analysis of the Polarization in a Li-Ion Battery Cell by Numerical Simulations. J. Electrochem. Soc. 2010, 157, 11. [Google Scholar]
- Jiaxin, Z.; Lu, J.; Amine, K.; Pan, F. Depolarization Effect to Enhance the Performance of Lithium Ions Batteries. Nano Energy 2017, 33, 497–507. [Google Scholar]
- Changsheng, Q.; He, G.; Shi, W.; Zou, M.; Liu, C. The Polarization Characteristics of Lithium-Ion Batteries under Cyclic Charge and Discharge. J. Solid State Electrochem. 2019, 23, 1887–1902. [Google Scholar]
- Guanhua, F.; Li, Z.; Mi, L.; Zheng, J.; Feng, X.; Chen, W. Polypropylene/Hydrophobic-Silica-Aerogel-Composite Separator Induced Enhanced Safety and Low Polarization for Lithium-Ion Batteries. J. Power Sources 2018, 376, 177–183. [Google Scholar]
- Hyun-seung, K.; Kim, J.; Jang, J.; Kim, N.; Ryu, J.H.; Yoon, S.; Oh, S.M. A Comparative Study of Polarization During the Initial Lithiation Step in Tungsten-Oxide Negative Electrodes for Lithium-Ion Batteries. Solid State Ion. 2017, 311, 1–5. [Google Scholar]
- Ying, S.; Wen, L.; Li, F.; Cheng, H. Nanosized Li4ti5o12/Graphene Hybrid Materials with Low Polarization for high rate lithium ion Batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar]
- Balasundaram, M.; Ramar, V.; Yap, C.; Balaya, P. Investigation of Physico-Chemical Processes in Lithium-Ion Batteries by Deconvolution of Electrochemical Impedance Spectra. J. Power Sources 2017, 361, 300–309. [Google Scholar]
- Woo-Jin, S.; Joo, S.H.; Kim, D.H.; Hwang, C.; Jung, G.Y.; Bae, S.; Son, Y.; Cho, J.; Song, H.; Kwak, S.K.; et al. Significance of Ferroelectric Polarization in Poly (Vinylidene Difluoride) Binder for High-Rate Li-Ion Diffusion. Nano Energy 2017, 32, 255–262. [Google Scholar]
- J, N.D.; Wang, M.; Le, A.V.; Shi, Y.; Qiao, Y. Internal Resistance and Polarization Dynamics of Lithium-Ion Batteries Upon Internal Shorting. Appl. Energy 2018, 212, 796–808. [Google Scholar]
- Yingxia, H.; Lai, H. Effects of Discharge Rate on Electrochemical and Thermal Characteristics of Lifepo4/Graphite Battery. Appl. Therm. Eng. 2019, 157, 113744. [Google Scholar]
- Noboru, S. Thermal Behavior Analysis of Lithium-Ion Batteries for Electric and Hybrid Vehicles. J. Power Sources 2001, 99, 70–77. [Google Scholar]
- Peyman, T.; Mansouri, A.; Yazdanpour, M.; Bahrami, M. Theoretical Analysis of Potential and Current Distributions in Planar Electrodes of Lithium-Ion Batteries. Electrochim. Acta 2014, 133, 197–208. [Google Scholar]
- Damian, G.; Faulkner, T.; Peterson, V.K.; Sharma, N. Structural Evidence for Mg-Doped Lifepo4 Electrode Polarisation in Commercial Li-Ion Batteries. J. Power Sources 2018, 394, 1–8. [Google Scholar]
- Haijun, R.; Jiang, J.; Sun, B.; Zhang, W.; Gao, W.; Wang, l.; Ma, Z. A Rapid Low-Temperature Internal Heating Strategy with Optimal Frequency Based on Constant Polarization Voltage for Lithium-Ion Batteries. Appl. Energy 2016, 177, 771–782. [Google Scholar]
- Xiaokang, L.; Wang, Q.; Yang, Y.; Kang, J. Correlation between Capacity Loss and Measurable Parameters of Lithium-Ion Batteries. Int. J. Electr. Power Energy Syst. 2019, 110, 819–826. [Google Scholar]
- Cyril, M.; Fullenwarth, J.; Monconduit, L.; Lestriez, B. Diagnostic of the Failure Mechanism in Nisb2 Electrode for Li Battery through Analysis of Its Polarization on Galvanostatic Cycling. Electrochim. Acta 2012, 78, 177–182. [Google Scholar]
- Bizhong, X.; Chen, G.; Zhou, J.; Yang, Y.; Huang, R.; Wang, W.; Lai, Y.; Wang, M.; Wang, H. Online Parameter Identification and Joint Estimation of the State of Charge and the State of Health of Lithium-Ion Batteries Considering the Degree of Polarization. Energies 2019, 12, 2939. [Google Scholar]
- Caiping, Z.; Jiang, J.; Gao, Y.; Zhang, W.; Liu, Q.; Hu, X. Polarization Based Charging Time and Temperature Rise Optimization for Lithium-Ion Batteries. Energy Procedia 2016, 88, 675–681. [Google Scholar]
- Zhang, C.; Jiang, J.; Gao, Y.; Zhang, W.; Liu, Q.; Hu, X. Charging Optimization in Lithium-Ion Batteries Based on Temperature Rise and Charge Time. Appl. Energy 2017, 194, 569–577. [Google Scholar] [CrossRef]
- Rong, X.; Yang, Y.; Yin, F.; Liu, P.; Cloetens, P.; Liu, Y.; Lin, F.; Zhao, K. Heterogeneous Damage in Li-Ion Batteries: Experimental Analysis and Theoretical Modeling. J. Mech. Phys. Solids 2019, 129, 160–183. [Google Scholar]
- Hosang, P.; Yoon, T.; Kim, Y.; Ryu, J.H.; Oh, S.M. Li2nio2 as a Sacrificing Positive Additive for Lithium-Ion Batteries. Electrochim. Acta 2013, 108, 591–595. [Google Scholar]
- Moya, A.A.; Castilla, J.; Horno, J. Ionic Transport in Electrochemical Cells Including Electrical Double-Layer Effects. A Network Thermodynamics Approach. J. Phys. Chem. 1995, 99, 1292–1298. [Google Scholar] [CrossRef]
- Bo, Y.; Lim, C.; Song, Z.; Zhu, L. Analysis of Polarization in Realistic Li Ion Battery Electrode Microstructure Using Numerical Simulation. Electrochim. Acta 2015, 185, 125–141. [Google Scholar]
- Junfu, L.; Wang, L.; Lyu, C.; Liu, E.; Xing, Y.; Pecht, M. A Parameter Estimation Method for a Simplified Electrochemical Model for Li-Ion Batteries. Electrochim. Acta 2018, 275, 50–58. [Google Scholar]
- Emil, N.; Torregrossa, D.; Cherkaoui, R.; Paolone, M. Parameter Identification of a Lithium-Ion Cell Single-Particle Model through Non-Invasive Testing. J. Energy Storage 2017, 12, 138–148. [Google Scholar]
- Thanh-Son, D.; Vyasarayani, C.P.; McPhee, J. Simplification and Order Reduction of Lithium-Ion Battery Model Based on Porous-Electrode Theory. J. Power Sources 2012, 198, 329–337. [Google Scholar]
- Xiaowei, Z.; Cai, Y.; Yang, L.; Deng, Z.; Qiang, J. State of Charge Estimation Based on a New Dual-Polarization-Resistance Model for Electric Vehicles. Energy 2017, 135, 40–52. [Google Scholar]
- Henrik, E.; Fridholm, B.; Lindbergh, G. Comparison of Lumped Diffusion Models for Voltage Prediction of a Lithium-Ion Battery Cell During Dynamic Loads. J. Power Sources 2018, 402, 296–300. [Google Scholar]
- Andre, D.; Meiler, M.; Steiner, K.; Wimmer, C.; Soczka-Guth, T.; Sauer, D.U. Characterization of High-Power Lithium-Ion Batteries by Electrochemical Impedance Spectroscopy. I. Experimental Investigation. J. Power Sources 2011, 196, 5334–5341. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Electrochemical Impedance Study on the Low Temperature of Li-Ion Batteries. Electrochim. Acta 2004, 49, 1057–1061. [Google Scholar] [CrossRef]
- Moya, A.A. Identification of Characteristic Time Constants in the Initial Dynamic Response of Electric Double Layer Capacitors from High-Frequency Electrochemical Impedance. J. Power Sources 2018, 397, 124–133. [Google Scholar] [CrossRef]
- Xing, Z.; Huang, J.; Pan, Z.; Ouyang, M. Impedance Characterization of Lithium-Ion Batteries Aging under High-Temperature Cycling: Importance of Electrolyte-Phase Diffusion. J. Power Sources 2019, 426, 216–222. [Google Scholar]
- Santhanagopalan, S.; Guo, Q.; Ramadass, P.; White, R.E. White. Review of Models for Predicting the Cycling Performance of Lithium Ion Batteries. J. Power Sources 2006, 156, 620–628. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Srinivasan, V.; Wang, C.Y. Analysis of Electrochemical and Thermal Behavior of Li-Ion Cells. J. Electrochem. Soc. 2003, 150, A98. [Google Scholar] [CrossRef] [Green Version]
- Botte, G.G.; Subramanian, V.R.; White, R.E. White. Mathematical Modeling of Secondary Lithium Batteries. Electrochim. Acta 2000, 45, 2595–2609. [Google Scholar] [CrossRef]
- Haran, B.S.; Popov, B.N.; White, R.E. White. Determination of the Hydrogen Diffusion Coefficient in Metal Hydrides by Impedance Spectroscopy. J. Power Sources 1998, 75, 56–63. [Google Scholar] [CrossRef]
- Diwakar, V.D. Towards Efficient Models for Lithium Ion Batteries. Ph.D. Thesis, Tennessee Technological University, Ann Arbor, MI, USA, 2009. [Google Scholar]
- Shen, W.-J.; Li, H.-X. Parameter Identification for the Electrochemical Model of Li-Ion Battery. In Proceedings of the 2016 International Conference on System Science and Engineering (ICSSE), Puli, Taiwan, 7–9 July 2016. [Google Scholar]
- Kennedy, J.; Eberhart, R. Particle Swarm Optimization. In Proceedings of the ICNN’95—International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995. [Google Scholar]
- Levenberg, K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Lyshevski, S.E. Engineering and Scientific Computations Using Matlab; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bizhong, X.; Huang, R.; Lao, Z.; Zhang, R.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. Online Parameter Identification of Lithium-Ion Batteries Using a Novel Multiple Forgetting Factor Recursive Least Square Algorithm. Energies 2018, 11, 11. [Google Scholar]
- Myounggu, P.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A Review of Conduction Phenomena in Li-Ion Batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar]



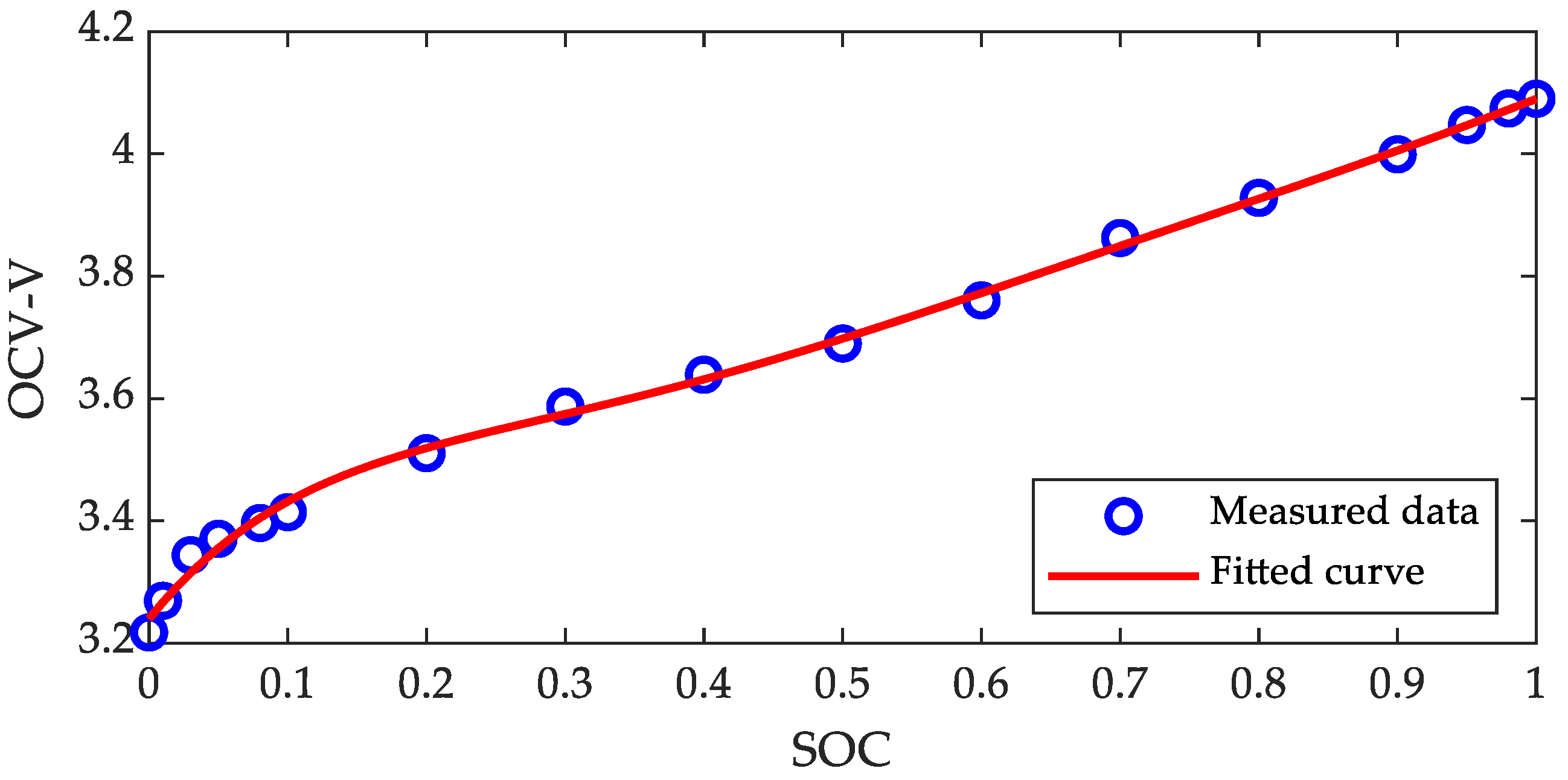


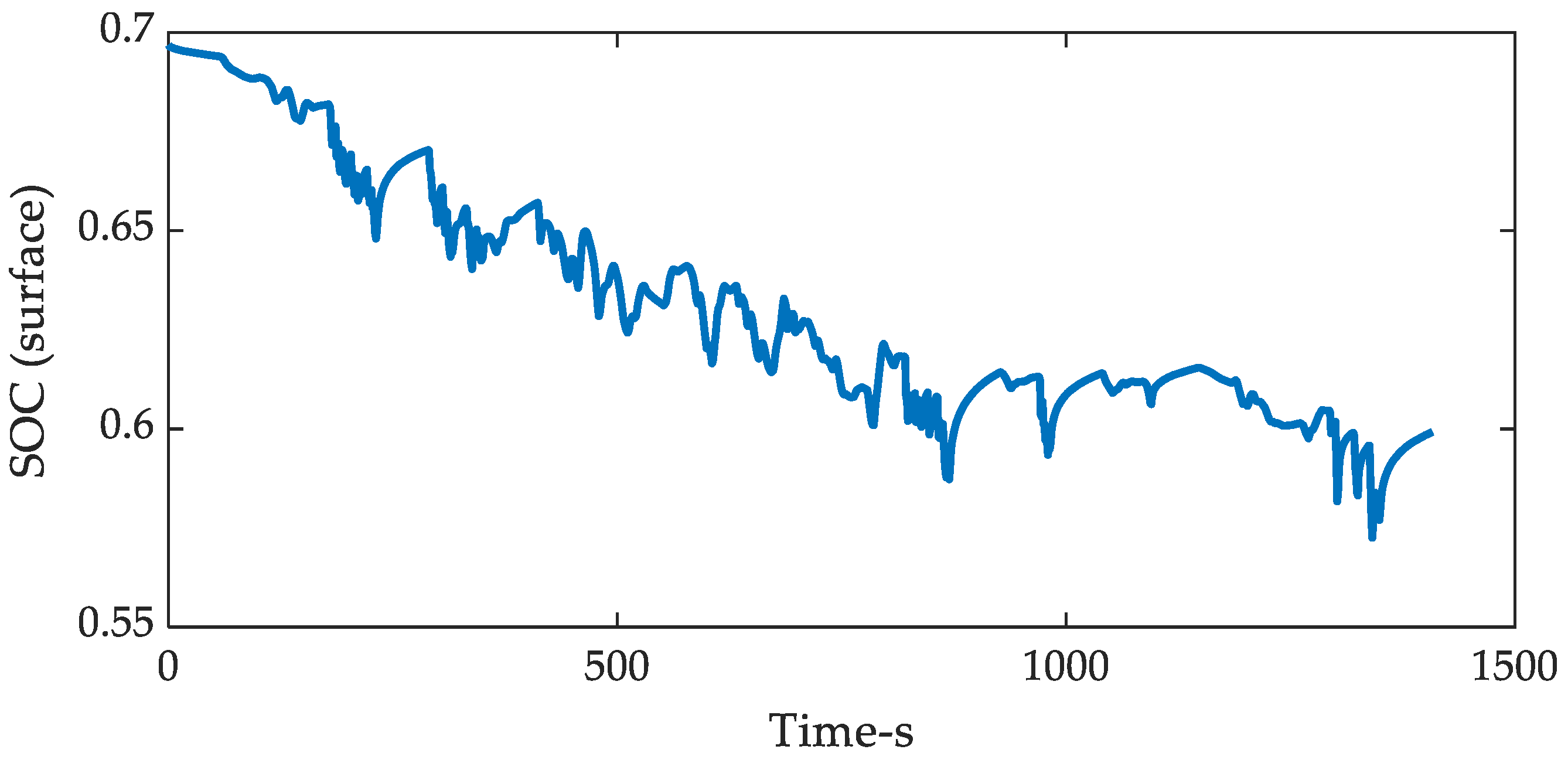
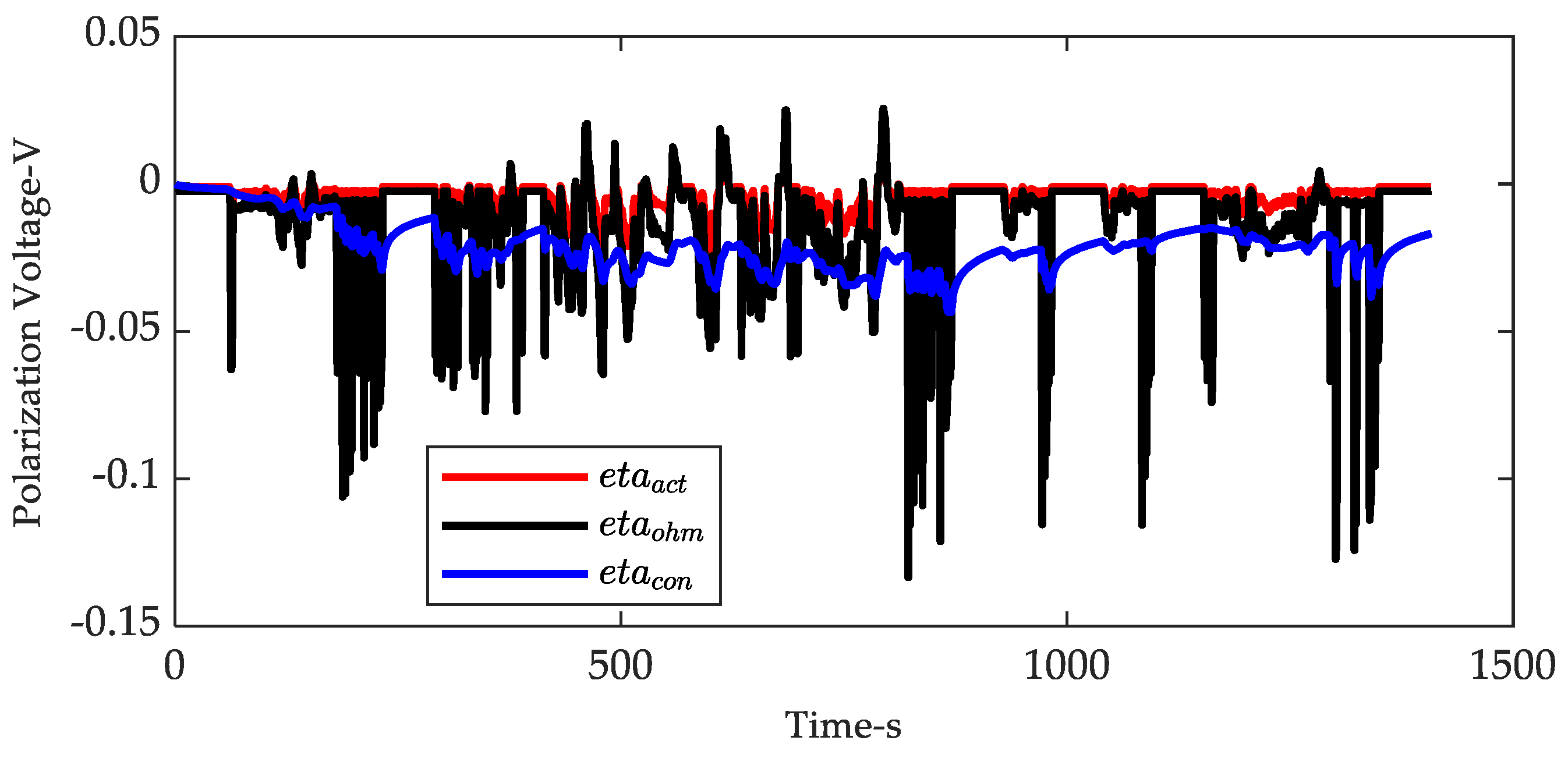
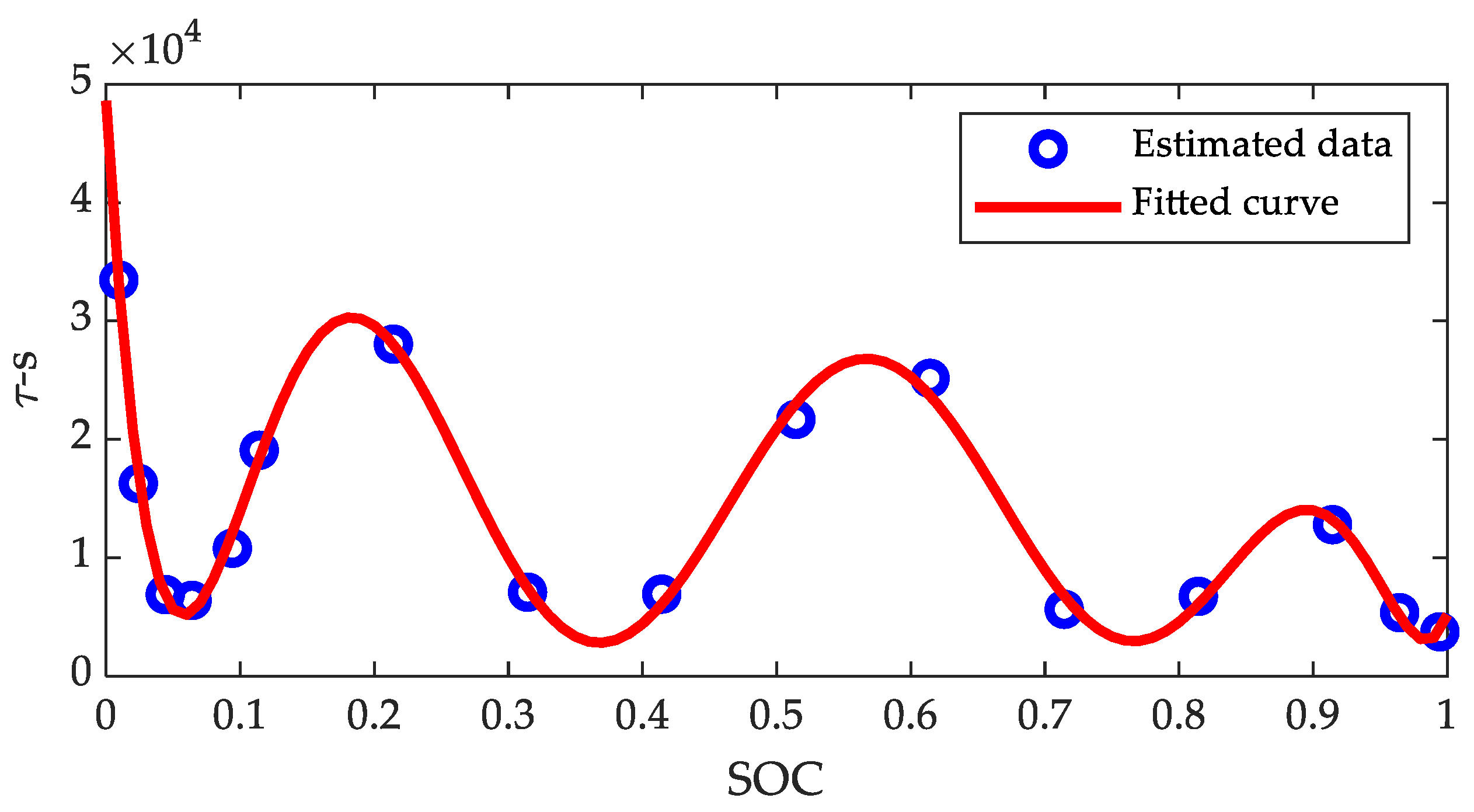
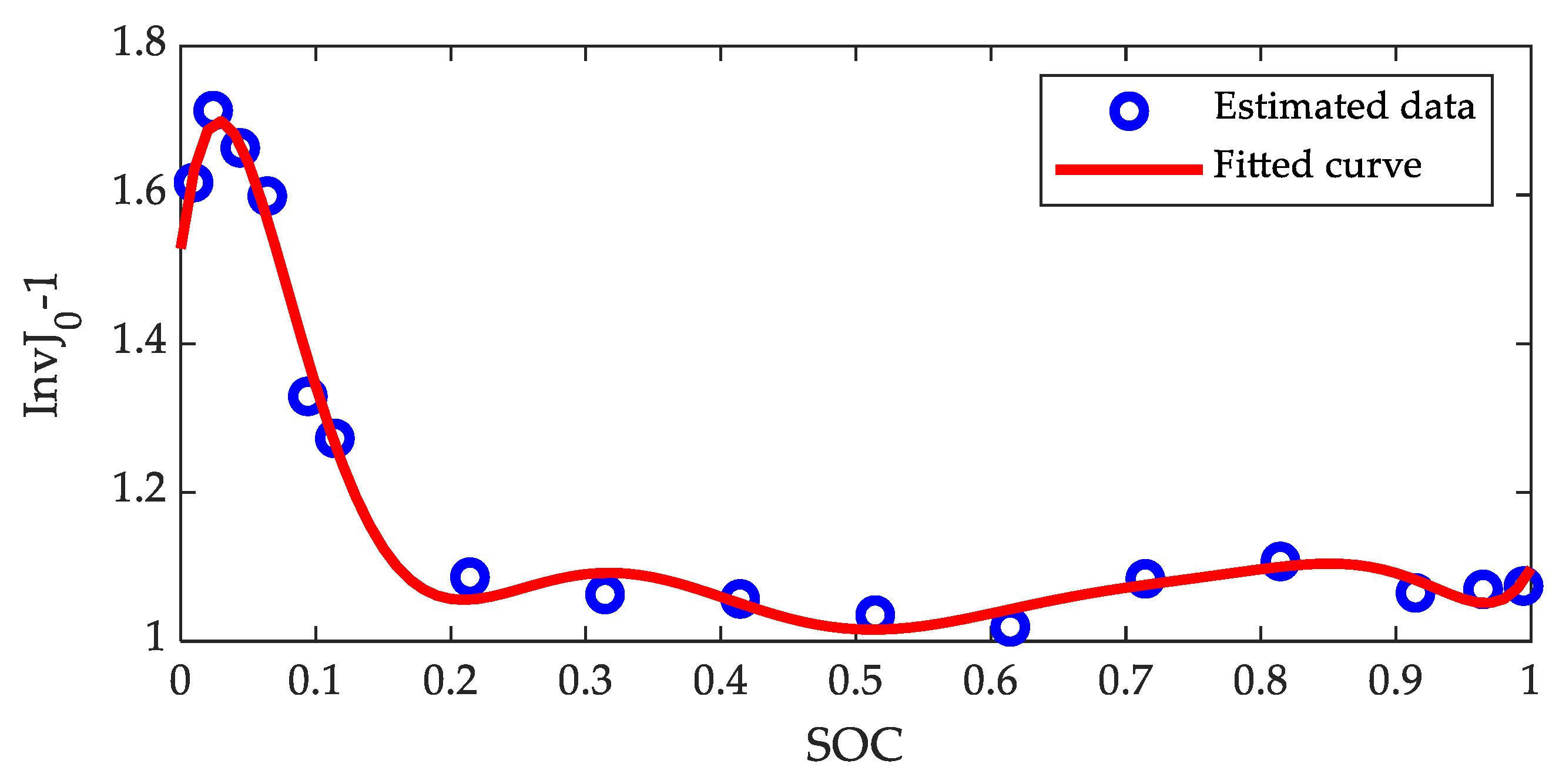

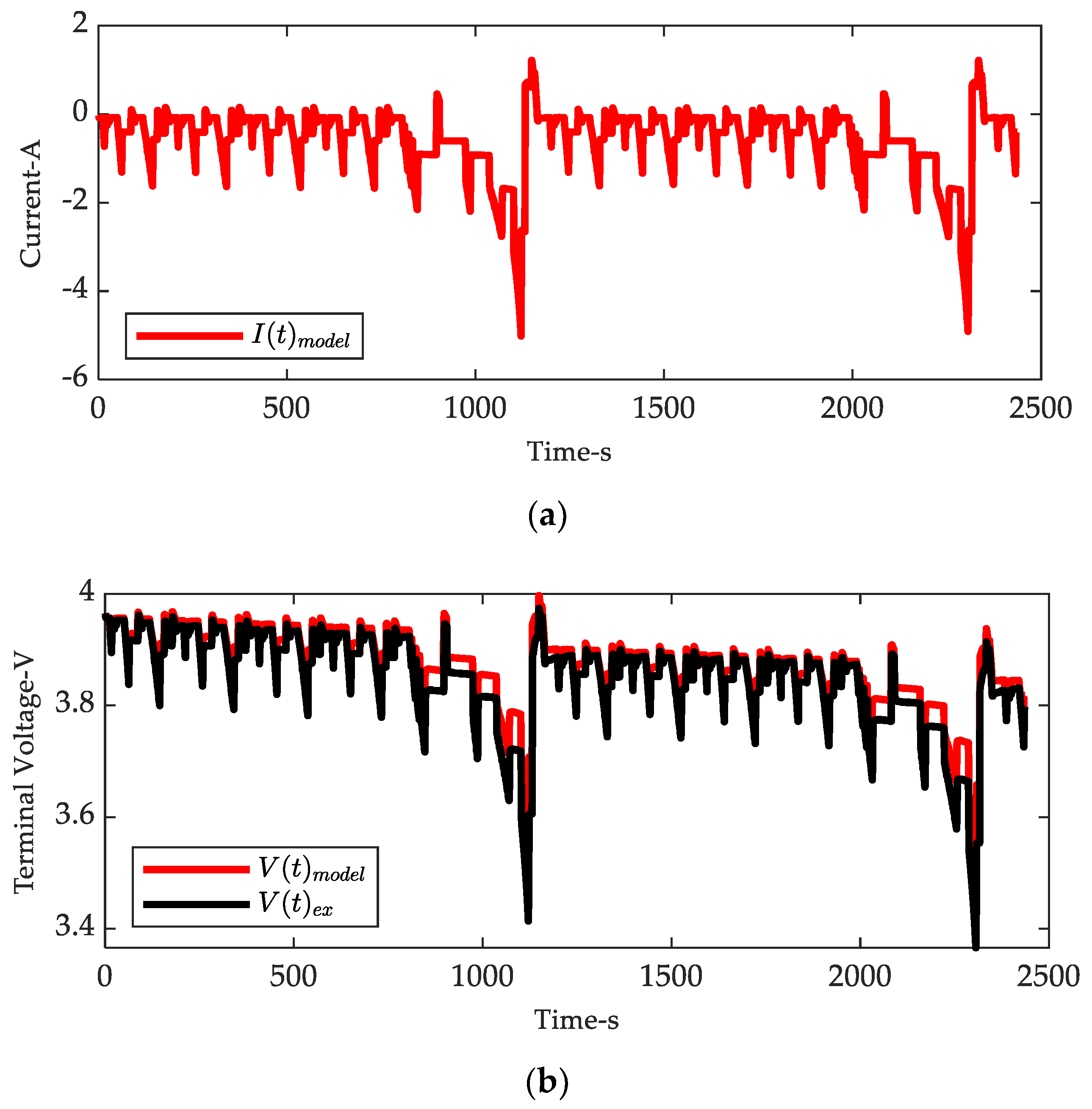
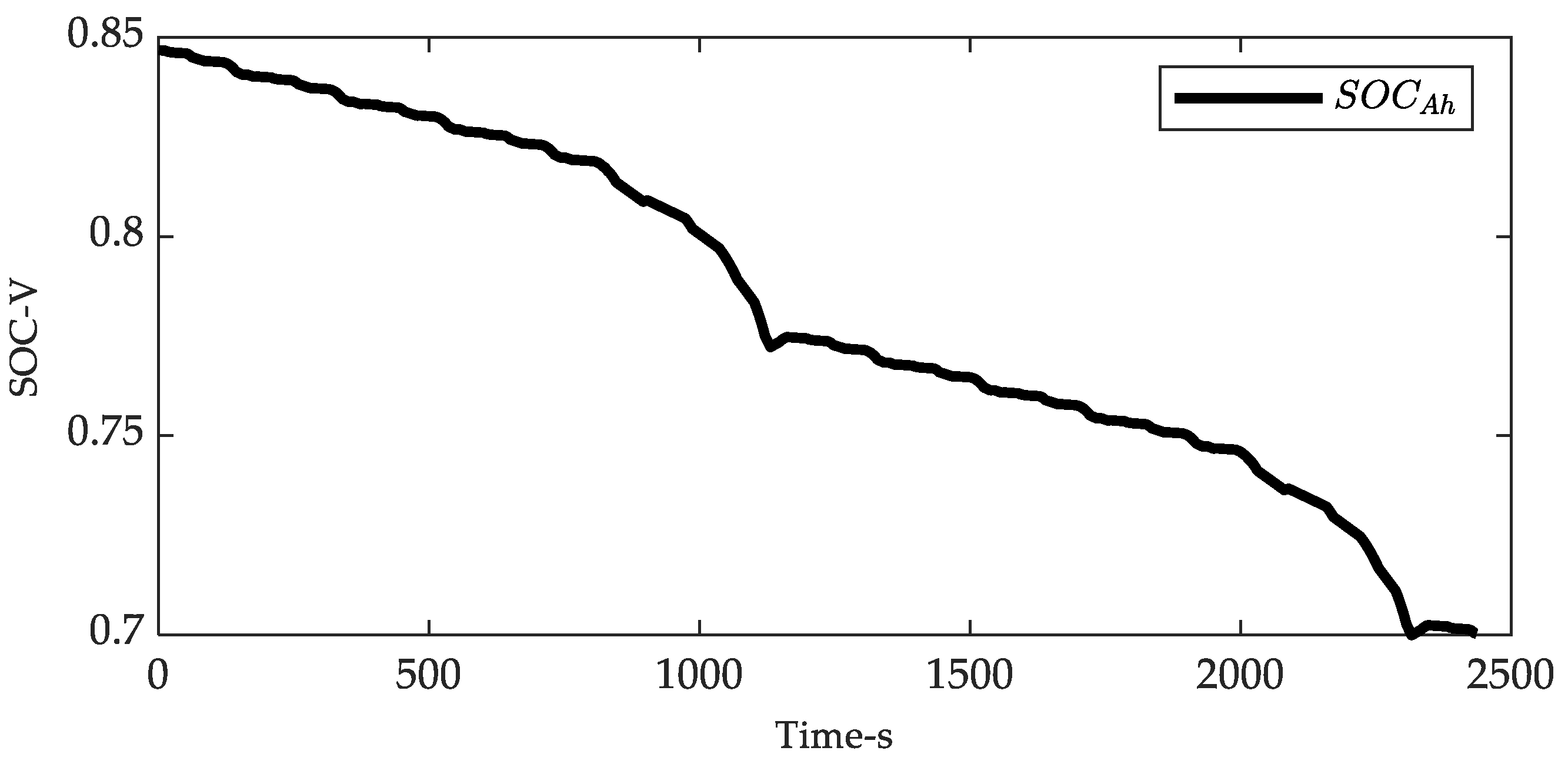
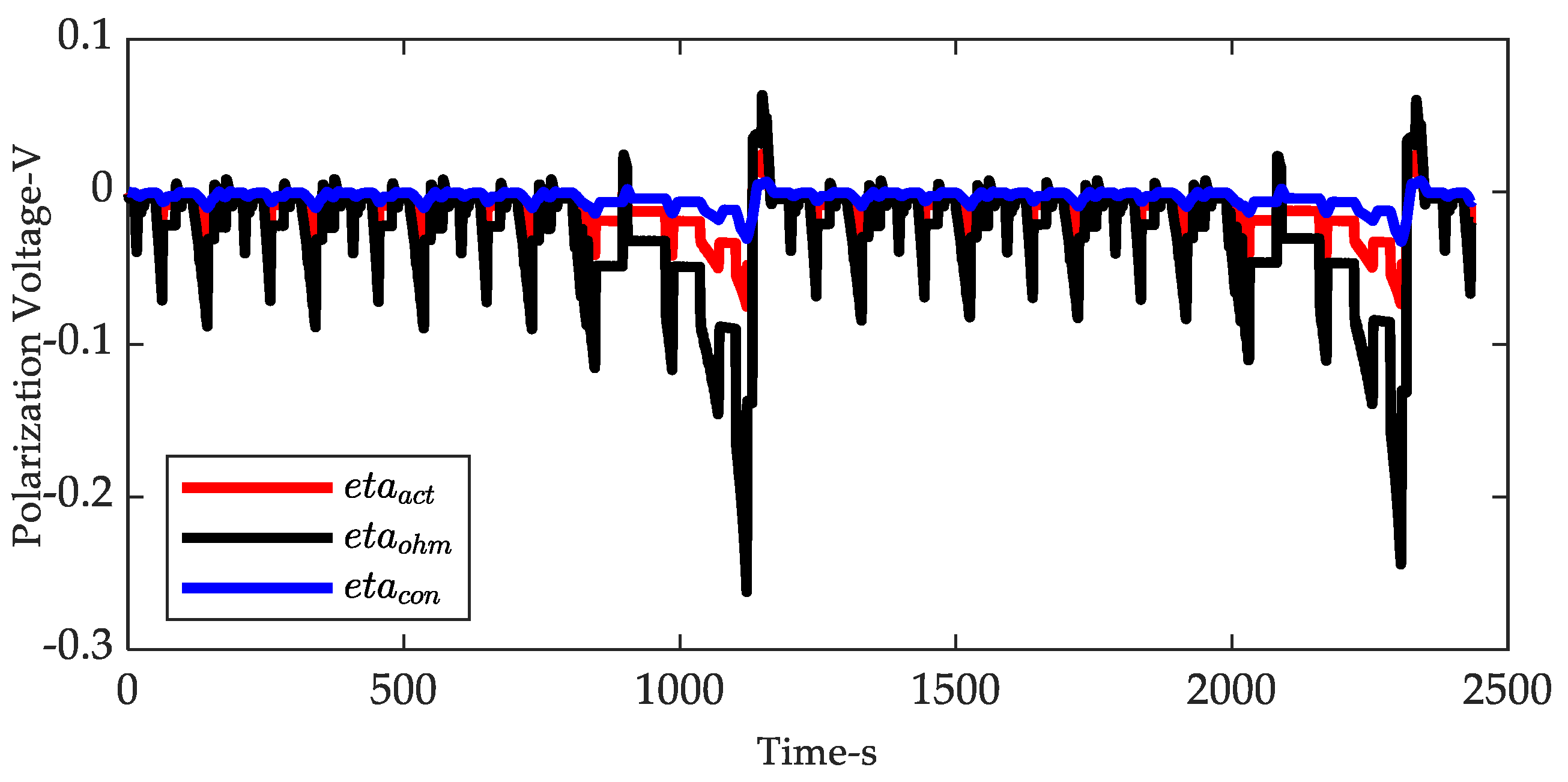
| Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Values | −5.0010 | 20.8142 | −34.1273 | 27.8136 | −11.4670 | 2.8176 | 3.2400 |
| Algorithm | (Dimensionless) | /mV | |
|---|---|---|---|
| PSO Algorithm | 17,447 | 2.6007 | 49.279 |
| L-M Method | 10,163 | 1.0765 | 69.642 |
| Joint Estimation Algorithm | 10,034 | 1.1412 | 69.62 |
| Algorithm | PSO Algorithm | L-M Method | Joint Estimation Algorithm |
|---|---|---|---|
| Mean Voltage Error/V | 0.0128780611 | 0.0104785487 | 0.0017621340 |
| Voltage RMSE/V | 0.0145556022 | 0.0120200028 | 0.0033290570 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.; Ye, B.; Cao, J. Polarization Voltage Characterization of Lithium-Ion Batteries Based on a Lumped Diffusion Model and Joint Parameter Estimation Algorithm. Energies 2022, 15, 1150. https://doi.org/10.3390/en15031150
Xia B, Ye B, Cao J. Polarization Voltage Characterization of Lithium-Ion Batteries Based on a Lumped Diffusion Model and Joint Parameter Estimation Algorithm. Energies. 2022; 15(3):1150. https://doi.org/10.3390/en15031150
Chicago/Turabian StyleXia, Bizhong, Bo Ye, and Jianwen Cao. 2022. "Polarization Voltage Characterization of Lithium-Ion Batteries Based on a Lumped Diffusion Model and Joint Parameter Estimation Algorithm" Energies 15, no. 3: 1150. https://doi.org/10.3390/en15031150







