Improvement in the Electrochemical Properties of Lithium Metal by Heat Treatment: Changes in the Chemical Composition of Native and Solid Electrolyte Interphase Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Lithium Metal at Various Heat-Treatment Temperatures and for Surface Analysis
2.2. Electrochemical Measurements
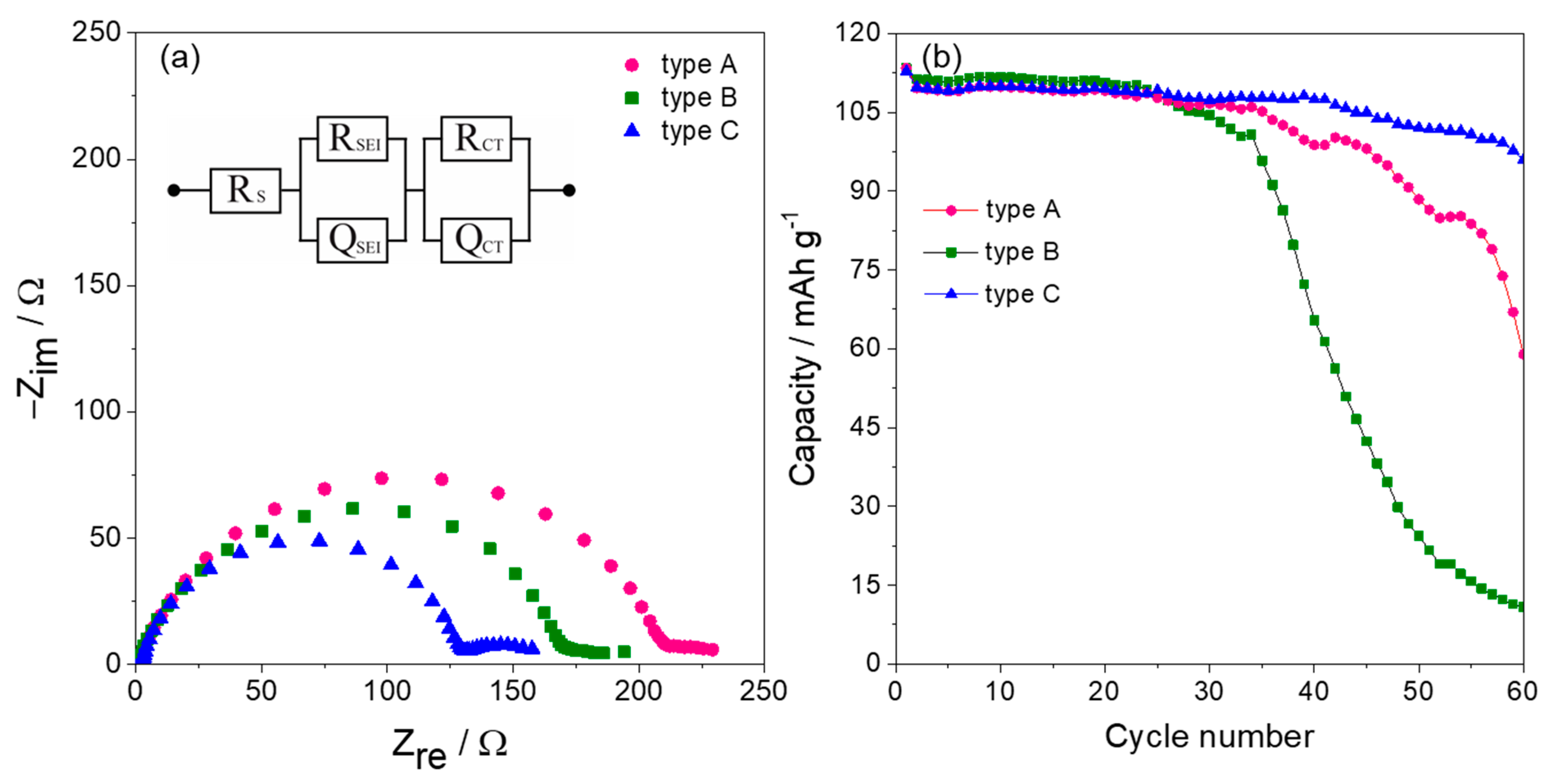
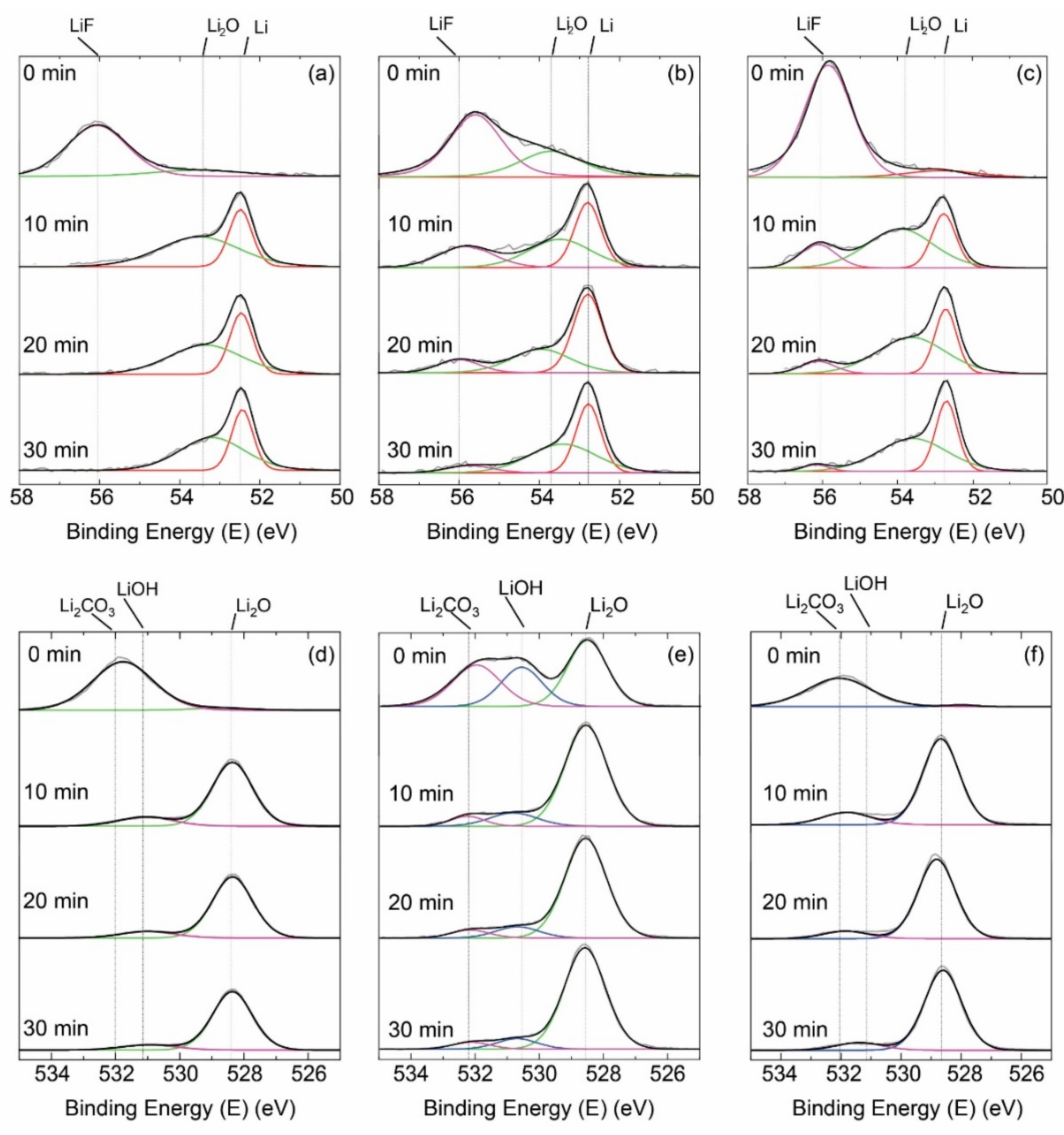
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, S.; Kim, J.H.; Yamada, Y.; Munakata, H.; Lee, Y.S.; Kim, S.S.; Kanamura, K. Improvement of rate capability by graphite foam anode for Li secondary batteries. J. Power Sources 2017, 355, 164–170. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Kanamura, K.; Shiraishi, S.; Takehara, Z.I. Electrochemical deposition of lithium metal in nonaqueous electrolyte containing (C2H5)4NF(HF)4 additive. J. Fluor. Chem. 1998, 87, 235–243. [Google Scholar] [CrossRef]
- Shin, K.H.; Jung, K.N.; Yoon, S.K.; Yeon, S.H.; Shim, J.M.; Joen, J.D.; Jeong, S.-K. Effects of electrolyte concentration on growth of dendritic zinc in aqueous solutions. Trans. Korean Hydrogen New Energy Soc. 2012, 23, 390–396. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Lopez, C.M.; Vaughey, J.T.; Dees, D.W. Morphological transitions on lithium metal anodes. J. Electrochem. Soc. 2009, 156, A726–A729. [Google Scholar] [CrossRef]
- Harry, K.J.; Hallinan, D.T.; Parkinson, D.Y.; MacDowell, A.A.; Balsara, N.P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 2014, 13, 69–73. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems-The solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Hao, F.; Verma, A.; Mukherjee, P. Mechanistic insight into dendrite–SEI interactions for lithium metal electrodes. J. Mater. Chem. A 2018, 6, 19664–19671. [Google Scholar] [CrossRef]
- Bieker, B.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.-K.; Seo, H.-Y.; Kim, D.-H.; Han, H.-K.; Kim, J.-G.; Lee, Y.B.; Iriyama, Y.; Abe, T.; Ogumi, Z. Suppression of dendritic lithium formation by using concentrated electrolyte solutions. Electrochem. Commun. 2008, 10, 635–638. [Google Scholar] [CrossRef]
- Qien, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-B.; Zhang, R.; Chao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Z. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Gmitter, A.J.; Plitz, I.; Amatucci, G.G. High concentration dinitrile, 3-alkoxypropionitrile, and linear carbonate electrolytes enabled by vinylene and monofluoroethylene carbonate additives. J. Electrochem. Soc. 2012, 159, A370–A379. [Google Scholar] [CrossRef]
- Hayashi, K.; Nemoto, Y.; Tobishima, S.I.; Yamaki, J.I. Mixed solvent electrolyte for high voltage lithium metal secondary cells. Electrochim. Acta 1999, 44, 2337–2344. [Google Scholar] [CrossRef]
- Liu, Q.C.; Xu, J.J.; Yuan, S.; Chang, Z.W.; Xu, D.; Yin, Y.B.; Zhang, X.B. Artificial protection film on lithium metal anode toward long-cycle-life lithium–oxygen batteries. Adv. Mater. 2015, 27, 5241–5247. [Google Scholar] [CrossRef]
- Kanamura, K.; Tamura, H.; Shiraishi, S.; Takehara, Z.-I. XPS analysis of lithium surfaces following immersion in various solvents containing LiBF4. J. Electrochem. Soc. 1995, 142, 340–347. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kanamura, K.; Takehara, Z.-I. Influence of initial surface condition of lithium metal anodes on surface modification with HF. J. Appl. Electrochem. 1999, 29, 867–879. [Google Scholar] [CrossRef]
- Kanamura, K.; Tamura, H.; Shiraishi, S.; Takehara, Z.-I. XPS analysis for the lithium surface immersed in γ-butyrolactone containing various salts. Electrochim. Acta 1995, 40, 913–921. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kanamura, K.; Takehara, Z.-I. Study of the surface composition of highly smooth lithium deposited in various carbonate electrolytes containing HF. Langmuir 1997, 13, 3542–3549. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kanamura, K.; Takehara, Z.-I. Surface condition changes in lithium metal deposited in nonaqueous electrolyte containing HF by dissolution-deposition cycles. J. Electrochem. Soc. 1999, 146, 1633–1639. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kanamura, K.; Takehara, Z. Effect of surface modification using various acids on electrodeposition of lithium. J. Appl. Electrochem. 1995, 25, 584–591. [Google Scholar] [CrossRef]
- Wu, B.; Lochala, J.; Taverne, T.; Xiao, J. The interplay between solid electrolyte interface (SEI) and dendritic lithium growth. Nano Energy 2017, 40, 34–41. [Google Scholar] [CrossRef]
- Conder, J.; Villevieille, C.; Trabesinger, S.; Novák, P.; Gubler, L.; Bouchet, R. Electrochemical impedance spectroscopy of a Li–S battery: Part 2. Influence of separator chemistry on the lithium electrode/electrolyte interface. Electrochim. Acta 2017, 255, 379–390. [Google Scholar] [CrossRef]
- Iermakova, D.I.; Dugas, R.; Palacín, M.R.; Ponrouch, A. On the comparative stability of Li and Na metal anode interfaces in conventional alkyl carbonate electrolytes. J. Electrochem. Soc. 2015, 162, A7060–A7066. [Google Scholar] [CrossRef]
- Kanamura, K.; Shiraishi, S.; Takehara, Z. Electrochemical deposition of very smooth lithium using nonaqueous electrolytes containing HF. J. Electrochem. Soc. 1996, 143, 2187–2197. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales, P.M.; Song, H.-Y.; Jo, M.-H.; Jeong, S.-K. Improvement in the Electrochemical Properties of Lithium Metal by Heat Treatment: Changes in the Chemical Composition of Native and Solid Electrolyte Interphase Films. Energies 2022, 15, 1419. https://doi.org/10.3390/en15041419
Nogales PM, Song H-Y, Jo M-H, Jeong S-K. Improvement in the Electrochemical Properties of Lithium Metal by Heat Treatment: Changes in the Chemical Composition of Native and Solid Electrolyte Interphase Films. Energies. 2022; 15(4):1419. https://doi.org/10.3390/en15041419
Chicago/Turabian StyleNogales, Paul Maldonado, Hee-Youb Song, Mun-Hui Jo, and Soon-Ki Jeong. 2022. "Improvement in the Electrochemical Properties of Lithium Metal by Heat Treatment: Changes in the Chemical Composition of Native and Solid Electrolyte Interphase Films" Energies 15, no. 4: 1419. https://doi.org/10.3390/en15041419






