Symmetrical Composite Supercapacitor Based on Activated Carbon and Cobalt Nanoparticles with High Cyclic Stability and Current Load
Abstract
:1. Introduction
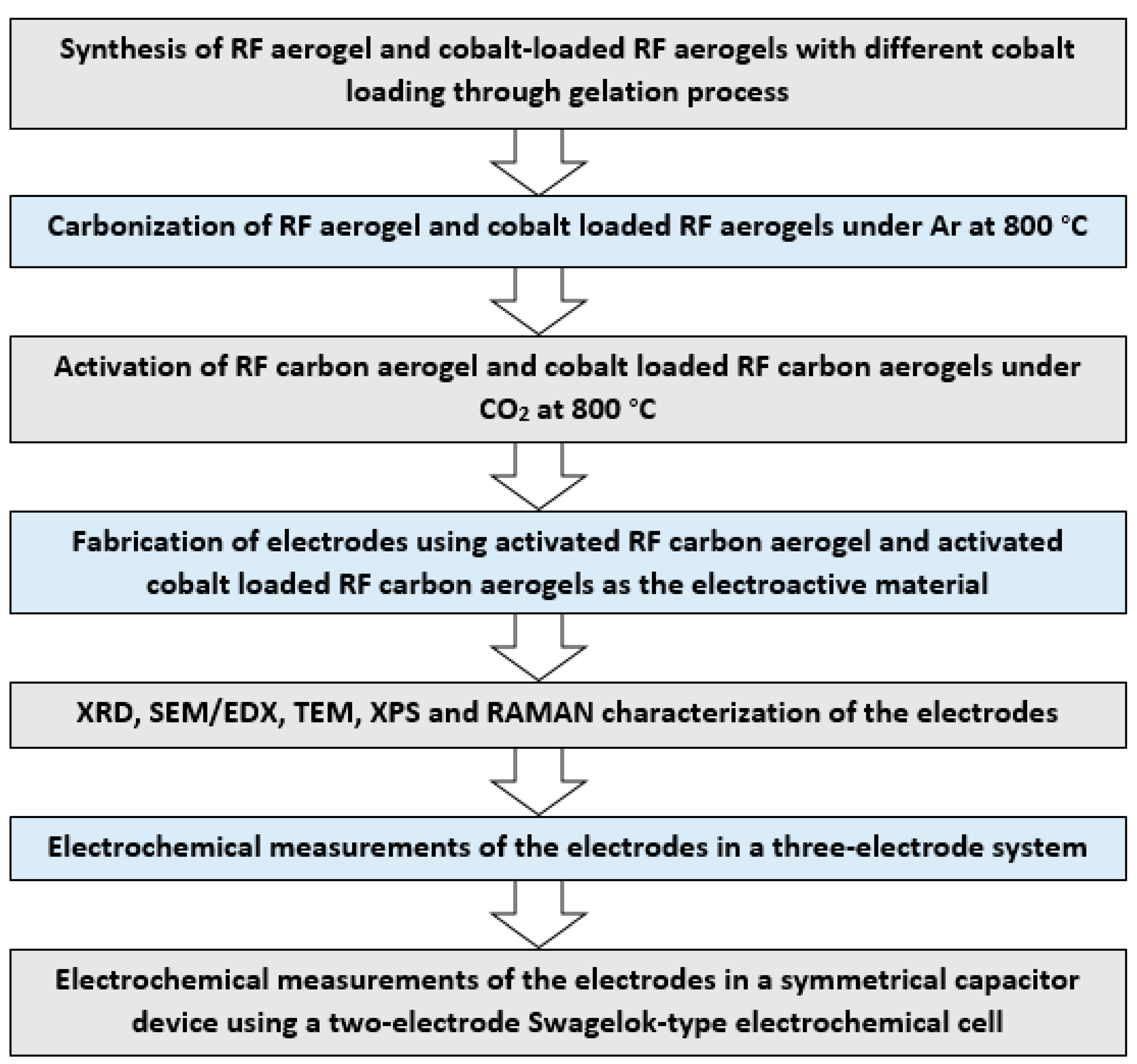
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Activated Carbon–Cobalt Composite (Co@AC)
2.3. Preparation of the Co@AC Electrode
2.4. Characterization
2.5. Electrochemical Measurements
3. Results
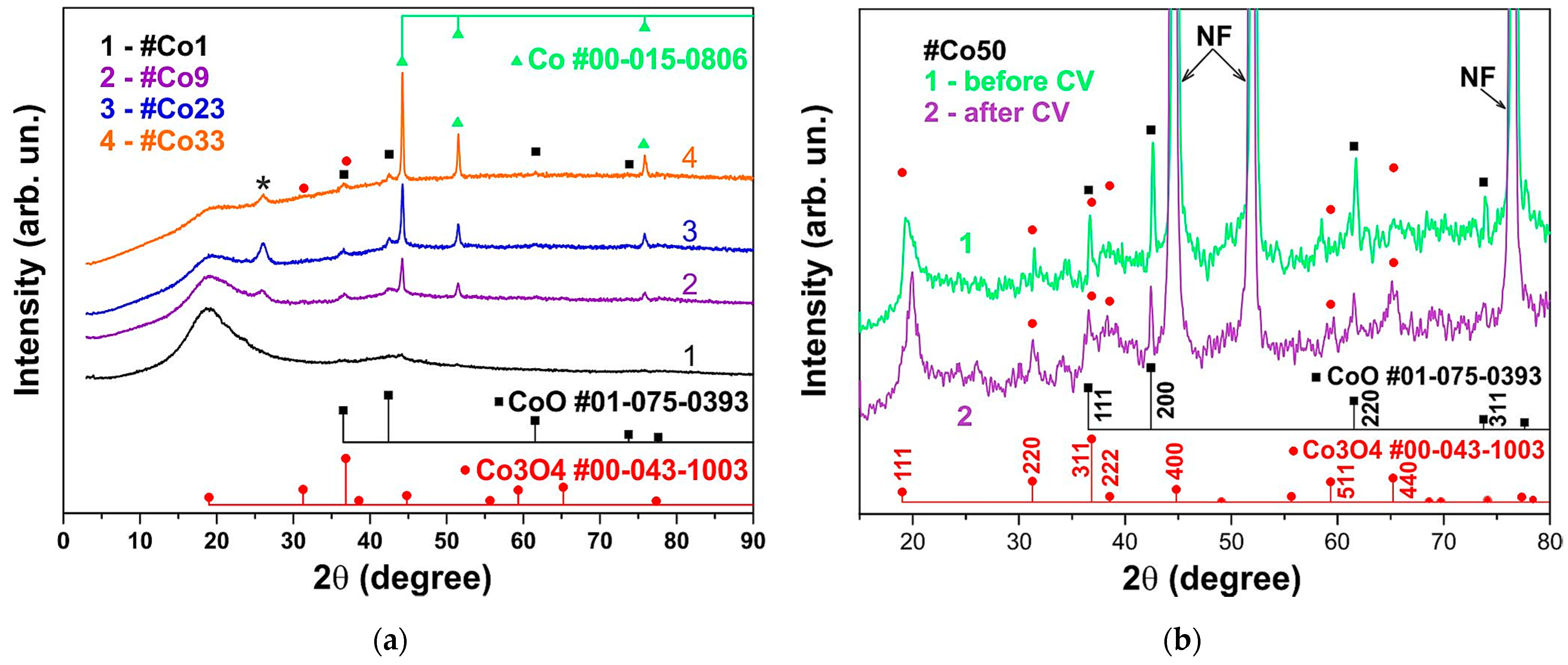
3.1. XRD Analysis of the Co@AC Composite
3.2. Surface Morphology Study
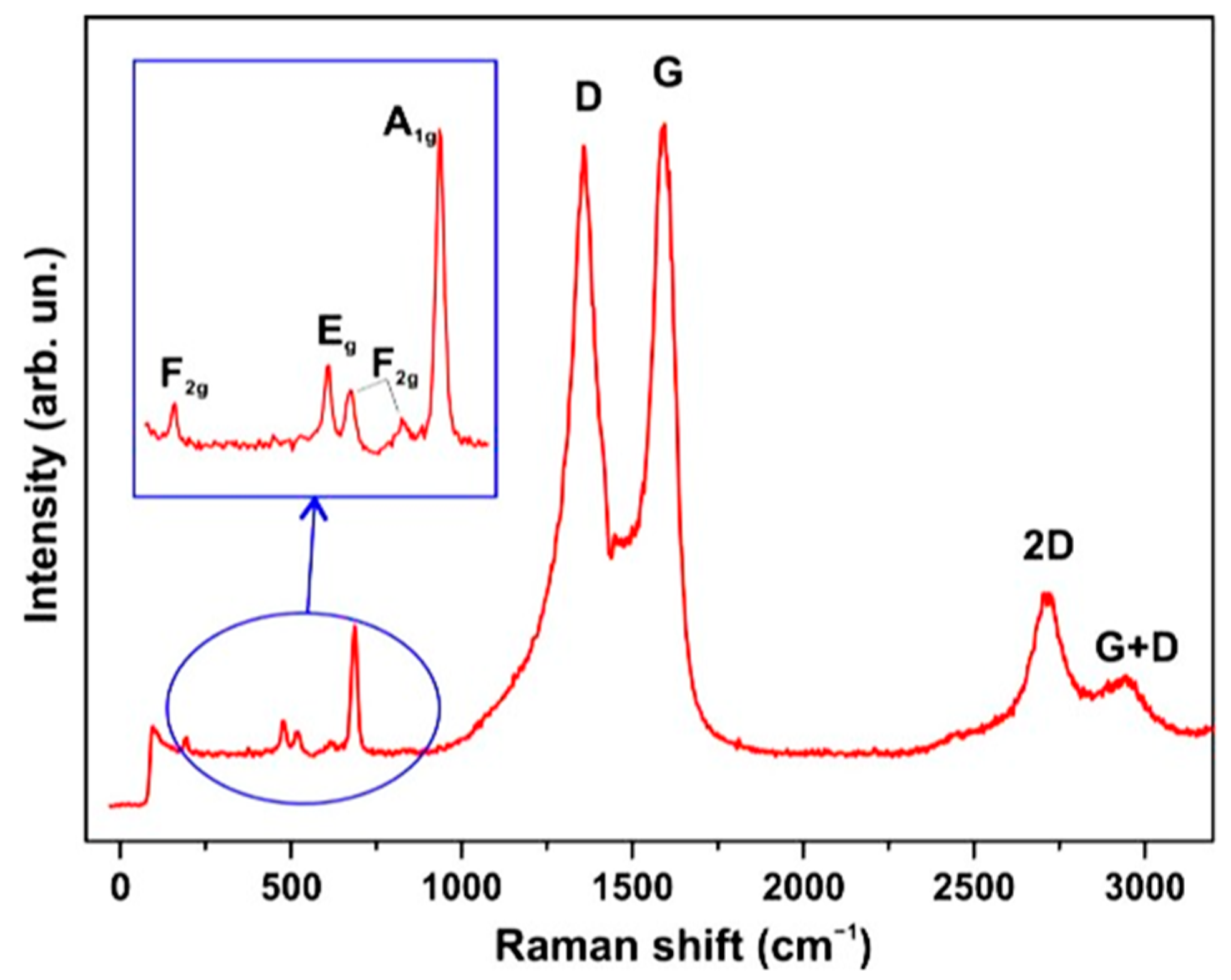
3.3. Raman Spectra
3.4. Chemical Characterization
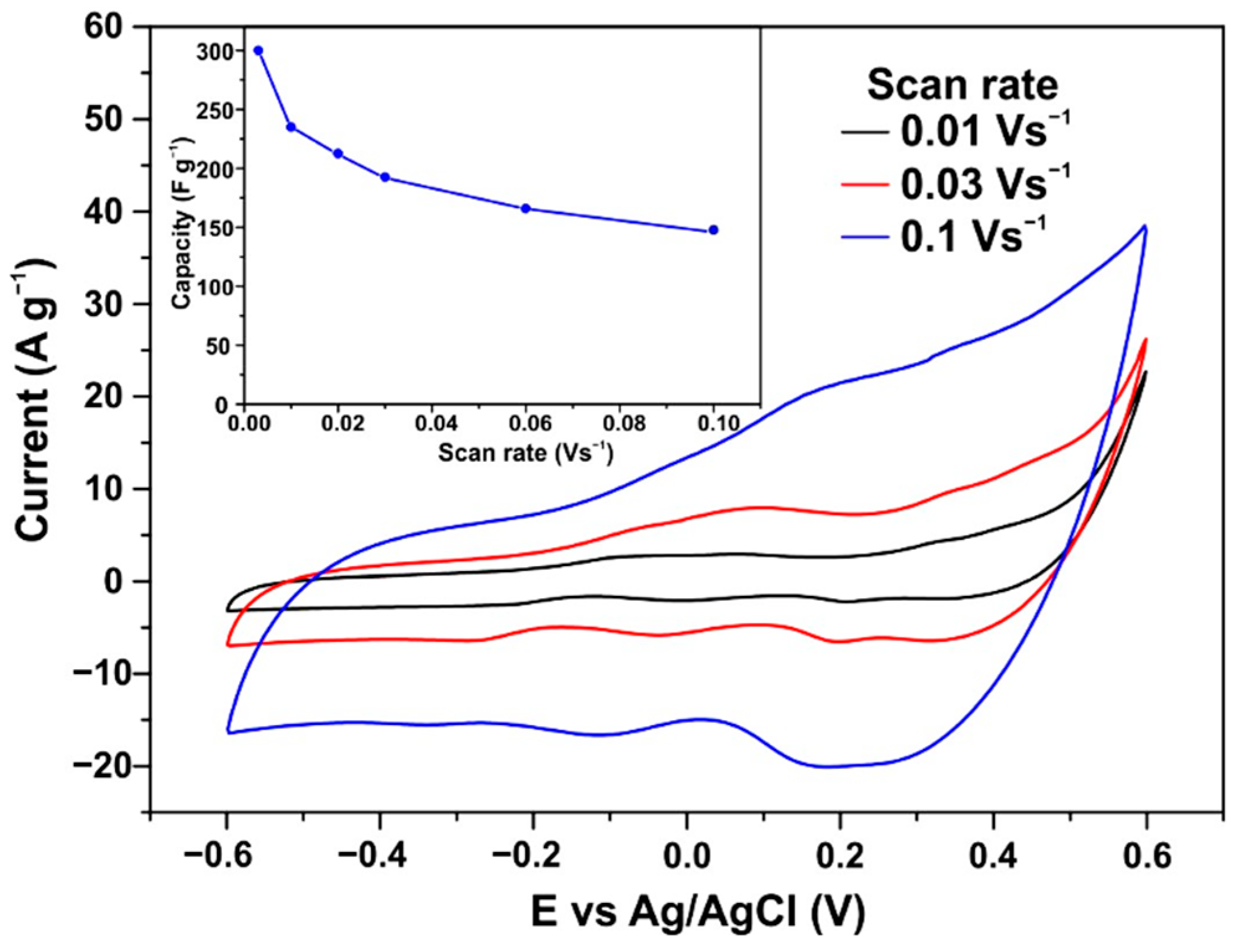
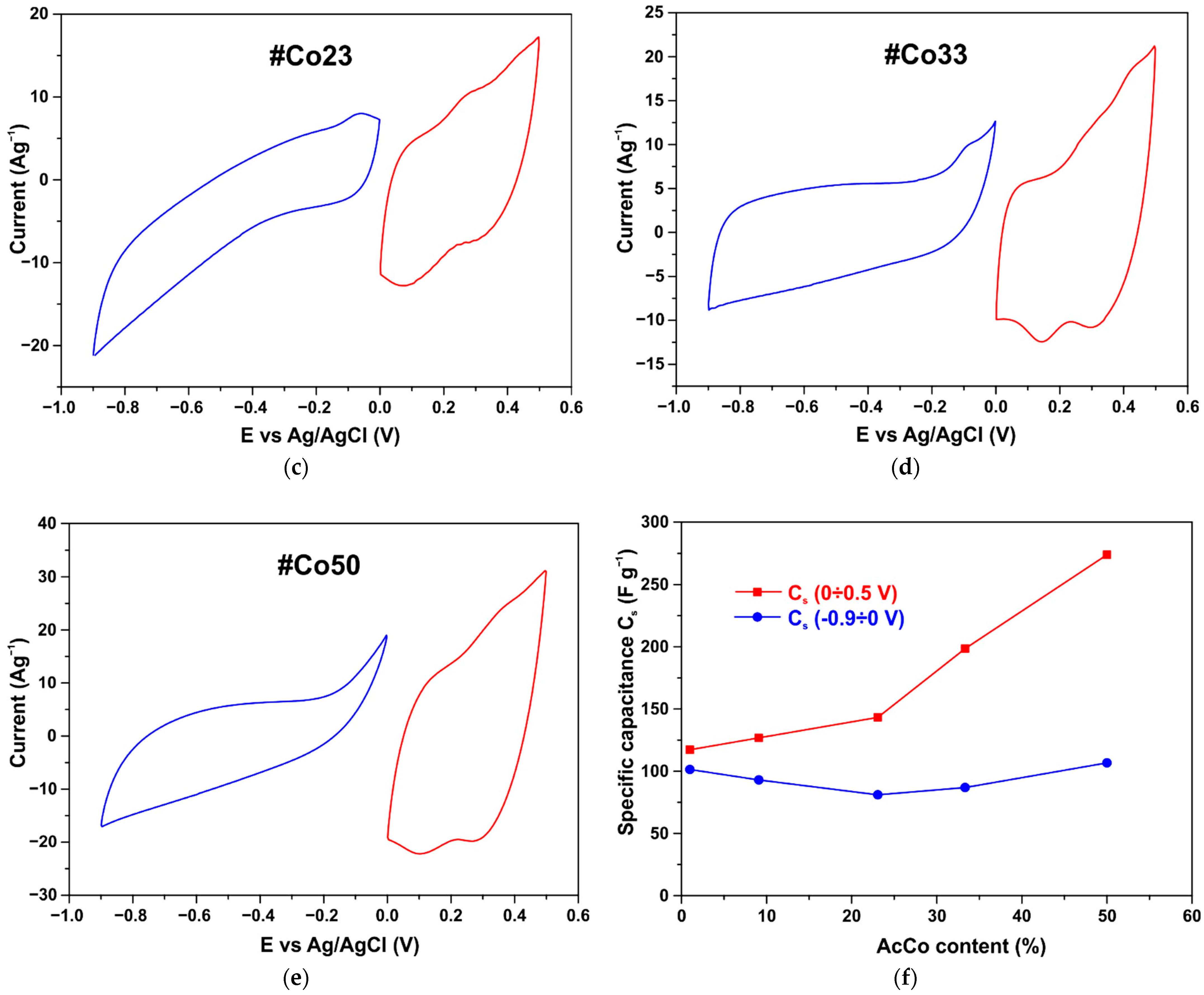
3.5. CV Measurements
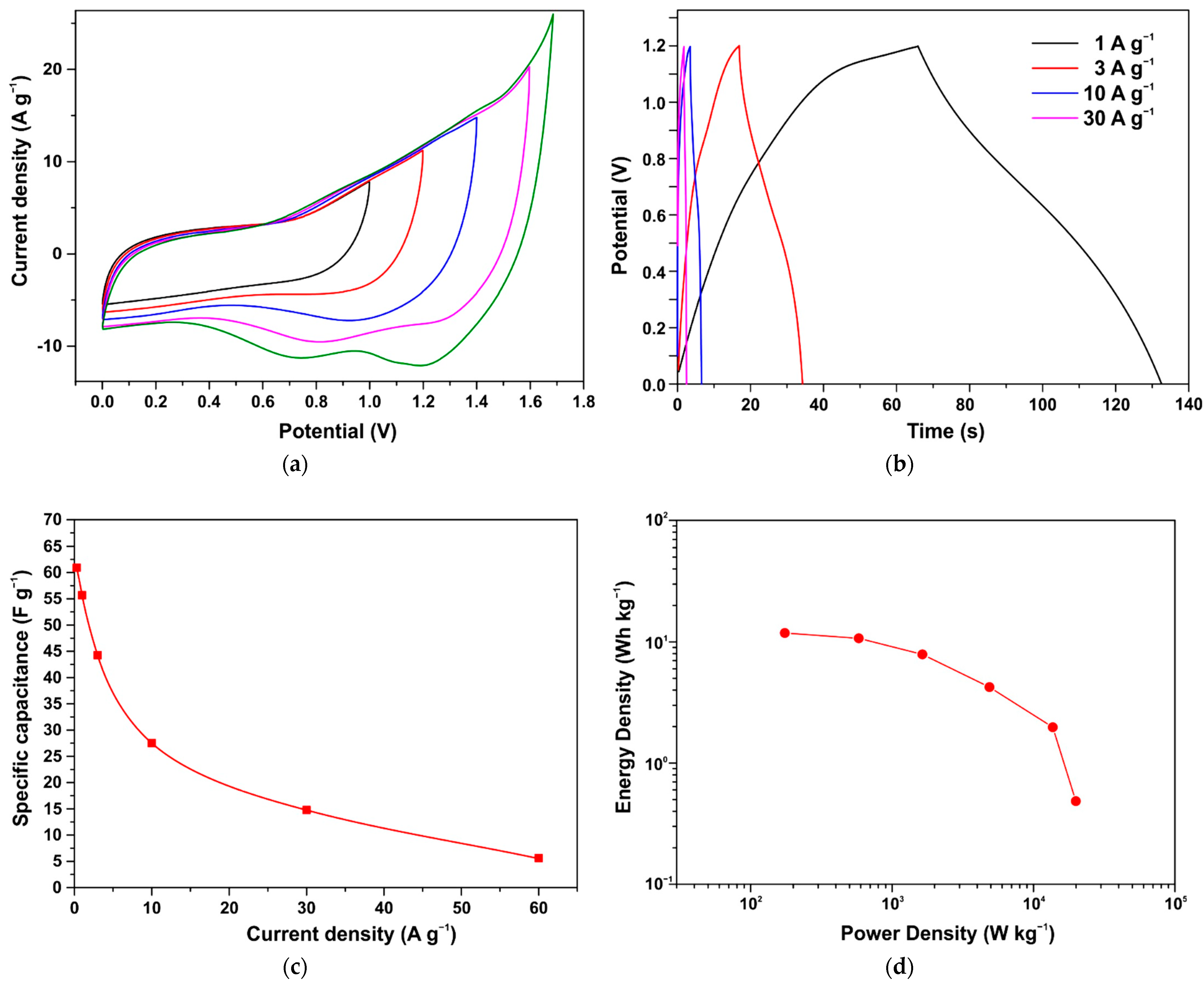
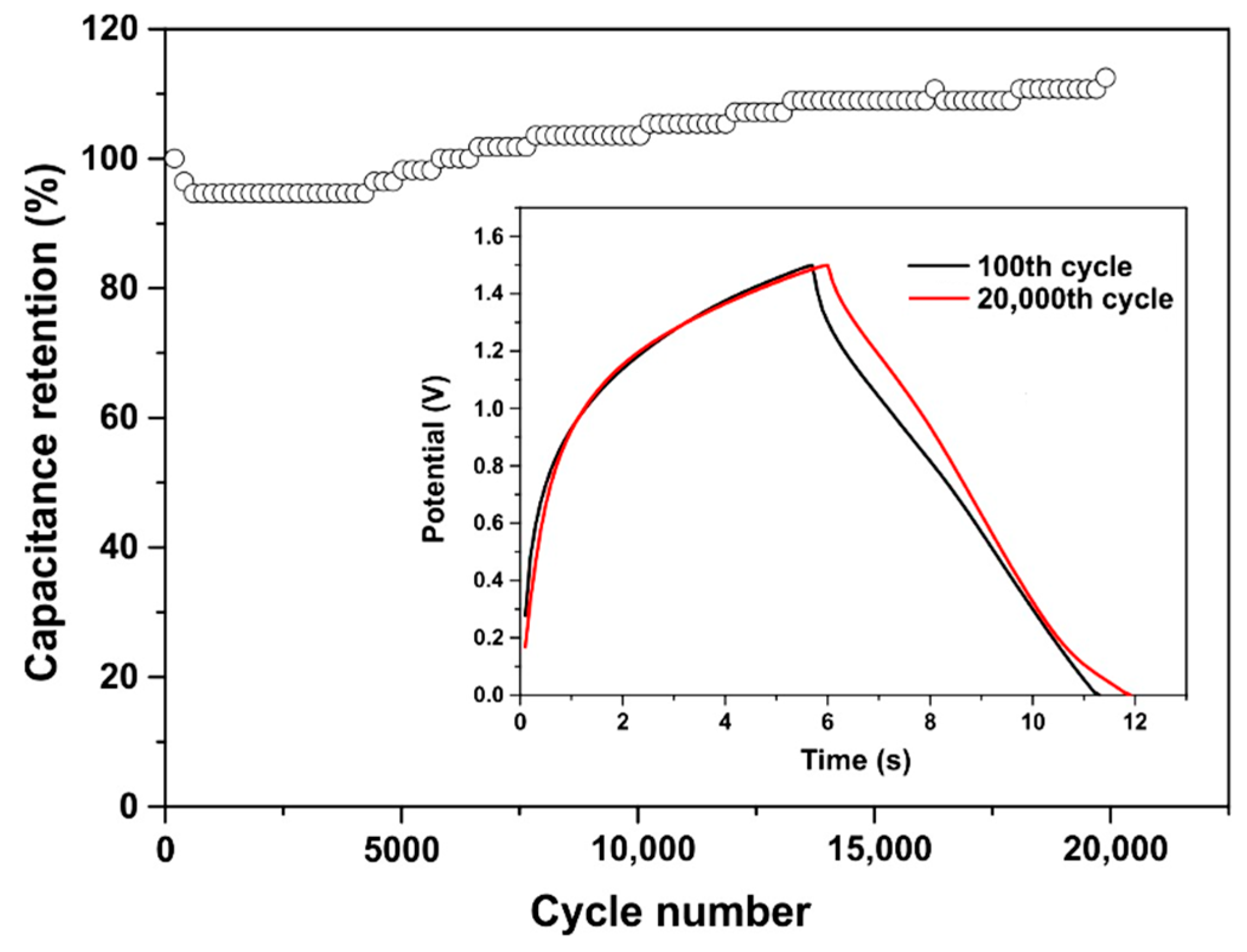
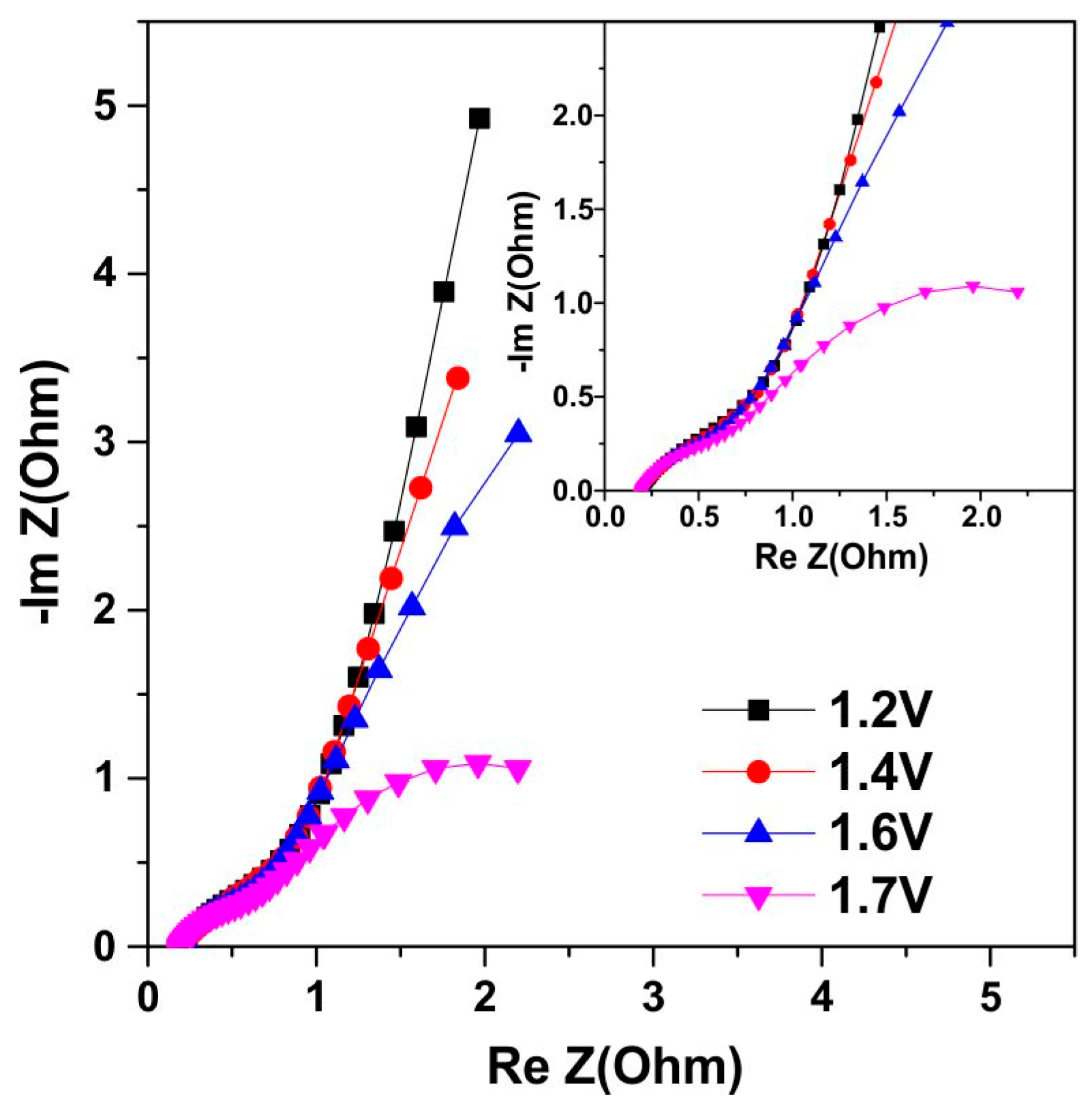
3.6. Symmetrical Capacitor Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- He, X.; Zhang, X. A comprehensive review of supercapacitors: Properties, electrodes, electrolytes and thermal management systems based on phase change materials. J. Energy Storage 2022, 56, 106023. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. Carbon dioxide sequestration in coal: Implications for CO2 disposal and CH4 displacement from coal seams. Geo-Environment and Landscape Evolution II. WIT Trans. Ecol. Environ. 2006, 89, 151–160. [Google Scholar]
- Hossain, E.; Resalat Faruque, H.M.; Haque Sunny, M.S.; Mohammad, N.; Nawar, N. A Comprehensive Review on Energy Storage Systems: Types, Comparison, Current Scenario, Applications, Barriers, and Potential Solutions, Policies, and Future Prospects. Energies 2020, 13, 3651. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium-Ion Batteries with High Rate Capabilities. ACS Sustain. Chem. Eng. 2017, 5, 2799–2816. [Google Scholar] [CrossRef]
- Tian, R.; Park, S.H.; King, P.J.; Cunningham, G.; Coelho, J.; Nicolosi, V.; Coleman, J.N. Quantifying the factors limiting rate performance in battery electrodes. Nat. Commun. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Ke, J.; Zhang, Y.; Wen, Z.; Huang, S.; Ye, M.; Tang, Y.; Liu, X.; Li, C.C. Designing strategies of advanced electrode materials for high-rate rechargeable batteries. J. Mater. Chem. A 2023, 11, 4428–4457. [Google Scholar] [CrossRef]
- Yu, H.; Xu, J.; Deng, C.; Xia, M.; Zhang, X.; Shu, J.; Wang, Z. The Nature of the Ultrahigh Initial Coulombic Efficiency of Ni2Fe(CN)6 in Aqueous Ammonium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 9594–9599. [Google Scholar] [CrossRef]
- Yu, H.; Deng, C.; Yan, H.; Xia, M.; Zhang, X.; Wang, Z.B.; Shu, J. Cu3(PO4)2: Novel Anion Convertor for Aqueous Dual-Ion Battery. Nanomicro Lett. 2021, 13, 41. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Xu, C.; Li, J.; Yang, Z.; Yan, H.; Yu, H.; Yan, L.; Zhang, L.; Shu, J. Aqueous non-metallic ion batteries: Materials, mechanisms and design strategies. Coord. Chem. Rev. 2023, 474, 214867. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, V.; Campagna, N.; Di Tommaso, A.O.; Miceli, R.; Pellitteri, F.; Puccio, C.; Viola, F. Modelling, Simulation and Characterization of a Supercapacitor in Automotive Applications. In Proceedings of the 2020 Fifteenth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 10–12 September 2020. [Google Scholar] [CrossRef]
- Carter, R.; Cruden, A.; Hall, P.J. Optimizing for Efficiency or Battery Life in a Battery/Supercapacitor Electric Vehicle. IEEE Trans. Veh. Technol. 2012, 61, 1526–1533. [Google Scholar] [CrossRef]
- Subasinghage, K.; Gunawardane, K.; Padmawansa, N.; Kularatna, N.; Moradian, M. Modern Supercapacitors Technologies and Their Applicability in Mature Electrical Engineering Applications. Energies 2022, 15, 7752. [Google Scholar] [CrossRef]
- Luta, D.N.; Raji, A.K. Optimal sizing of hybrid fuel cell-supercapacitor storage system for off-grid renewable applications. Energy 2019, 166, 530–540. [Google Scholar] [CrossRef]
- Glavin, M.E.; Chan, P.K.W.; Armstrong, S.; Hurley, W.G.A. Stand-alone photovoltaic supercapacitor battery hybrid energy storage system. In Proceedings of the 2008 13th International Power Electronics and Motion Control Conference, Poznan, Poland, 1–3 September 2008; pp. 1688–1695. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.A. Hybrid PV-Battery/Supercapacitor System and a Basic Active Power Control Proposal in MATLAB/Simulink. Electronics 2020, 9, 129. [Google Scholar] [CrossRef]
- Jayananda, D.; Kularatna, N.; Steyn-Ross, D.A. Supercapacitor-assisted LED (SCALED) technique for renewable energy systems: A very low frequency design approach with short-term DC-UPS capability eliminating battery banks. IET Renew. Power Gener. 2020, 14, 1559–1570. [Google Scholar] [CrossRef]
- Yun, J.; Song, C.; Lee, H.; Park, H.; Jeong, Y.R.; Kim, J.W.; Jin, S.W.; Oh, S.Y.; Sun, L.; Zi, G.; et al. Stretchable array of high-performance micro-supercapacitors charged with solar cells for wireless powering of an integrated strain sensor. Nano Energy 2018, 49, 644–654. [Google Scholar] [CrossRef]
- De Carvalho, W.C.; Bataglioli, R.P.; Fernandes, R.A.S.; Coury, D.V. Fuzzy-based approach for power smoothing of a full-converter wind turbine generator using a supercapacitor energy storage. Electr. Power Syst. Res. 2020, 184, 106287. [Google Scholar] [CrossRef]
- Hall, P.J.; Bain, E.J. Energy-storage technologies and electricity generation. Energy Policy 2008, 36, 4352–4355. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 1230002. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.; Goldin, M.; Mirzaeian, M.; Jirandehi, H.F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2017, 42, 25565–25587. [Google Scholar] [CrossRef]
- Obreja, V.V.; Dinescu, A.; Obreja, A.C. Activated carbon-based electrodes in commercial supercapacitors and their performance. Int. Rev. Electr. Eng. 2010, 5, 272–281. Available online: https://www.researchgate.net/publication/256457223 (accessed on 7 April 2023).
- Abbas, Q.; Mirzaeian, M.; Ogwu, A.A.; Mazur, M.; Gibson, D. Effect of physical activation/surface functional groups on wettability and electrochemical performance of carbon/activated carbon aerogels-based electrode materials for electrochemical capacitors. Int. J. Hydrogen Energy 2018, 45, 13586–13595. [Google Scholar] [CrossRef]
- Sillars, F.B.; Fletcher, S.I.; Mirzaeian, M.; Hall, P.J. Effect of activated carbon xerogel pore size on the capacitance performance of ionic liquid electrolytes. Energy Environ. Sci. 2011, 4, 695–706. [Google Scholar] [CrossRef]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Conway, B.; Bockris, J.M.; Ammar, I. The dielectric constant of the solution in the diffuse and Helmholtz double layers at a charged interface in aqueous solution. Trans. Faraday Soc. 1951, 47, 756–766. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Rudge, A.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Fauvarque, J.-F. Chemical synthesis and characterization of fluorinated polyphenylthiophenes: Application to energy storage. Synth. Met. 2001, 123, 311–319. [Google Scholar] [CrossRef]
- Naoi, K.; Suematsu, S.; Manago, A. Electrochemistry of poly(1,5-diaminoanthraquinone) and its application in electrochemical capacitor materials. J. Electrochem. Soc. 2000, 147, 420–426. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hou, Y.; Kang, J.L.; Hirata, A.; Fujita, T.; Chen, M.W. Toward the Theoretical Capacitance of RuO2 Reinforced by Highly Conductive Nanoporous Gold. Adv. Energy Mater. 2013, 3, 851–856. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, W.; Wang, Y.; Zhang, B.; Luo, S.; Zhou, X.; Zhang, C.; Gu, X.; Hu, C. Porous Fe2O3 Nanospheres Anchored on Activated Carbon Cloth for High-Performance Symmetric Super-capacitors. Nano Energy 2019, 57, 379–387. [Google Scholar] [CrossRef]
- Long, X.; Zeng, Z.; Guo, E.; Shi, X.; Zhou, H.; Wang, X. Facile Fabrication of All-Solid-State Flexible Interdigitated MnO2 Super-capacitor via in-Situ Catalytic Solution Route. J. Power Sources 2016, 325, 264–272. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Z.; Wang, W.; Li, Z.; Zhang, Z. Fabrication of MoOx Film as a Conductive Anode Material for Micro-Supercapacitors by Electrodeposition and Annealing. J. Electrochem. Soc. 2014, 161, A1051–A1057. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, L.; Wu, X. Facile Synthesis of NiO Nanocubes for Photocatalysts and Supercapacitor Electrodes. J. Alloys Compd. 2019, 772, 465–471. [Google Scholar] [CrossRef]
- Lee, S.-M.; Park, Y.-J.; Lam, D.V.; Kim, J.-H.; Lee, K. Effects of Annealing on Electrochemical Performance in Graphene/V2O5 Supercapacitor. Appl. Surf. Sci. 2020, 512, 145626. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Kalkozova, Z.K.; Nurbolat, S.T.; Mirzaeian, M. Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications. Nanomaterials 2022, 12, 3669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.; Miao, X.; Yang, W.; Wang, C.; Pan, Q. ZIF-L-Co@ carbon fiber paper composite derived Co/Co3O4@C electrocatalyst for ORR in alkali/acidic media and overall seawater splitting. Int. J. Hydrogen Energy 2020, 45, 33028–33036. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, S. Electrocatalysts based on metal@ carbon core@ shell nanocomposites: An overview. Green Energy Environ. 2018, 3, 335–351. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, D.; Zhu, X.; Wang, S.; Zou, Y.; Lan, L.; Chen, R.; Yang, Y.; Liao, Q. ZIF-67-derived Co nanoparticles embedded in N-doped porous carbon composite interconnected by MWCNTs as highly efficient ORR electrocatalysts for a flexible direct formate fuel cell. Chem. Eng. J. 2022, 432, 134192. [Google Scholar] [CrossRef]
- Raoof, J.-B.; Hosseini, S.R.; Ojani, R.; Mandegarzad, S. MOF-derived Cu/nanoporous carbon composite and its application for electro-catalysis of hydrogen evolution reaction. Energy 2015, 90, 1075–1081. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, J.; Lin, C.; Xie, A.; Lin, B.; He, L.; Sun, D. Porous Carbon Derived from Rice Husks as Sustainable Bioresources: Insights into the Role of Micro/Mesoporous Hierarchy in Co3O4/C Composite for Asymmetric Supercapacitors. Microporous Mesoporous Mater. 2020, 291, 109709. [Google Scholar] [CrossRef]
- Zallouz, C.; Réty, B.; Vidal, L.; Meins, J.M.L.; Ghimbeu, C.M. Nanoparticles Embedded in Mesoporous Carbon for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 5022–5037. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Salunkhe, R.; Kaneti, Y.; Yamauchi, Y. Metal–Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Liu, T.; Jaroniec, M.; Yu, J. Nickel-based materials for supercapacitors. Mater. Today 2019, 25, 35–65. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Xu, Y.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis of hierarchical nanocage MnCo2O4 for high performance supercapacitor. Electrochim. Acta 2017, 225, 39–46. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Jeanmariet, G.; Rotenberg, B.; Salanne, M. Microscopic Simulations of Electrochemical Double-Layer Capacitors. Chem. Rev. 2022, 122, 10860–10898. [Google Scholar] [CrossRef]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef] [PubMed]
- Kops, L.; Ruschhaupt, P.; Guhrenz, C.; Schlee, P.; Pohlmann, S.; Varzi, A.; Passerini, S.; Balducci, A. Development of a high-energy electrical double-layer capacitor demonstrator with 5000 F in an industrial cell format. J. Power Sources 2023, 571, 233016. [Google Scholar] [CrossRef]
- Pilon, L.; Wang, H.; d’Entremont, A. Recent Advances in Continuum Modeling of Interfacial and Transport Phenomena in Electric Double Layer Capacitors. J. Electrochem. Soc. 2015, 162, A5158–A5178. [Google Scholar] [CrossRef]
- Kondrat, S.; Kornyshev, A. Corrigendum: Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 2013, 25, 022201. [Google Scholar] [CrossRef]
- Allaguri, A.; Freeborn, T.; Elwakil, A.S.; Maundy, B.J. Reevaluation of Performance of Electric Double-layer Capacitors from Constant-current Charge/ Discharge and Cyclic Voltammetry. Sci. Rep. 2016, 6, 38568. [Google Scholar] [CrossRef]
- Kierzek, K.; Gryglewicz, G. Activated Carbons and Their Evaluation in Electric Double Layer Capacitor. Molecules 2020, 25, 4255. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Lei, Y. Review on Nanoarchitectured Current Collectors for Pseudocapacitors. Small Methods 2019, 3, 180034. [Google Scholar] [CrossRef]
- Gao, S.; Fan, H. A review on the metal-oxide-based pseudocapacitors. Chem. Bull. 2013, 76, 202–209. [Google Scholar]
- Kumar, N.; Kim, S.B.; Lee, S.-Y.; Park, S.-J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z. Intercalation pseudocapacitance in electrochemical energy storage- recent advances in fundamental understanding and materials development. Mater. Adv. 2020, 7, 100072. [Google Scholar]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Jithul, K.P.; Singh Samara, K. Cupric Oxide based Supercapacitors: A Review. J. Phys. Conf. Ser. 2022, 2267, 012120. [Google Scholar] [CrossRef]
- Khot, M.; Kiani, A. A review on the advances in electrochemical capacitive charge storage in transition metal oxide electrodes for pseudocapacitors. Int. J. Energy Res. 2022, 46, 21757–21796. [Google Scholar] [CrossRef]
- Ehsani, A.; Heidari, A.A.; Shiri, H.M. Electrochemical Pseudocapacitors Based on Ternary Nanocomposite of Conductive Polymer/Graphene/Metal Oxide: An Introduction and Review to it in Recent Studies. Chem. Rec. 2019, 19, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Kiran Mangalampalli, S.R.N.; Dobbidi, P.; Ramasubramanian, L.N.; Korimilli, E.P.; Perumal, S.; Bakshi, S.R. Advances in functional and structural ceramics: Development, characterization, and applications. Ceram. Int. 2022, 48, 28763–28765. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Nguyen, L.N.; Nakate, U.T.; Lingamdinne, L.P.; Koduru, J.R.; Auti, P.S.; Vattikuti, S.V.P.; Shin, D.K. Electrochemical supercapacitor performance of NiCo2O4 nanoballs structured electrodes prepared via hydrthermal route with varying reaction time. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129901. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhvar, A.A.; Kharade, R.B.; Kadam, R.A.; Kumar, V.; Nakate, U.T.; Shelke, P.B.; Bobade, D.H.; Teli, A.M.; Dhas, S.D.; et al. Hydrothermally synthesized Ni3V2O8 nanoparticles with horny surfaces for HER and supercapacitor application. Mater. Lett. 2023, 338, 134033. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Koduru, J.R.; Velhal, N.B.; Nakate, U.T.; Jadhavar, A.A.; Sali, N.D.; Shin, D.K. Interconnected plate-like NiCo2O4 microstructures for supercapacitor application. Mater. Sci. Eng. B 2023, 287, 116072. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; He, S.; Du, H.; Liu, K.; Zhang, C.; Jiang, S. Facile Electrodeposition of NiCo2O4 Nanosheets on Porous Carbonized Wood for Wood-Derived Asymmetric Supercapacitors. Polymers 2022, 14, 2521. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zhang, H.; Zhang, C.; Jiang, S.; Hou, H. High mass-loading α-Fe2O3 nanoparticles anchored on nitrogen-doped wood carbon for high-energy-density supercapacitor. Chin. Chem. Lett. 2023, 108283. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Ihrar, M.; Hussain, S.B.; Oh, W.C.; Ullah, K. A review on graphene based transition metal oxide composites and its application towards supercapacitor electrodes. SN Appl. Sci. 2020, 2, 764. [Google Scholar] [CrossRef]
- Naskar, P.; Kundu, D.; Maiti, A.; Chakraborty, P.; Biswas, B.; Banerjee, A. Frontiers in Hybrid Ion Capacitors: A Review on Advanced Materials and Emerging Devices. ChemElectroChem 2021, 8, 1393–1429. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Zhu, K.; Maganti, S.; Zhao, Z.; Bai, P. Three-dimensional printing of the copper sulfate hybrid composites for supercapacitor electrodes with ultra-high areal and volumetric capacitances. Adv. Compos. Hybrid Mater. 2022, 5, 1537–1547. [Google Scholar] [CrossRef]
- Sufyan Javad, M.; Asim, S.; Najam, T.; Khalid, M.; Hussain, I.; Ahmad, A.; Assiri, M.A.; Han, W. Recent progress in flexible Zn-ion hybrid supercapacitors: Fundamentals, fabrication designs, and applications. Carbon Energy 2023, 5, e271. [Google Scholar]
- Lakshmi, K.C.; Vadhanarayanan. High-Performance Supercapacitors: A Comprehensive Review on Paradigm Shift of Conventional Energy Storage Devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, A.; Wu, X.; Liang, H.; Zhang, W. Status and Opportunities of Zinc Ion Hybrid Capacitors: Focus on Carbon Materials, Current Collectors, and Separators. Nano-Micro Lett. 2023, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Z.; Liu, J.; Li, C.; Mao, F.; Wu, H.; Zhang, Q. Enhancing the Performance of Battery-Supercapacitor-Hybrid Energy Device Through Narrowing the Capacitance Difference Between Two Electrodes via the Utilization of 2D MOF-Nanosheet-Derived Ni@nitrogen-Doped-Carbon Core-Shell Rings as Both Negative and Positive Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 47482–47489. [Google Scholar] [PubMed]
- Guo, Y.; Chen, C.; Wang, Y.; Hong, Y.; Wu, H.; Wang, K.; Niu, D.; Zhang, C.; Zhang, Q. Cu/CuxO@C nanocomposites as efficient electrodes for high-performance supercapacitor devices. Dalton Trans. 2022, 51, 14551–14556. [Google Scholar] [CrossRef]
- Wang, K.; Chen, C.; Li, Y.; Hong, Y.; Wu, H.; Zhang, C.; Zhang, Q. Insight into Electrochemical Performance of Nitrogen-Doped Carbon/NiCo-Alloy Active Nanocomposites. Small 2023, 2300054. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Prado, I.; Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Design of Activated Carbon/Activated Carbon Asymmetric Capacitors. Front. Mater. 2016, 3, 16. [Google Scholar] [CrossRef]
- Akinwolemiwaa, B.; Weia, C.; Chen, G.Z. Mechanisms and Designs of Asymmetrical Electrochemical Capacitors. Electrochim. Acta 2017, 247, 344–357. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahe-dron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Ravindra, A.V.; Behera, B.C.; Padhan, P.; Lebedev, O.I.; Prellier, W. Tailoring of crystal phase and Nel temperature of cobalt monoxides nanocrystals with synthetic approach conditions. J. Appl. Phys. 2014, 116, 033912. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Akhanova, N.; Gabdullin, M.; Kalkozova, Z.; Tulegenova, A.; Nurbolat, S.; Abdullin, K. Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors. Energies 2020, 13, 5228. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and De-vices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Nunes, W.G.; Freitas, B.G.A.; Beraldo, R.M.; Filho, R.M. A rational experimental approach to identify correctly the working voltage window of aqueous-based supercapacitors. Sci. Rep. 2020, 10, 19195. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullin, K.A.; Gabdullin, M.T.; Kalkozova, Z.K.; Nurbolat, S.T.; Mirzaeian, M. Symmetrical Composite Supercapacitor Based on Activated Carbon and Cobalt Nanoparticles with High Cyclic Stability and Current Load. Energies 2023, 16, 4287. https://doi.org/10.3390/en16114287
Abdullin KA, Gabdullin MT, Kalkozova ZK, Nurbolat ST, Mirzaeian M. Symmetrical Composite Supercapacitor Based on Activated Carbon and Cobalt Nanoparticles with High Cyclic Stability and Current Load. Energies. 2023; 16(11):4287. https://doi.org/10.3390/en16114287
Chicago/Turabian StyleAbdullin, Khabibulla A., Maratbek T. Gabdullin, Zhanar K. Kalkozova, Shyryn T. Nurbolat, and Mojtaba Mirzaeian. 2023. "Symmetrical Composite Supercapacitor Based on Activated Carbon and Cobalt Nanoparticles with High Cyclic Stability and Current Load" Energies 16, no. 11: 4287. https://doi.org/10.3390/en16114287






