Determination of the Electrical Parameters of Iodine-Doped Polymer Solar Cells at the Macro- and Nanoscale for Indoor Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
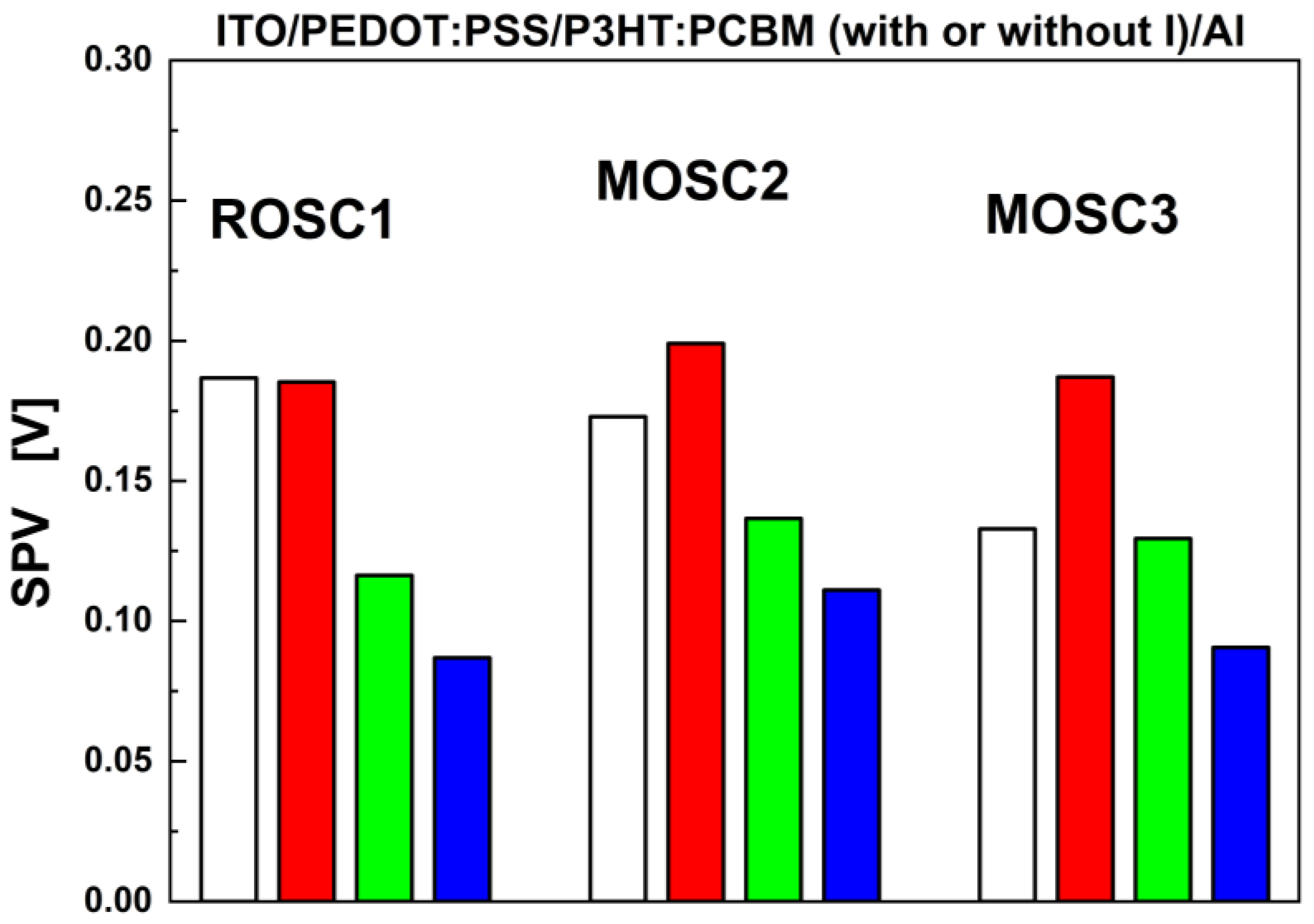
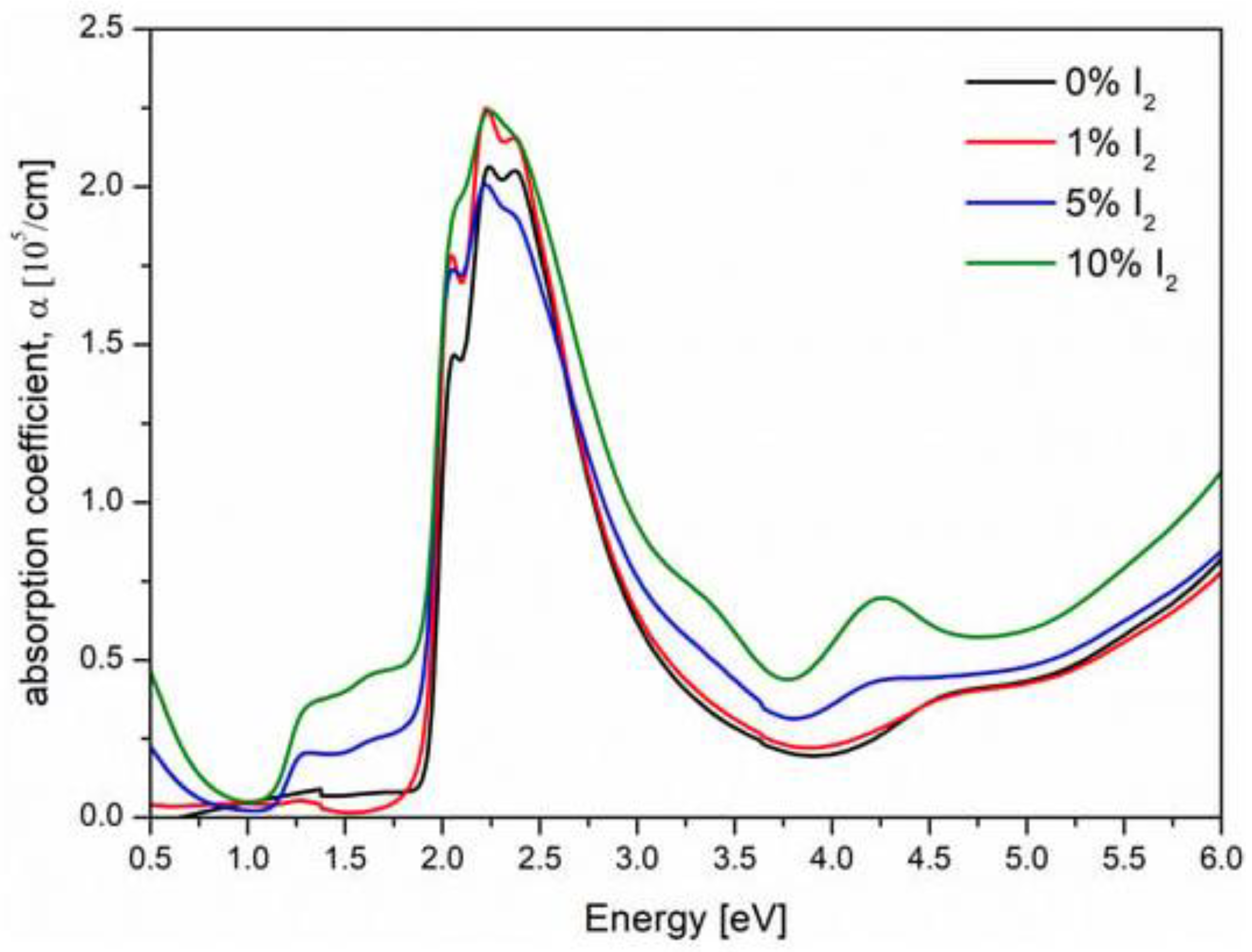
3.1. Photoactive Kelvin Probe Force Microscopy (p–KPFM)
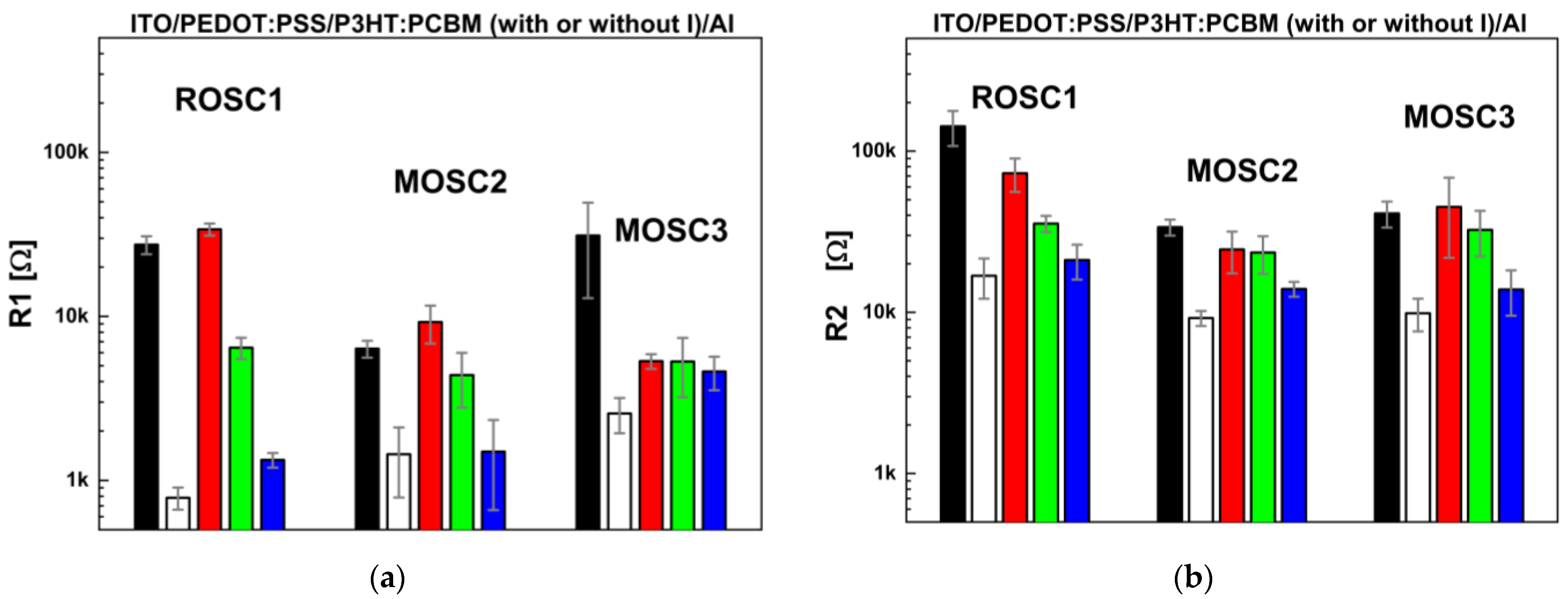
3.2. Photoactive Impedance Spectroscopy Experiments and Equivalent Electrical Circuit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Ren, Z.; Zhang, Y.; Liu, K.; Zhang, H.; Tang, H.; Yan, C.; Zheng, Z.; Li, G. Stretchable ITO-Free Organic Solar Cells with Intrinsic Anti-Reflection Substrate for High-Efficiency Outdoor and Indoor Energy Harvesting. Adv. Funct. Mater. 2021, 31, 2010172. [Google Scholar] [CrossRef]
- Lechêne, B.P.; Cowell, M.; Pierre, A.; Evans, J.W.; Wright, P.K.; Arias, A.C. Organic solar cells and fully printed super-capacitors optimized for indoor light energy harvesting. Nano Energy 2016, 26, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Xu, T.; Lee, J.Y.; Ledbetter, A.; Guo, L.J. Photonic Color Filters Integrated with Organic Solar Cells for Energy Harvesting. ACS Nano 2011, 5, 7055–7060. [Google Scholar] [CrossRef] [PubMed]
- Mola, G.T.; Mthethwa, M.C.; Hamed, M.S.G.; Adedeji, M.A.; Mbuyise, X.G.; Kumar, A.; Sharma, G.; Zang, Y. Local surface plasmon resonance assisted energy harvesting in thin film organic solar cells. J. Alloys Compd. 2021, 856, 158172. [Google Scholar] [CrossRef]
- Agnihotri, P.; Sahu, S.; Tiwari, S. Recent Advances & Perspectives in Electron Transport Layer of Organic Solar Cells for Efficient Solar Energy Harvesting. In Proceedings of the International Conference on Energy, Communication, Data Analytics and Soft Computing, Chennai, India, 1–2 August 2017. [Google Scholar]
- Balasankar, A.; Arthiya, S.E.; Ramasundaram, S.; Sumathi, P.; Arokiyaraj, S.; Oh, T.; Aruchamy, K.; Sriram, G.; Kurkuri, M.D. Recent Advances in the Preparation and Performance of Porous Titanium-Based Anode Materials for Sodium-Ion Batteries. Energies 2022, 15, 9495. [Google Scholar] [CrossRef]
- Rajendhiran, R.; Atchudan, R.; Palanisamy, J.; Balasankar, A.; Oh, T.H.; Deivasigamani, V.; Ramasundaram, S. Prickly Pear Fruit Extract: Capping Agent for the Sol–Gel Synthesis of Discrete Titanium Dioxide Nanoparticles and Sensitizer for Dye-Sensitized Solar Cell. Coatings 2023, 13, 579. [Google Scholar] [CrossRef]
- Gnida, P.; Amin, M.F.; Pająk, A.K.; Jarząbek, B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef]
- Caballero-Quintana, I.; Amargós-Reyes, O.; Maldonado, J.L.; Nicasio-Collazo, J.; Romero-Borja, D.; Barreiro-Arguelles, D.; Molnar, G.; Bousseksou, A. Scanning Probe Microscopy Analysis of Nonfullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 29520–29527. [Google Scholar] [CrossRef]
- Khatiwada, D.; Venkatesan, S.; Chen, Q.; Chen, J.; Adhikari, N.; Dubey, A.; Mitul, A.F.; Mohammed, L.; Qiao, Q. Improved performance by morphology control via fullerenes in PBDT-TBT-alkoBT based organic solar cells. J. Mater. Chem. A 2015, 3, 15307–15313. [Google Scholar] [CrossRef]
- Scherer, M.; Saive, R.; Daume, D.; Kröger, M.; Kowalsky, W. Sample preparation for scanning Kelvin probe microscopy studies on cross sections of organic solar cells. Appl. Phys. Lett. Adv. 2013, 3, 092134. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Mao, L.; Li, Y.; Kong, T.; Wu, N.; Ma, C.; Bai, S.; Jin, Y.; Wu, D.; Lu, W.; et al. Quantitative operando visualization of the energy band depth profile in solar cells. Nat. Commun. 2015, 6, 7745. [Google Scholar] [CrossRef]
- Saive, R.; Scherer, M.; Mueller, C.; Daume, D.; Schinke, J.; Kroeger, M.; Kowalsky, W. Imaging the Electric Potential within Organic Solar Cells. Adv. Funct. Mater. 2013, 23, 5854–5860. [Google Scholar] [CrossRef]
- Chen, Q.; Ye, F.; Lai, J.; Dai, P.; Lu, S.; Ma, C.; Zhao, Y.; Xie, Y.; Chen, L. Energy band alignment in operando inverted structure P3HT:PCBM organic solar cells. Nano Energy 2017, 40, 454–461. [Google Scholar] [CrossRef]
- Akaike, K. Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics. Materials 2020, 13, 2411. [Google Scholar] [CrossRef]
- Kong, J.; Lee, J.; Jeong, Y.; Kim, M.; Kang, S.O.; Lee, K. Biased internal potential distributions in a bulk heterojunction organic solar cell incorporated with a TiOx interlayer. Appl. Phys. Lett. 2012, 100, 213305. [Google Scholar] [CrossRef]
- Glatzel, T.; Hoppe, H.; Sariciftci, N.S.; Lux-Steiner, M.C.; Komiyama, M. Kelvin Probe Force Microscopy Study of Conjugated Polymer/Fullerene Organic Solar Cells. Jpn. J. Appl. Phys. 2005, 44, 5370–5373. [Google Scholar] [CrossRef]
- Hoppe, H.; Glatzel, T.; Niggemann, M.; Hinsch, A.; Lux-Steiner, M.C.; Sariciftci, N.S. Kelvin Probe Force Microscopy Study on Conjugated Polymer/Fullerene Bulk Heterojunction Organic Solar Cells. Nano Lett. 2005, 5, 269–274. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy Theory, Experiment, and Applications; John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bisquert, J. Theory of the Impedance of Electron Diffusion and Recombination in a Thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Bisquert, J. Interpretation of electron diffusion coefficient in organic and inorganic semiconductors with broad distributions of states. Phys. Chem. Chem. Phys. 2008, 10, 3175–3194. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J. Characterization of nanostructured hybrid and organic solar cells by impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 9083–9118. [Google Scholar] [CrossRef]
- Bisquert, J.; Mora-Sero, I.; Fabregat-Santiago, F. Diffusion–Recombination Impedance Model for Solar Cells with Disorder and Nonlinear Recombination. Chem. Electro. Chem. 2014, 1, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Bisquert, J.; Bertoluzzi, L.; Mora-Sero, I.; Garcia-Belmonte, G. Theory of Impedance and Capacitance Spectroscopy of Solar Cells with Dielectric Relaxation, Drift-Diffusion Transport, and Recombination. J. Phys. Chem. C 2014, 118, 18983–18991. [Google Scholar] [CrossRef]
- Glatthaar, M.; Mingirulli, N.; Zimmermann, B.; Ziegler, T.; Kern, R.; Niggemann, M.; Hinsch, A.; Gombert, A. Impedance spectroscopy on organic bulk-heterojunction solar cells. Phys. Status Solidi 2005, 202, R125–R127. [Google Scholar] [CrossRef]
- Lu, L.; Luo, Z.; Xu, T.; Yu, L. Cooperative Plasmonic Effect of Ag and Au Nanoparticles on Enhancing Performance of Polymer Solar Cells. Nano Lett. 2013, 13, 59–64. [Google Scholar] [CrossRef]
- Glatthaar, M.; Riede, M.; Keegan, N.; Sylvester-Hvid, K.; Zimmermann, B.; Niggemann, M.; Hinsch, A.; Gombert, A. Efficiency limiting factors of organic bulk heterojunction solar cells identified by electrical impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2007, 91, 390–393. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Boix, P.P.; Bisquert, J.; Sessolo, M.; Bolink, H. Simultaneous determination of carrier lifetime and electron density-of-states in P3HT:PCBM organic solar cells under illumination by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2010, 94, 366–375. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Munar, A.; Barea, E.M.; Bisquert, J.; Ugarte, I.; Pacios, R. Charge carrier mobility and lifetime of organic bulk heterojunctions analyzed by impedance spectroscopy. Org. Electron. 2008, 9, 847–851. [Google Scholar] [CrossRef]
- Kuwabara, T.; Kawahara, Y.; Yamaguchi, T.; Takahashi, K. Characterization of Inverted-Type Organic Solar Cells with a ZnO Layer as the Electron Collection Electrode by ac Impedance Spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 2107–2110. [Google Scholar] [CrossRef]
- Yo, J.; Chen, C.C.; Dou, L.; Murase, S.; Duan, H.S.; Hawks, S.A.; Xu, T.; Son, H.J.; Yu, L.; Li, G.; et al. Metal Oxide Nanoparticles as an Electron-Transport Layer in High-Performance and Stable Inverted Polymer Solar Cells. Adv. Mater. 2012, 24, 5267–5272. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.L.; Baek, N.S.; Zou, J.; O’Malley, K.; Jen, A.K.Y. Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer. Appl. Phys. Lett. 2008, 92, 253301. [Google Scholar] [CrossRef]
- Boix, P.P.; Ajuria, J.; Pacios, R.; Garcia-Belmonte, G. Carrier recombination losses in inverted polymer: Fullerene solar cells with ZnO holeblocking layer from transient photovoltage and impedance spectroscopy techniques. J. Appl. Phys. 2011, 109, 074514. [Google Scholar] [CrossRef]
- Zimmermann, B.; Wurfel, U.; Niggemann, M. Long term stability of efficient inverted P3HT:PCBM solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 491–496. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Chuchmała, A.; Gorecki, L.; Sikora, A.; Mazurek, B.; Pasciak, G. Opto(electrical) properties of new aromatic polyazomethines with fluorene moieties in the main chain for polymeric photovoltaic devices. Synth. Met. 2012, 162, 143–153. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Chuchmała, A.; Sikora, A.; Gorecki, L.; Sek, D. Opto(electrical) properties of triphenylamine-based polyazomethine and its blend with [6,6]-phenyl C61 butyric acid methyl ester. High Perform. Polym. 2013, 25, 832–842. [Google Scholar] [CrossRef]
- Jarząbek, B.; Nitschke, P.; Godzierz, M.; Palewicz, M.; Piasecki, T.; Gotszalk, T.P. Thermo-Optical and Structural Studies of Iodine-Doped Polymer: Fullerene Blend Films, Used in Photovoltaic Structures. Polymers 2022, 14, 858. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Iwan, A.; Sikora, A.; Hamplová, V.; Bubnov, A. AFM study of advanced composite materials for organic photovoltaic cells with active layer based on P3HT:PCBM and chiral photosensitive liquid crystalline dopants. Liq. Cryst. 2015, 42, 964–972. [Google Scholar] [CrossRef]
- Basta, M.; Dusza, M.; Palewicz, M.; Nawrot, U.; Granek, F. Method to analyze the ability of bulk heterojunctions of organic and hybrid solar cells to dissociate photogenerated excitons and collect free carriers. J. Appl. Phys. 2014, 115, 174504. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Xiao, T.; Shen, Z.; Ren, Y.; Lu, G.; Bu, L. In Situ Measuring Film-Depth-Dependent Light Absorption Spectra for Organic Photovoltaics. Front. Chem. 2020, 8, 211. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, C.; Sun, Y.; Li, Q.; Islam, M.R.; Liu, K.; Wang, Z.; Qu, S.; Wang, Z. Optical management in organic photovoltaic devices. Carbon Energy 2021, 3, 4–23. [Google Scholar] [CrossRef]
- Zhuo, Z.; Zhang, F.; Wang, J.; Wang, J.; Xu, X.; Xu, Z.; Wang, Y.; Tang, W. Efficiency improvement of polymer solar cells by iodine doping. Solid-State Electron. 2011, 63, 83–88. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Xiang, J.; Sun, Z.; Ji, R.; Lai, S.K.; Lau, S.P.; Kong, J.; Zhao, J.; Yang, C.; et al. Solution processable high-performance infrared organic photodetector by iodine doping. RSC Adv. 2016, 6, 45166–45171. [Google Scholar] [CrossRef]
- Bubnov, A.; Iwan, A.; Cigl, M.; Boharewicz, B.; Tazbir, I.; Wójcik, K.; Sikora, A.; Hamplova, V. Photosensitive self-assembling materials as functional dopants for organic photovoltaic cells. RSC Adv. 2016, 6, 11577–11590. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Sikora, A.; Maliński, M.; Chrobak, Ł.; Mad, W. Laser Beam Induced Current Technique of Polymer Solar Cells Based on New Poly (Azomethine) or Poly (3-Hexylthiophene). Chem. Sci. Rev. Lett. 2015, 4, 597–607. [Google Scholar]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.P.; bin Mohd Yusoff, A.R.; Jang, J. Understanding the role of organic polar solvent induced nanoscale morphology and electrical evolutions of P3HT:PCBM composite film. Org. Electron. 2015, 25, 50–56. [Google Scholar] [CrossRef]
- Spadafora, E.J.; Demadrille, R.; Ratier, B.; Grevin, B. Imaging the Carrier Photogeneration in Nanoscale Phase Segregated Organic Heterojunctions by Kelvin Probe Force Microscopy. Am. Chem. Soc. Nano Lett. 2010, 10, 3337–3342. [Google Scholar] [CrossRef]
- Grévin, B.; Bardagot, O.; Demadrille, R. Implementation of data-cube pump–probe KPFM on organic solar cells. Beilstein J. Nanotechnol. 2020, 11, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Romero, B.; del Pozo, G.; Arredondo, B.; Reinhardt, J.P.; Sessler, M.; Wurfel, U. Circuital Model Validation for S-Shaped Organic Solar Cells by Means of Impedance Spectroscopy. IEEE J. Photovolt. 2015, 5, 234–237. [Google Scholar] [CrossRef]
- Arredondo, B.; Romero, B.; Del Pozo, G.; Sessler, M.; Veit, C.; Würfel, U. Impedance spectroscopy analysis of small molecule solution processed organic solar cell. Sol. Energy Mater. Sol. Cells 2014, 128, 351–356. [Google Scholar] [CrossRef]
- Knipper, M.; Parisi, J.; Coakley, K.; Waldauf, C.; Brabec, C.J.; Dyakonov, V. Impedance Spectroscopy on Polymer-Fullerene Solar Cells. Z. Naturforsch. 2007, 62, 490–494. [Google Scholar] [CrossRef]
- Perrier, G.; de Bettignies, R.; Berson, S.; Lemaître, N.; Guillerez, S. Impedance spectrometry of optimized standard and inverted P3HT-PCBM organic solar cells. Sol. Energy Mater. Sol. Cells. 2012, 101, 210–216. [Google Scholar] [CrossRef]
- Mullenbach, T.K.; Zou, Y.; Holst, J.; Holmes, R.J. Interpreting impedance spectra of organic photovoltaic cells—Extracting charge transit and recombination rates. J. Appl. Phys. 2014, 116, 124513. [Google Scholar] [CrossRef]
- Grancharov, G.; Atanasova, M.D.; Kalinova, R.; Gergova, R.; Popkirov, G.; Dikov, C.; Sendova-Vassileva, M. Flexible Polymer–Organic Solar Cells Based on P3HT:PCBM Bulk Heterojunction Active Layer Constructed under Environmental Conditions. Molecules 2021, 26, 6890. [Google Scholar] [CrossRef]
- Jarząbek, B.; Nitschke, P.; Hajduk, B.; Domanski, M.; Bednarski, H. In situ thermo-optical studies of polymer:fullerene blend films. Polym. Test. 2020, 88, 106573. [Google Scholar] [CrossRef]
- Tian, S.; Huang, T.; Han, F.; Wang, F.; Tan, Z.; Bai, Y. Optimization of tail state Urbach energy enables efficient organic solar cells and perovskite/organic tandem solar cells. Org. Electron. 2023, 113, 106714. [Google Scholar] [CrossRef]






| Type of Layer/Layers | ROSC1 | MOSC2 | MOSC3 |
|---|---|---|---|
| Aluminium electrode 1 Organic layers: x and y ITO | 120.06 nm | 122.37 nm | 119.11 nm |
| 85.48 nm | 98.04 nm | 111.09 nm | |
| 111.74 nm | 115.76 nm | 110.31 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palewicz, M.; Sikora, A.; Piasecki, T.; Gacka, E.; Nitschke, P.; Gnida, P.; Jarząbek, B.; Gotszalk, T. Determination of the Electrical Parameters of Iodine-Doped Polymer Solar Cells at the Macro- and Nanoscale for Indoor Applications. Energies 2023, 16, 4741. https://doi.org/10.3390/en16124741
Palewicz M, Sikora A, Piasecki T, Gacka E, Nitschke P, Gnida P, Jarząbek B, Gotszalk T. Determination of the Electrical Parameters of Iodine-Doped Polymer Solar Cells at the Macro- and Nanoscale for Indoor Applications. Energies. 2023; 16(12):4741. https://doi.org/10.3390/en16124741
Chicago/Turabian StylePalewicz, Marcin, Andrzej Sikora, Tomasz Piasecki, Ewelina Gacka, Paweł Nitschke, Paweł Gnida, Bożena Jarząbek, and Teodor Gotszalk. 2023. "Determination of the Electrical Parameters of Iodine-Doped Polymer Solar Cells at the Macro- and Nanoscale for Indoor Applications" Energies 16, no. 12: 4741. https://doi.org/10.3390/en16124741





