Synthesis and Hydrogenation of the Ti45−xVxZr38Ni17 (5 ≤ x ≤ 40) Mechanically Alloyed Materials
Abstract
1. Introduction
2. Experimental Process
3. Results
3.1. The Microstructure and the Composition
3.2. Structural Properties
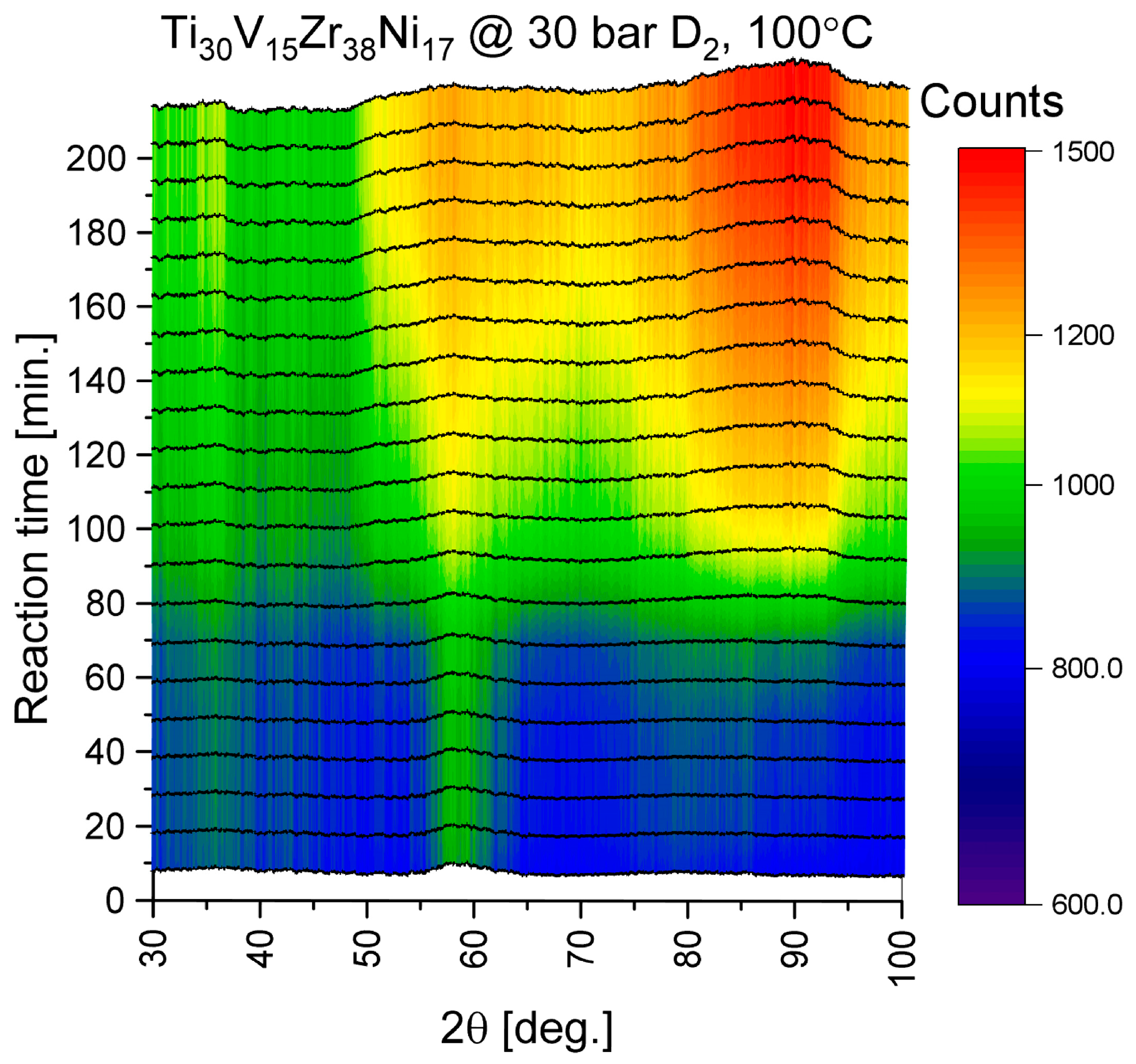
3.3. Hydrogen Sorption
3.4. Desorption of the Deuterided Samples
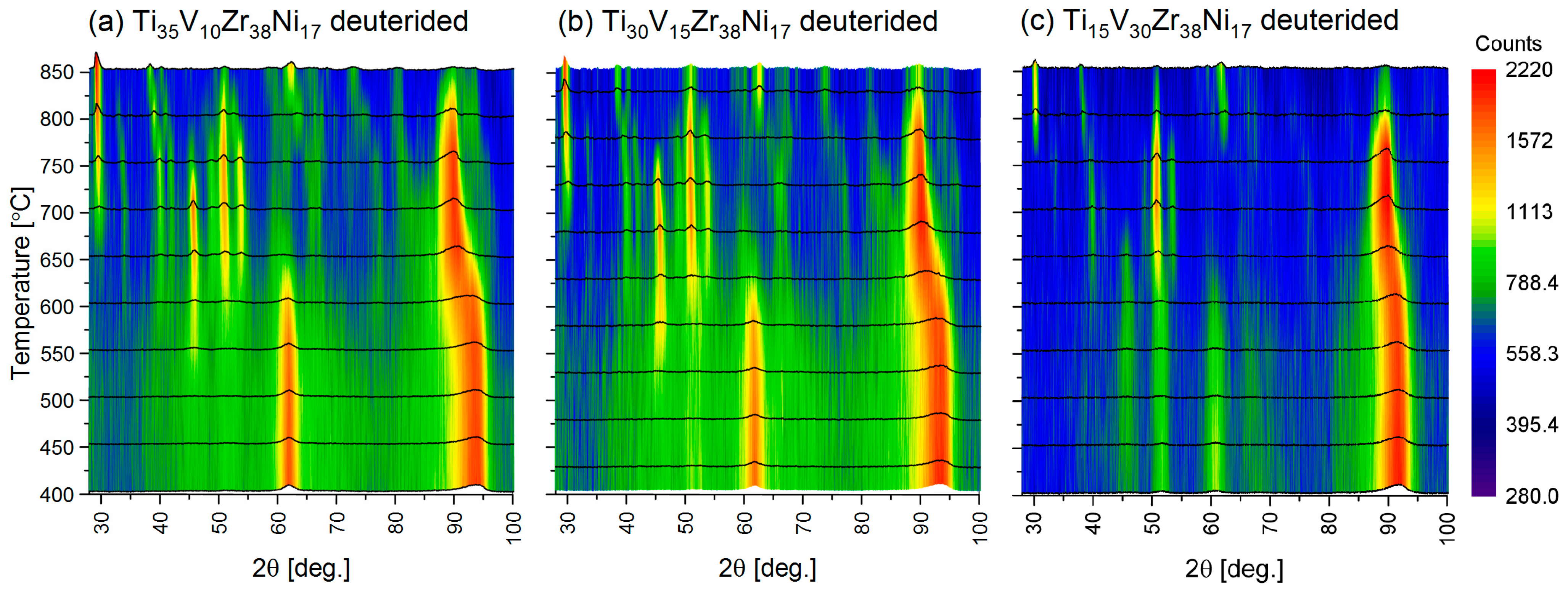
3.5. In Situ Neutron Diffraction under High Deuterium Pressure
3.6. Lattice Expansion for the Amorphous Deuteride
4. Concluding Remarks and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energ. Ecol. Environ. 2016, 1, 10. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sust. Energ. Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179. [Google Scholar] [CrossRef]
- Huang, L.J.; Lin, H.J.; Wang, H.; Ouyang, L.Z.; Zhu, M.J. Amorphous alloys for hydrogen storage. Alloys Compd. 2023, 941, 168945. [Google Scholar] [CrossRef]
- Zuo, S.; Wu, R.; Pang, G.; Yang, Y.; Jin, M. High temperature internal friction in Ni50.3Ti29.7Zr20 shape memory alloy. Intermetallics 2019, 109, 174. [Google Scholar] [CrossRef]
- Liens, A.; Ter-Ovanessian, B.; Courtois, N.; Fabregue, D.; Wada, T.; Kato, H.; Chevalier, J. Effect of alloying elements on the microstructure and corrosion behavior of TiZr-based bulk metallic glasses. Corros. Sci. 2020, 177, 108854. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Faverani, L.P.; Grandini, C.R.; Rangel, E.C.; da Cruz, N.C.; Junior, F.H.N.; Almeida, A.B.; Vicente, F.B.; Morais, B.R.; Barão, V.A.; et al. Characterization of chemically treated Ti-Zr system alloys for dental implant application. Mater. Sci. Eng. C 2018, 92, 849. [Google Scholar] [CrossRef]
- Guiose, B.; Cuevas, F.; Décamps, B.; Leroy, E.; Percheron-Guégan, A. Microstructural analysis of the ageing of pseudo-binary (Ti,Zr)Ni intermetallic compounds as negative electrodes of Ni-MH batteries. Electrochim. Acta 2009, 54, 2781. [Google Scholar] [CrossRef]
- Inoue, S.; Sawada, N.; Namazu, T. Effect of Zr content on mechanical properties of Ti–Ni–Zr shape memory alloy films prepared by dc magnetron sputtering. Vacuum 2008, 83, 664. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, J.-G.; Yan, C.-W. Novel Ti/Zr Based Non-Chromium Chemical Conversion Coating for the Corrosion Protection of Electrogalvanized Steel. Int. J. Electrochem. Sci. 2011, 6, 4853. [Google Scholar] [CrossRef]
- Lefaix, H.; Prima, F.; Zanna, S.; Vermaut, P.; Dubot, P.; Marcus, P.; Janičkovič, D.; Švec, P. Surface Properties of a Nano-Quasicrystalline Forming Ti Based System. Mater. Trans. 2007, 48, 278. [Google Scholar] [CrossRef][Green Version]
- Zhernovenkova, Y.V.; Andreev, L.A.; Kaloshkin, S.D.; Sviridova, T.A.; Tcherdyntsev, V.V.; Tomilin, I.A. Hydrogen absorption in amorphous and quasicrystalline Ti45Ni17Zr38 powders synthesized by mechanical alloying. J. Alloys Compd. 2007, 434–435, 747. [Google Scholar] [CrossRef]
- Takasaki, A.; Kelton, K.F. High-pressure hydrogen loading in Ti45Zr38Ni17 amorphous and quasicrystal powders synthesized by mechanical alloying. J. Alloys Compd. 2002, 347, 295. [Google Scholar] [CrossRef]
- Shahi, R.R.; Yadav, T.P.; Shaz, M.A.; Srivastava, O.N.; van Smaalen, S. Effect of processing parameter on hydrogen storage characteristics of as quenched Ti45Zr38Ni17 quasicrystalline alloys. Int. J. Hydrogen Energy 2011, 36, 592. [Google Scholar] [CrossRef]
- Kocjan, A.; McGuiness, P.J.; Kobe, S. Desorption of hydrogen from Ti–Zr–Ni hydrides using a mass spectrometer. Int. J. Hydrogen Energy 2010, 35, 259. [Google Scholar] [CrossRef]
- Huang, H.; Meng, D.; Lai, X.; Zhao, Y.; Zhou, P.; Wang, Q.; Huang, H.; Qiang, J. TiZrNi quasicrystal film prepared by magnetron sputtering. Vacuum 2015, 122, 147. [Google Scholar] [CrossRef]
- Takasaki, A.; Huett, V.T.; Kelton, F.K. Hydrogen Pressure-Composition Isotherms for Ti45Zr38Ni17 Amorphous and Quasicrystal Powders Produced by Mechanical Alloying. Mat. Trans. 2002, 43, 2165. [Google Scholar] [CrossRef][Green Version]
- Viano, A.M.; Stroud, R.M.; Gibbons, P.C.; McDowell, A.F.; Conradi, M.S.; Kelton, K.F. Hydrogenation of titanium-based quasicrystals. Phys. Rev. B 1995, 51, 12026. [Google Scholar] [CrossRef]
- Tominaga, T.; Takasaki, A.; Shibato, T.; Świerczek, K.J. HREM observation and high-pressure composition isotherm measurement of Ti45Zr38Ni17 quasicrystal powders synthesized by mechanical alloying. J. Alloys Compd. 2015, 645, S292. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, S.-H.; Shin, H.S.; Kim, J.J. Analysis of Structure and P–c–T Curve of Hydrogenated Ti53Zr27−xNi20Pdx Quasicrystals. J. Nanosci. Nanotechnol. 2013, 13, 7959. [Google Scholar] [CrossRef] [PubMed]
- Huogen, H.; Liang, C.; Qinying, X. Effect of Pd Substitution on Phase Formation and Stability in Ti-Zr-Ni Quasicrystalline Alloys. Rare Met. Mat. Eng. 2015, 44, 821. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. Structure and hydrogen absorption properties of Ti53Zr27Ni20(Pd,V) quasicrystals. Int. J. Hydrogen Energy 2018, 43, 19130. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Liu, F.; Xu, J. Nonlinear dynamic characteristics and bifurcation analysis of Ti–Zr–Ni quasicrystal as hydrogen storage material. Int. J. Hydrogen Energy 2021, 46, 16667. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Lemus, L.F.; Santos, D.S.D. Hydrogen absorption study of Ti-based alloys performed by melt-spinning. Mat. Res. 2013, 16, 679. [Google Scholar] [CrossRef]
- Shahi, R.R.; Yadav, T.P.; Shaz, M.A.; Srivastava, O.N. Synthesis characterization and hydrogenation behaviour of as quenched Ti41.5+XZr41.5-XNi17 (x=0, 3.5, 11.5 and 13.5) nano quasicrystalline ribbons. J. Phys. Conf. Ser. 2017, 809, 012011. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhang, T.; Kou, H.; Hu, R.; Xue, X. Hydrogen storage properties of non-stoichiometric Zr0.9TixV2 melt-spun ribbons. Energy 2016, 114, 1147. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.; Zepon, G.; Li, H.-W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, T.P.; Mukhopadhyay, N.K. Notable hydrogen storage in Ti–Zr–V–Cr–Ni high entropy alloy. Int. J. Hydrogen Energy 2022, 47, 22893. [Google Scholar] [CrossRef]
- Ma, X.; Ding, X.; Chen, R.; Zhang, J.; Song, Q.; Cui, H. Microstructural features and improved reversible hydrogen storage properties of ZrTiVFe high-entropy alloy via Cu alloying. Int. J. Hydrogen Energy 2023, 48, 2718. [Google Scholar] [CrossRef]
- Kazemipour, M.; Jazi, H.S.; Saidi, A.; Saatchi, A. Hydrogen storage properties of Ti0.72Zr0.28Mn1.6V0.4 alloy prepared by mechanical alloying and copper boat induction melting. Int. J. Hydrogen Energy 2014, 39, 12784. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, R.R.; Ding, X.; Fang, H.Z.; Li, X.Z.; Ding, H.S.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Effect of phase formation on hydrogen storage properties in Ti-V-Mn alloys by zirconium substitution. Energy 2019, 166, 587. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, H.; Li, D.; Li, X.; Yang, B.; Li, S.J. Microstructure and hydrogen storage properties of Ti–V–Mn alloy with Zr, Ni, and Zr7Ni10 addition. Mater. Res. 2022, 37, 1591. [Google Scholar] [CrossRef]
- Hang, Z.; Chen, L.; Xiao, X.; Yao, Z.; Shi, L.; Feng, Y.; Yang, L. Microstructure and hydrogen storage properties of Ti10+xV80-xFe6Zr4 (x=0~15) alloys. Int. J. Hydrogen Energy 2021, 46, 27622. [Google Scholar] [CrossRef]
- Kefi, C.; Huot, J. Microstructure and First Hydrogenation Properties of Ti30V60Mn(10−x)Crx (x = 0, 3.3, 6.6, 10) + 4 wt.% Zr. Metals 2023, 13, 1119. [Google Scholar] [CrossRef]
- Takasaki, A.; Gondek, Ł.; Czub, J.; Klimkowicz, A.; Żywczak, A.; Świerczek, K. Hydrogen Storage in Ti/Zr-Based Amorphous and Quasicrystal Alloys in Hydrogen Storage Technologies; Sankir, M., Sankir, N.D., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2018; pp. 117–146. [Google Scholar]
- Żywczak, A.; Shinya, D.; Gondek, Ł.; Takasaki, A.; Figiel, H. Hydriding of Ti45Zr38Ni17−xFex nanocompounds. Sol. State Commun. 2010, 150, 1. [Google Scholar] [CrossRef]
- Rusinek, D.; Czub, J.; Niewolski, J.; Gondek, Ł.; Gajewska, M.; Takasaki, A.; Hoser, A.; Żywczak, A.J. Structural phase transitions in the Ti45Zr38Ni17-xFex nano-alloys and their deuterides. J. Alloys Compd. 2015, 646, 90. [Google Scholar] [CrossRef]
- Żywczak, A.; Rusinek, D.; Czub, J.; Sikora, M.; Stępień, J.; Gondek, Ł.; Takasaki, A.; Hoser, A. Amorphous hydrides of the Ti45Zr38Ni17-xCox nano-powders. Int. J. Hydrogen Energy 2015, 40, 15534. [Google Scholar] [CrossRef]
- Sayetat, F.; Fertey, P.; Kessler, M.J. An Easy Method for the Determination of Debye Temperature from Thermal Expansion Analyses. App. Cryst. 1998, 31, 121. [Google Scholar] [CrossRef]
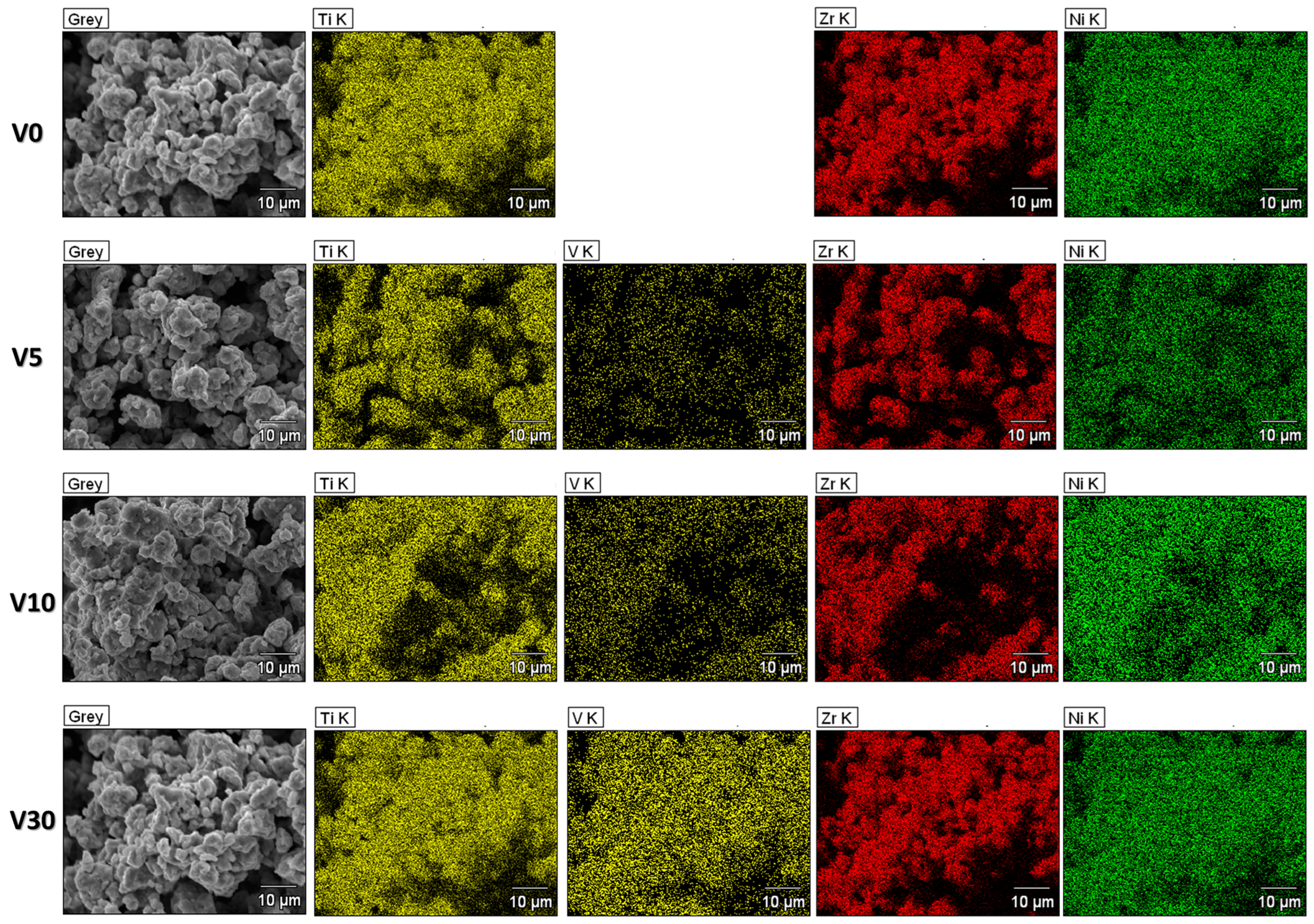
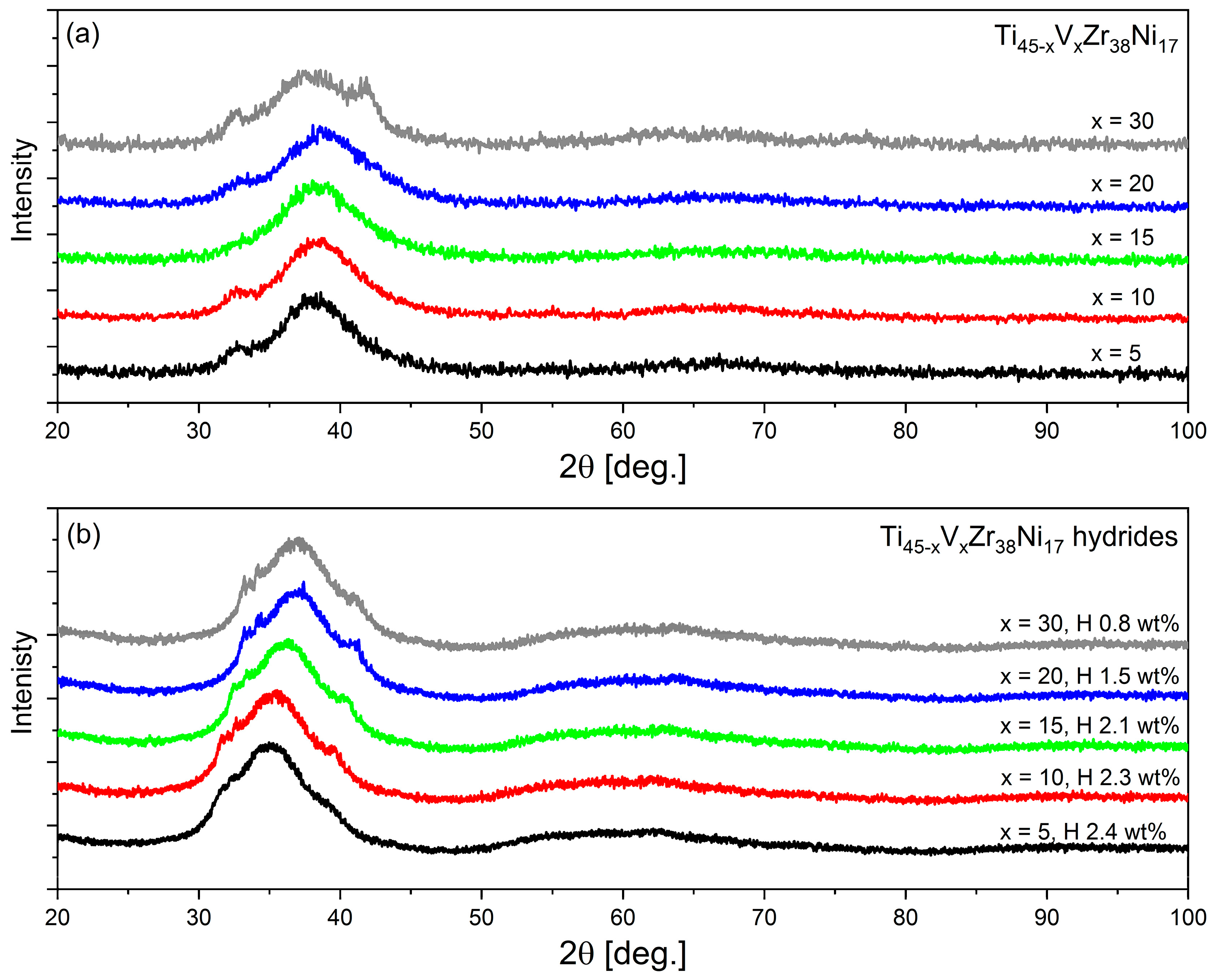
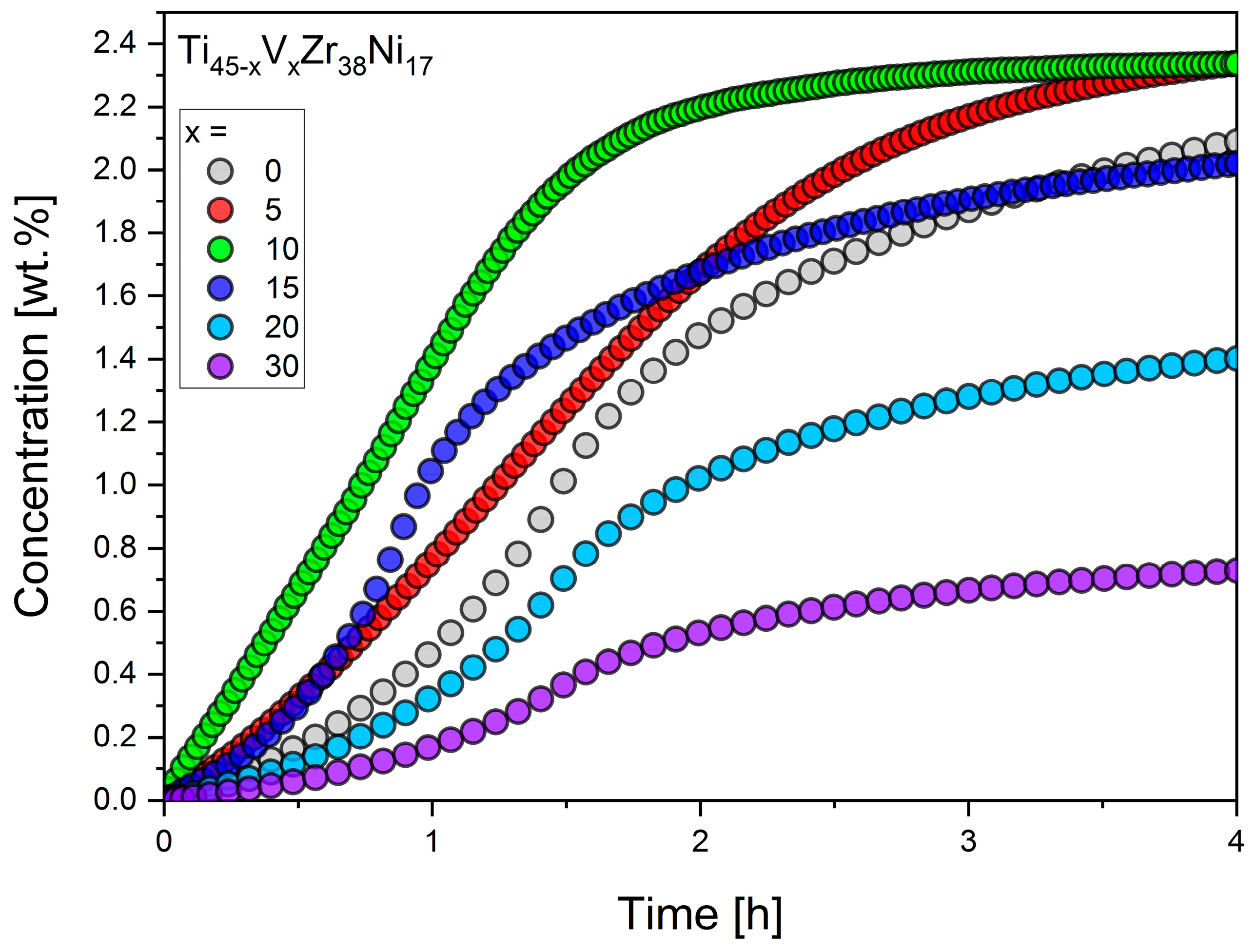
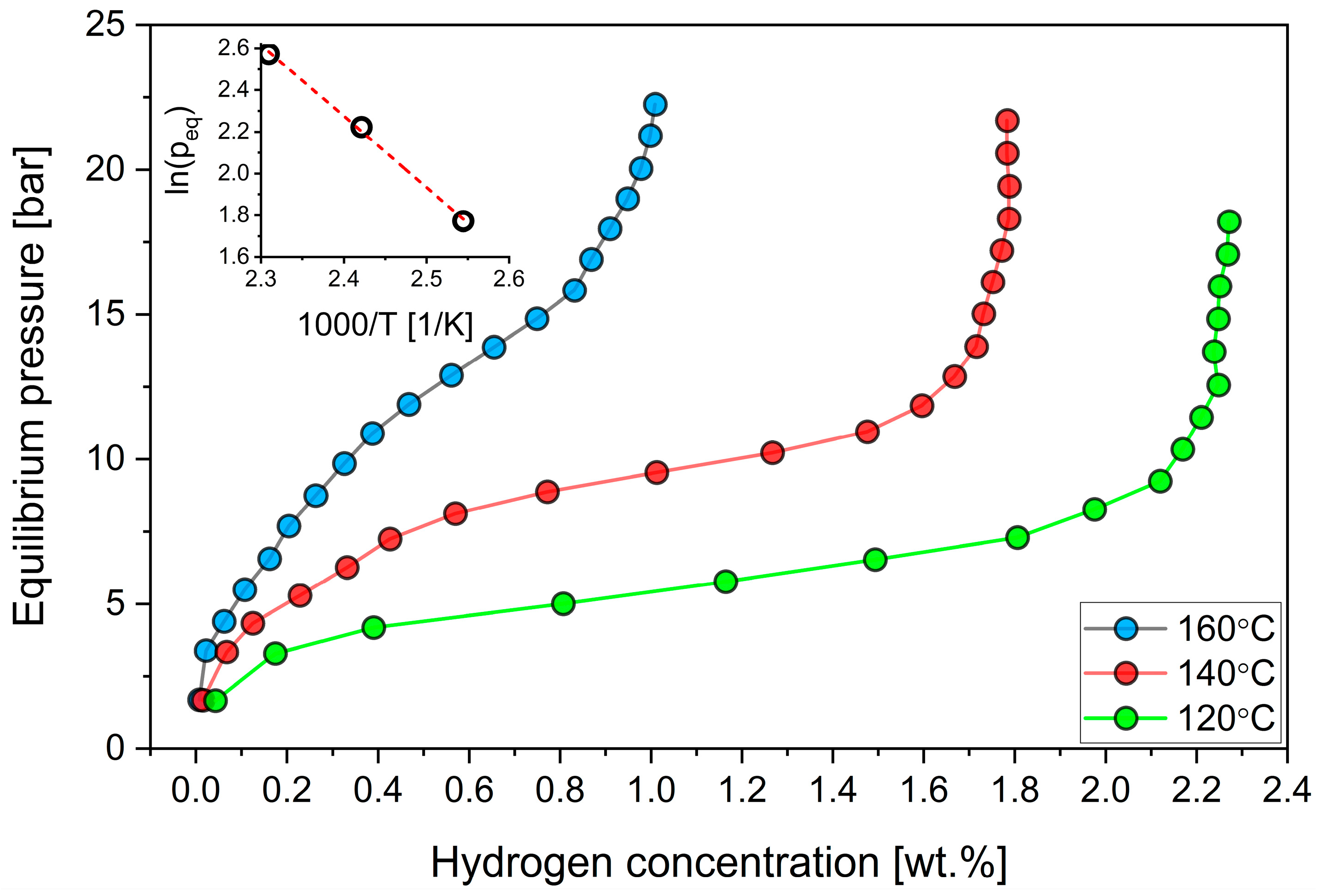

| Compound | Concentration [wt.%] |
|---|---|
| Ti40V5Zr38Ni17 | 2.4 |
| Ti35V10Zr38Ni17 | 2.3 |
| Ti30V15Zr38Ni17 | 2.1 |
| Ti25V20Zr38Ni17 | 1.5 |
| Ti15V30Zr38Ni17 | 0.8 |
| Compound: | Ti [at.%] | V [at.%] | Zr [at.%] | Ni [at.%] |
|---|---|---|---|---|
| Ti40V5Zr38Ni17 | 40.4 | 5.3 | 37.1 | 17.2 |
| Ti35V10Zr38Ni17 | 35.4 | 9.7 | 37.4 | 17.5 |
| Ti30V15Zr38Ni17 | 31.0 | 14.1 | 37.7 | 17.2 |
| Ti25V20Zr38Ni17 | 25.9 | 19.8 | 37.2 | 17.1 |
| Ti15V30Zr38Ni17 | 14.7 | 30.8 | 36.9 | 17.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czub, J.; Takasaki, A.; Hoser, A.; Reehuis, M.; Gondek, Ł. Synthesis and Hydrogenation of the Ti45−xVxZr38Ni17 (5 ≤ x ≤ 40) Mechanically Alloyed Materials. Energies 2023, 16, 5857. https://doi.org/10.3390/en16165857
Czub J, Takasaki A, Hoser A, Reehuis M, Gondek Ł. Synthesis and Hydrogenation of the Ti45−xVxZr38Ni17 (5 ≤ x ≤ 40) Mechanically Alloyed Materials. Energies. 2023; 16(16):5857. https://doi.org/10.3390/en16165857
Chicago/Turabian StyleCzub, Joanna, Akito Takasaki, Andreas Hoser, Manfred Reehuis, and Łukasz Gondek. 2023. "Synthesis and Hydrogenation of the Ti45−xVxZr38Ni17 (5 ≤ x ≤ 40) Mechanically Alloyed Materials" Energies 16, no. 16: 5857. https://doi.org/10.3390/en16165857
APA StyleCzub, J., Takasaki, A., Hoser, A., Reehuis, M., & Gondek, Ł. (2023). Synthesis and Hydrogenation of the Ti45−xVxZr38Ni17 (5 ≤ x ≤ 40) Mechanically Alloyed Materials. Energies, 16(16), 5857. https://doi.org/10.3390/en16165857







