Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkaline Pretreatment of Rice Husk
2.3. Thermogravimetric Analysis (TGA) of Pretreated RH
2.4. Torrefaction Experiments
2.5. Determination of Fuel and Related Properties of Torrefied RH Products
3. Results and Discussion
3.1. Thermochemical Characteristics of Rice Husk
3.2. Fuel Properties of Torrefied RH Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988; pp. 1–26. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: London, UK, 2013; pp. 87–145. [Google Scholar]
- Glaser, B.; Parr, M.; Braun, C.; Kopolo, G. Biochar is carbon negative. Nat. Geosci. 2009, 2, 2. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Oyedeji, O.; Leal, J.H.; Donohoe, B.S.; Semelsberger, T.A.; Li, C.; Hoover, A.N.; Webb, E.; Bose, E.A.; Zeng, Y.; et al. Characterizing variability in lignocellulosic biomass: A review. ACS Sustain. Chem. Eng. 2020, 8, 8059–8085. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.d.O.; Nunes, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the-art and research development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Negi, S.; Jaswal, G.; Dass, K.; Mazumder, K.; Elumalai, S.; Roy, J.K. Torrefaction: A sustainable method for transforming of agri-wastes to high energy density solids (biocoal). Rev. Environ. Sci. Biotechnol. 2020, 19, 463–488. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass torrefaction for the production of high-grade solid biofuels: A review. BioEnergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Knapczyk, A.; Francik, S.; Jewiarz, M.; Zawi’slak, A.; Francik, R. Thermal treatment of biomass: A bibliometric analysis—The torrefaction case. Energies 2021, 14, 162. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K.; Meda, V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. BioEnergy Res. 2021, 14, 645–669. [Google Scholar] [CrossRef]
- Kaniapan, S.; Pasupuleti, J.; Patma Nesan, K.; Abubackar, H.N.; Umar, H.A.; Oladosu, T.L.; Bello, S.R.; Rene, E.R. A Review of the sustainable utilization of rice residues for bioenergy conversion using different valorization techniques, their challenges, and techno-economic assessment. Int. J. Environ. Res. Public Health 2022, 19, 3427. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: A comprehensive utilization of rice husk biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Lu, Q. Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.; Sadrul Islam, A.K.M. Energy yield of torrefied rice husk at atmospheric conditions. Procedia Eng. 2015, 105, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gao, A.; Ma, Z.; Fei, D.; Chang, Y.; Shen, C. In-depth study of rice husk torrefaction: Characterization of solid, liquid and gaseous products, oxygen migration and energy yield. Bioresour. Technol. 2018, 253, 148–153. [Google Scholar] [CrossRef]
- Manatura, K.; Lu, J.H.; Wu, K.T.; Hsu, H.T. Exergy analysis on torrefied rice husk pellet in fluidized bed gasification. Appl. Therm. Eng. 2017, 111, 1016–1024. [Google Scholar] [CrossRef]
- Aslam, U.; Ramzan, N.; Lqbal, T.; Sharif, S.; Hasan, S.W.; Malik, A. Enhancement of fuel characteristics of rice husk via torrefaction process. Waste Manag. Res. 2019, 37, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yu, D.; Yu, X.; Liu, F.; Wu, J.; Zeng, X.; Yu, G.; Xu, M. Effect of the torrefaction on the emission of PM10 from combustion of rice husk and its blends with a lignite. Proc. Combust. Inst. 2019, 37, 2733–2740. [Google Scholar] [CrossRef]
- Qi, R.; Chen, Z.; Wang, M.; Wu, R.; Jiang, E. Prediction method for torrefied rice husk based on gray-scale analysis. ACS Omega 2019, 4, 17837–17842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Su, Y.; Xiong, Y.; Zhang, H. Physicochemical structure and reactivity of char from torrefied rice husk: Effects of inorganic species and torrefaction temperature. Fuel 2020, 262, 116667. [Google Scholar] [CrossRef]
- Chen, C.; Ji, G.; Mu, L.; Zhang, Y.; Li, A. Comprehensive research on the solid, liquid, and gaseous products of rice husk and rice straw torrefaction. Sustain. Energy Fuels 2021, 5, 687–697. [Google Scholar] [CrossRef]
- Tsai, W.T.; Jiang, T.J.; Tang, M.S.; Chang, C.H.; Kuo, T.H. Enhancement of thermochemical properties on rice husk under a wide range of torrefaction conditions. Biomass Conv. Bioref. 2021, 2021, 455. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Zhu, Y.; Niu, Y.; Tan, H.; Wang, X. Short review on the origin and countermeasure of biomass slagging in grate furnace. Front. Environ. Res. 2014, 2, 7. [Google Scholar]
- Chen, C.; Bi, Y.; Huang, Y.; Huang, H. Review on slagging evaluation methods of biomass fuel combustion. J. Anal. Appl. Pyrolysis 2021, 135, 105082. [Google Scholar] [CrossRef]
- Lachman, J.; Balas, M.; Lisy, M.; Lisa, H.; Milcak, P.; Elbl, P. An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Usmani, T.H.; Ahmad, T.W.; Yousufzai, A.H.K. Preparation and liquid-phase characterization of granular activated-carbon from rice husk. Bioresour. Technol. 1994, 48, 31–35. [Google Scholar] [CrossRef]
- Hsieh, Y.; Du, Y.; Jin, F.; Zhou, Z.; Enomoto, H. Alkaline pre-treatment of rice hulls for hydrothermal production of acetic acid. Chem. Eng. Res. Des. 2009, 87, 13–18. [Google Scholar] [CrossRef]
- Liou, T.H.; Wu, S.J. Characteristics of microporous/mesoporous carbons prepared from rice husk under base- and acid-treated conditions. J. Hazard. Mater. 2009, 171, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, A.; Bazargan, M.; McKay, G. Optimization of rice husk pretreatment for energy production. Renew. Energy 2015, 77, 512–520. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Characterization and alkaline pretreatment of rice husk varieties in Uganda for potential utilization as precursors in the production of activated carbon and other value-added products. Waste Manag. 2018, 81, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y.; John, M.J. Effect of alkaline pretreatment on the thermal behavior and chemical properties of rice husk varieties in relation to activated carbon production. J. Therm. Anal. Calorim. 2020, 139, 1681–1691. [Google Scholar] [CrossRef]
- Park, J.Y.; Gu, Y.M.; Park, S.Y.; Hwang, E.T.; Sang, B.-I.; Chun, J.; Lee, J.H. Two-stage continuous process for the extraction of silica from rice husk using attrition ball milling and alkaline leaching methods. Sustainability 2021, 13, 7350. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice husk: Product yields and compositions. Bioresour. Technol. 2007, 98, 22–28. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Huang, H.J. Valorization of rice husk for the production of porous biochar materials. Fermentation 2021, 7, 70. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chung, M.H.; Chu, M.H.; Huang, H.J.; Jao, Y.H.; Yeh, S.I. Conversion of water caltrop husk into biochar by torrefaction. Energy 2020, 195, 116967. [Google Scholar] [CrossRef]
- Almeida, G.; Brito, J.O.; Perre, P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: The potential of mass loss as a synthetic indicator. Bioresour. Technol. 2010, 101, 9778–9784. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, M.; Adi, A.M.; Suhada, N.; Malek, N.H.; Saringat, M.I.; Azdarpour, A. Optimization of palm kernel shell torrefaction to produce energy densified bio-coal. Energy Convers. Manag. 2014, 88, 1086–1093. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A. Fuel property enhancement of lignocellulosic and nonlignocellulosic biomass through torrefaction. Biomass Conv. Bioref. 2016, 6, 139–149. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, P.C.; Lin, Y.Q. Reusing Cow manure for the production of activated carbon using potassium hydroxide (KOH) activation process and its liquid-phase adsorption performance. Processes 2019, 7, 737. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.S.; Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chang, Y.T. Production of Highly Porous Biochar Materials from Spent Mushroom Composts. Horticulturae 2022, 8, 46. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of animal-origin ash—A valuable material for circular economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Johnston, C.T. Biochar analysis by Fourier-transform infra-red spectroscopy. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 199–228. [Google Scholar]

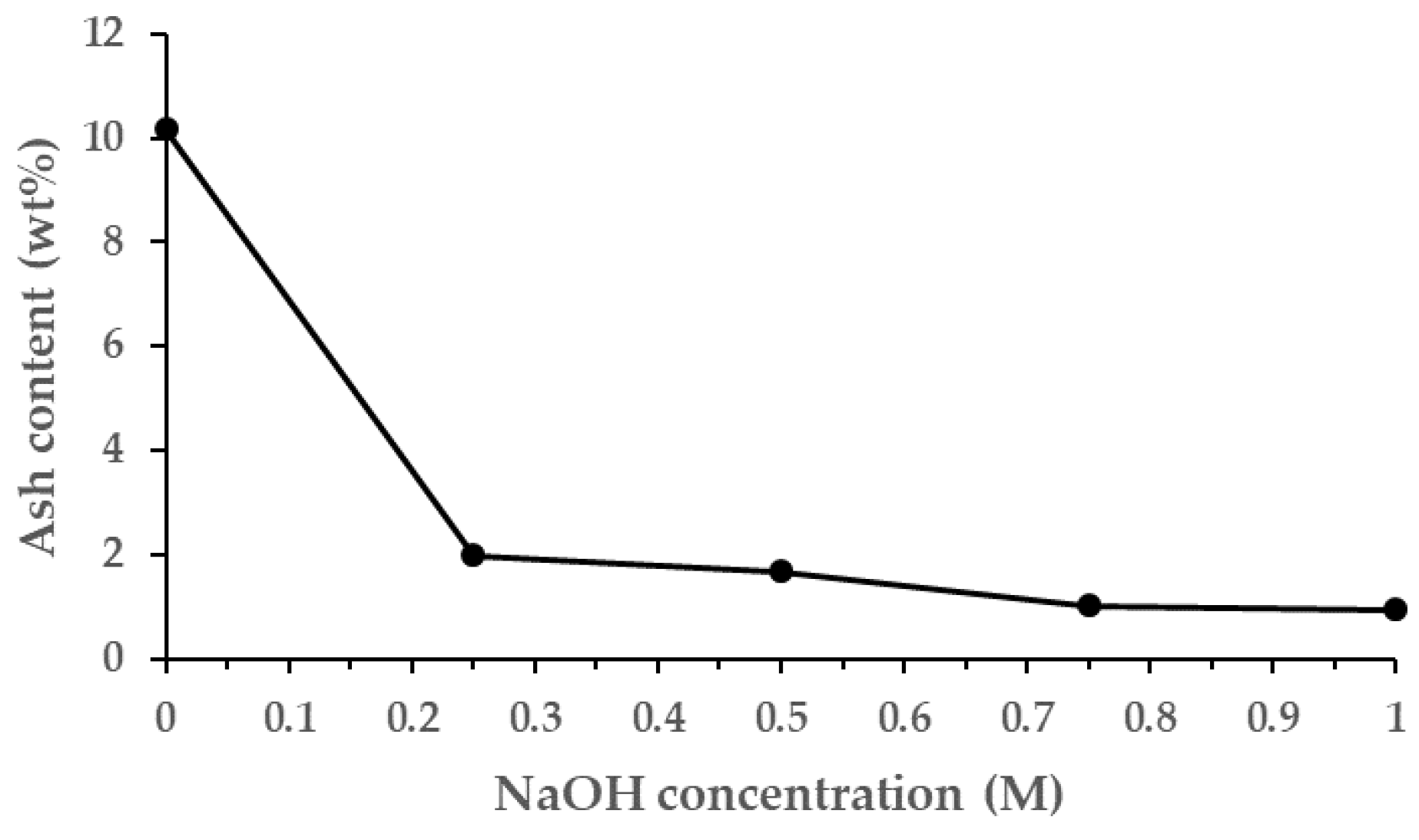

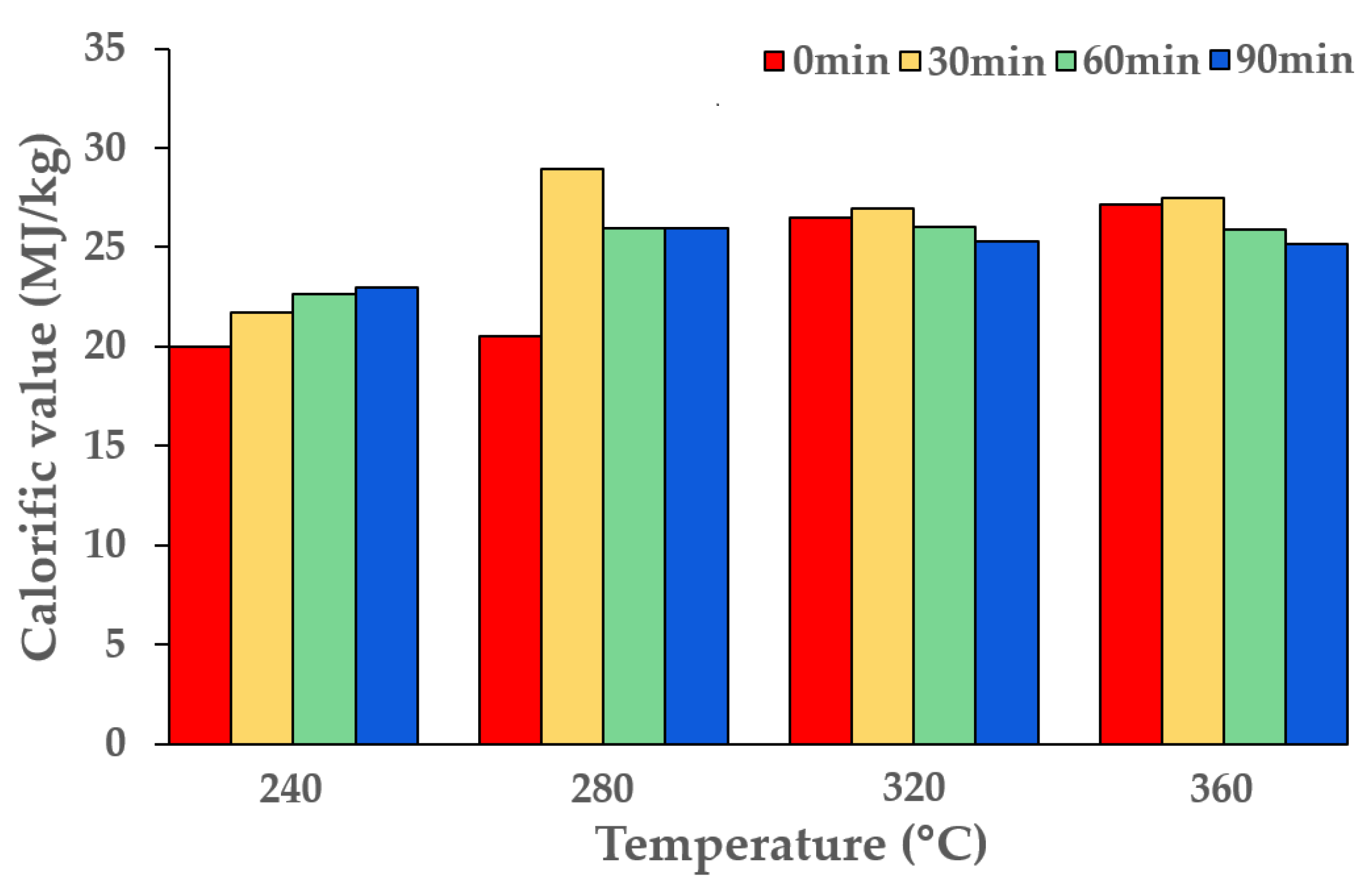

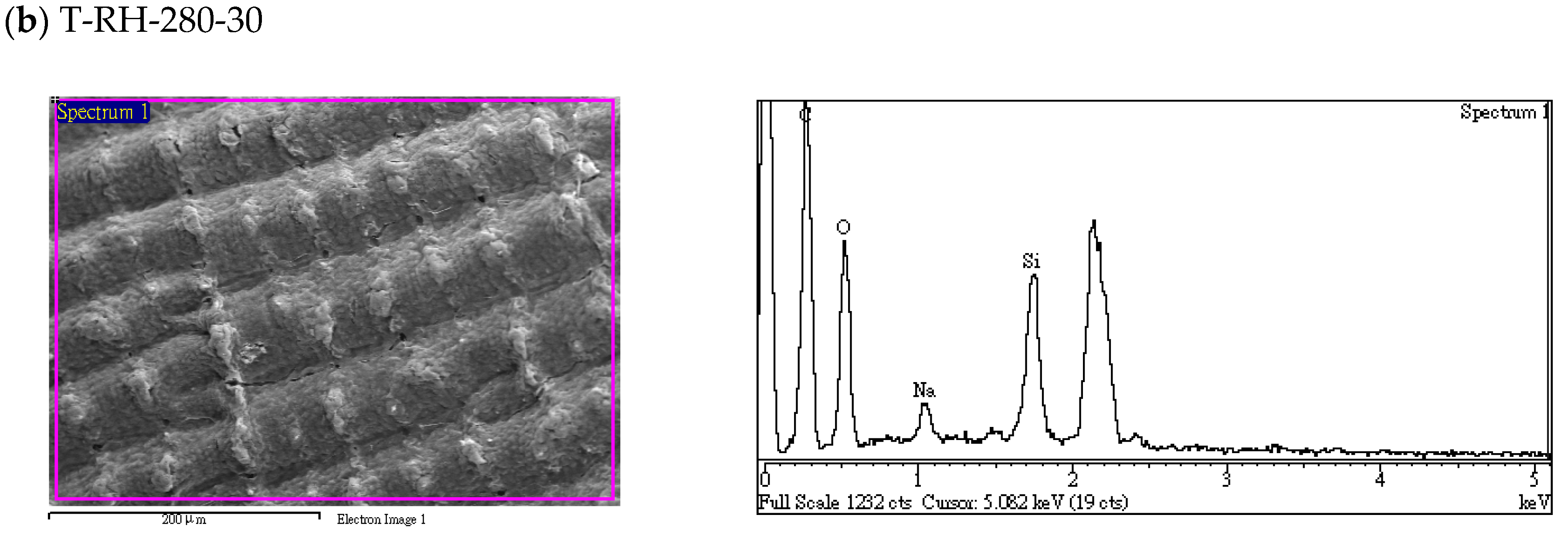

| RH-Torrefied Product a | Mass Yield (MYT-RH, -) | Calorific Value a (MJ/kg) | Enhancement Factor b (EFT-SP, -) | Energy Yield c (EYT-SP, -) |
|---|---|---|---|---|
| T-RH-240-0 d | 0.981 | 19.98 | 1.07 | 1.05 |
| T-RH-240-30 | 0.812 | 21.72 | 1.16 | 0.95 |
| T-RH-240-60 | 0.727 | 22.68 | 1.21 | 0.88 |
| T-RH-240-90 | 0.695 | 22.97 | 1.23 | 0.85 |
| T-RH-280-0 | 0.861 | 20.52 | 1.09 | 0.94 |
| T-RH-280-30 | 0.588 | 28.97 | 1.55 | 0.91 |
| T-RH-280-60 | 0.508 | 25.96 | 1.38 | 0.70 |
| T-RH-280-90 | 0.478 | 25.94 | 1.38 | 0.66 |
| T-RH-320-0 | 0.551 | 26.46 | 1.41 | 0.78 |
| T-RH-320-30 | 0.449 | 26.93 | 1.44 | 0.65 |
| T-RH-320-60 | 0.411 | 26.02 | 1.39 | 0.57 |
| T-RH-320-90 | 0.371 | 25.27 | 1.35 | 0.50 |
| T-RH-360-0 | 0.479 | 27.13 | 1.45 | 0.69 |
| T-RH-360-30 | 0.382 | 27.47 | 1.47 | 0.56 |
| T-RH-360-60 | 0.324 | 25.91 | 1.38 | 0.45 |
| T-RH-360-90 | 0.285 | 25.15 | 1.34 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Shen, Y.-H.; Tsai, W.-T. Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk. Energies 2023, 16, 679. https://doi.org/10.3390/en16020679
Tsai C-H, Shen Y-H, Tsai W-T. Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk. Energies. 2023; 16(2):679. https://doi.org/10.3390/en16020679
Chicago/Turabian StyleTsai, Chi-Hung, Yun-Hwei Shen, and Wen-Tien Tsai. 2023. "Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk" Energies 16, no. 2: 679. https://doi.org/10.3390/en16020679






