Hydrogen-Rich Syngas Production via Dry and Steam Reforming of Methane in Simulated Producer Gas over ZSM-5-Supported Trimetallic Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Test Gases
2.3. Experimental System and Method
- Conversion,
- Hydrogen yield,
- Hydrogen selectivity,
2.4. Catalyst Characterization
3. Results and Discussion
3.1. Catalyst Characterization
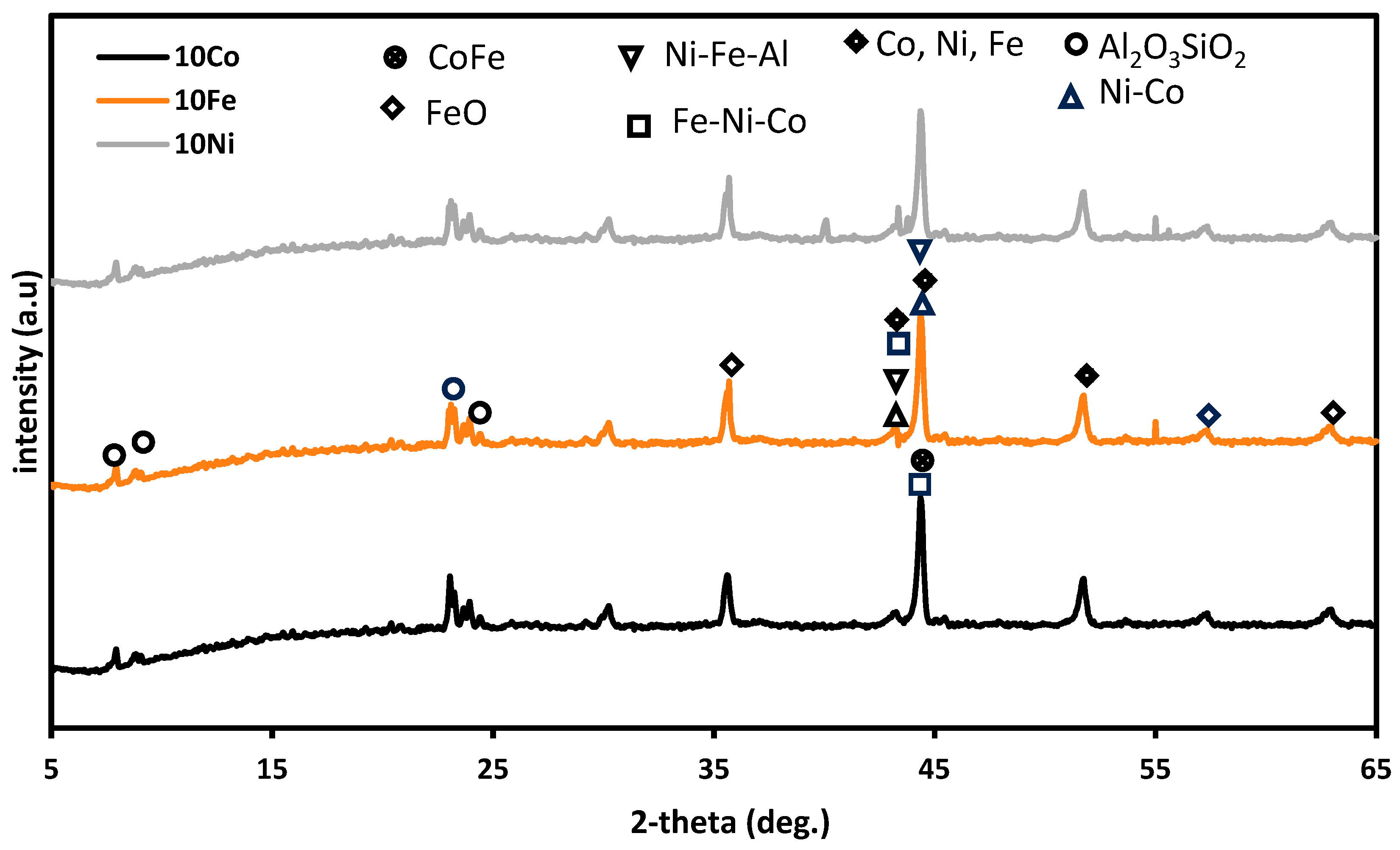
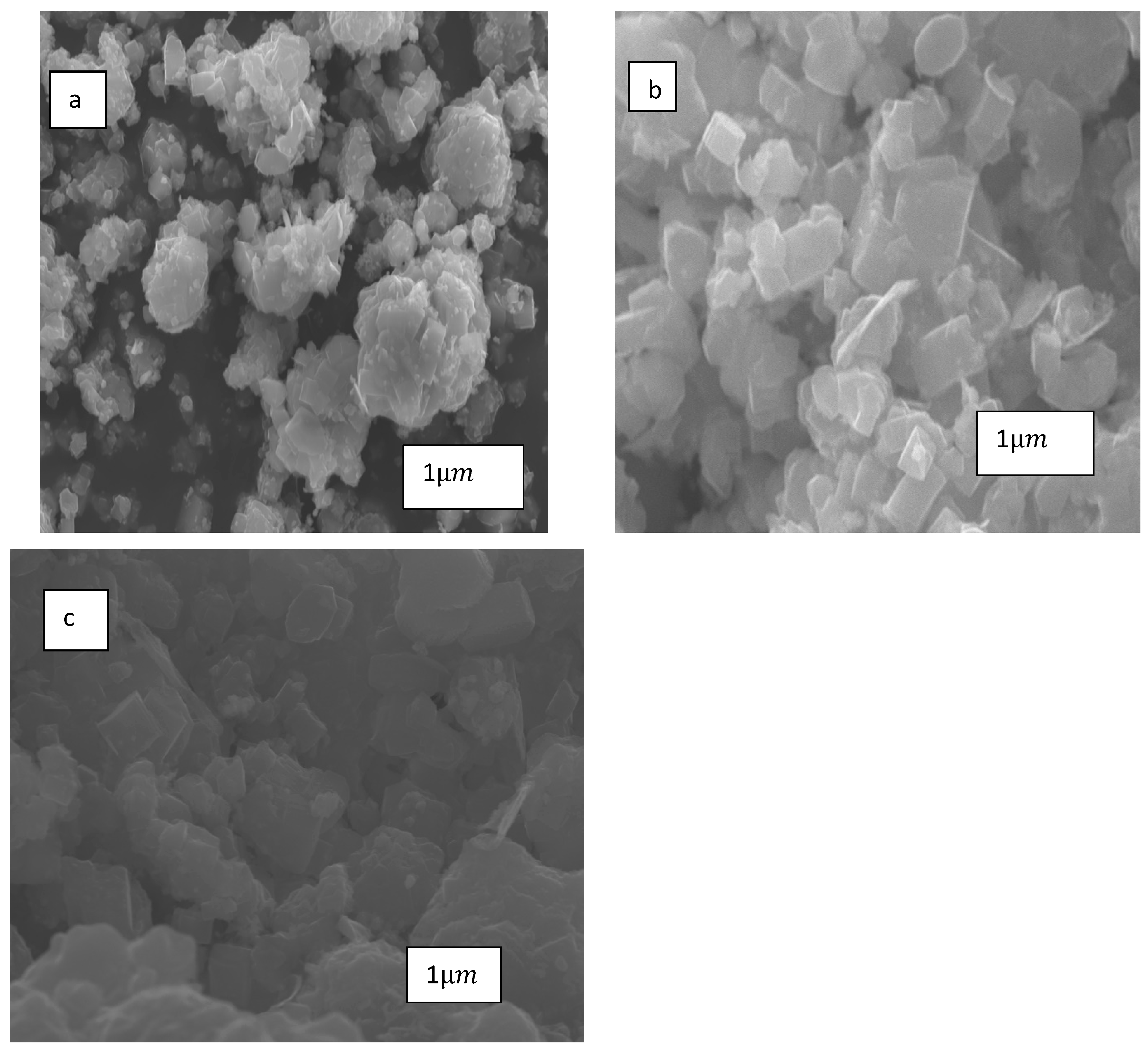
3.1.1. X-ray Diffraction (XRD)
3.1.2. Temperature Programmed Reduction (TPR)
3.1.3. N2-Adsorption/Desorption Analysis
3.2. Performance of Catalysts in Methane Reforming Reactions
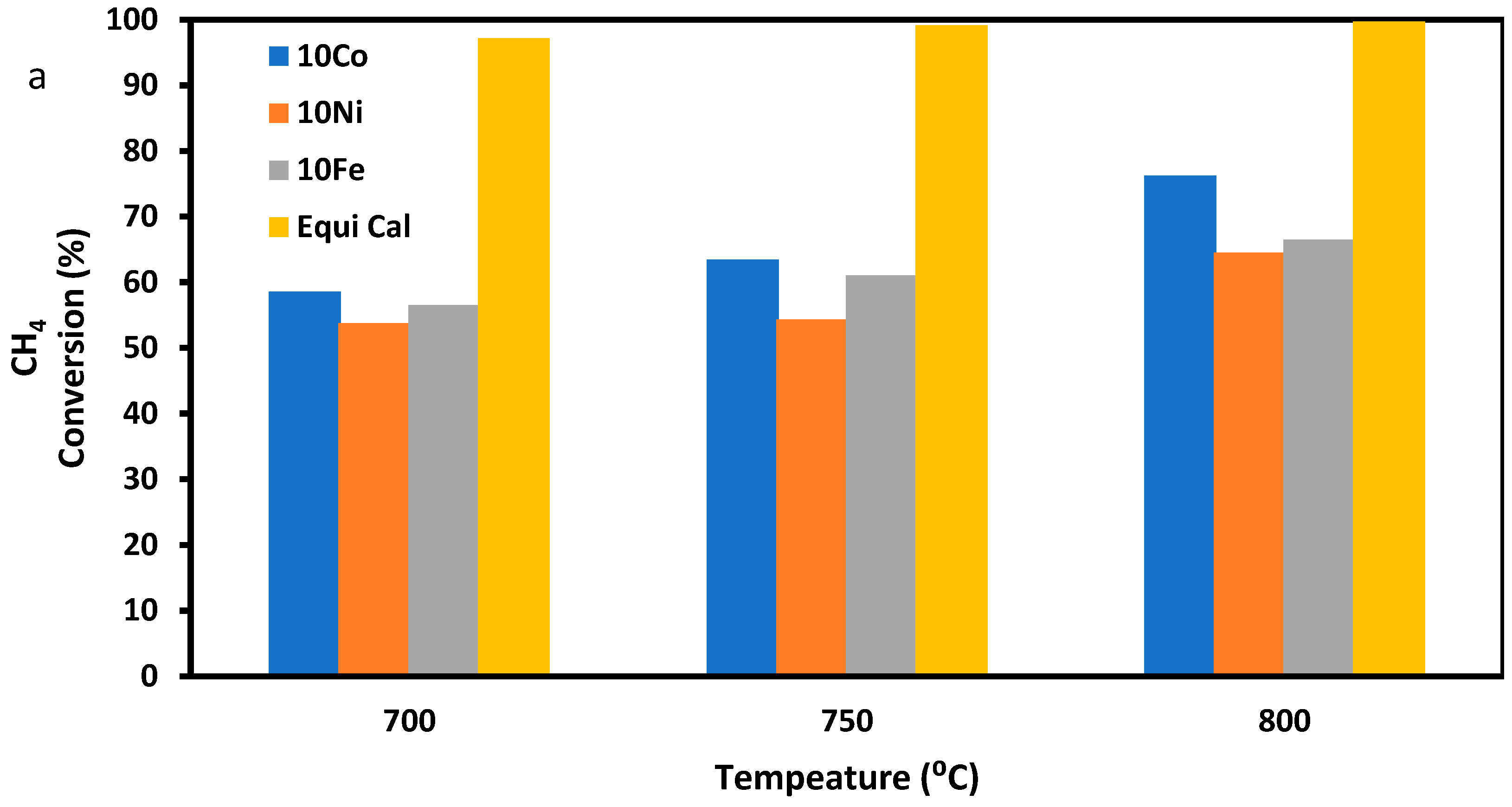
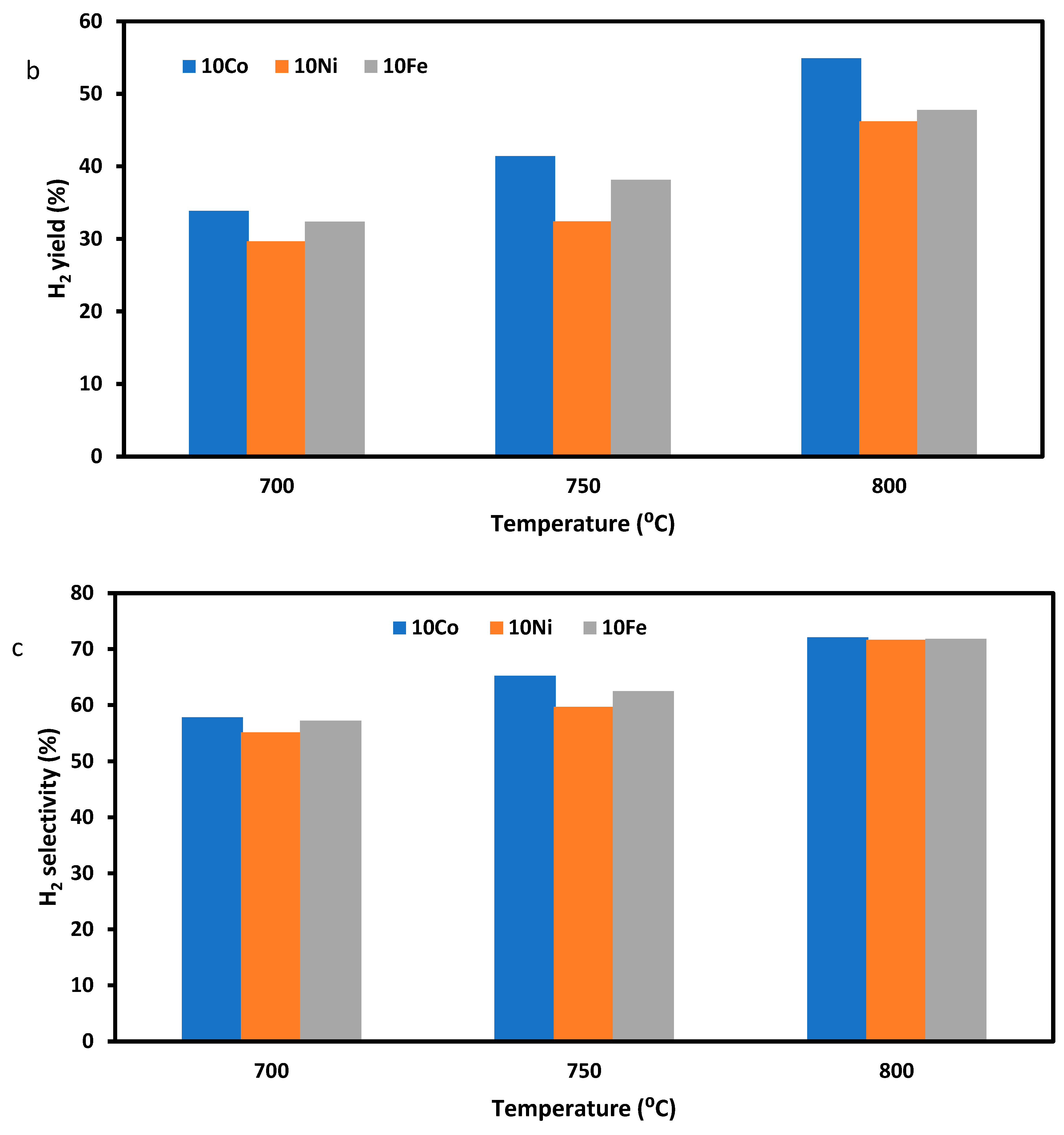
3.2.1. SRM with Pure Methane as the Feeding Gas
3.2.2. Catalyst Stability Performance
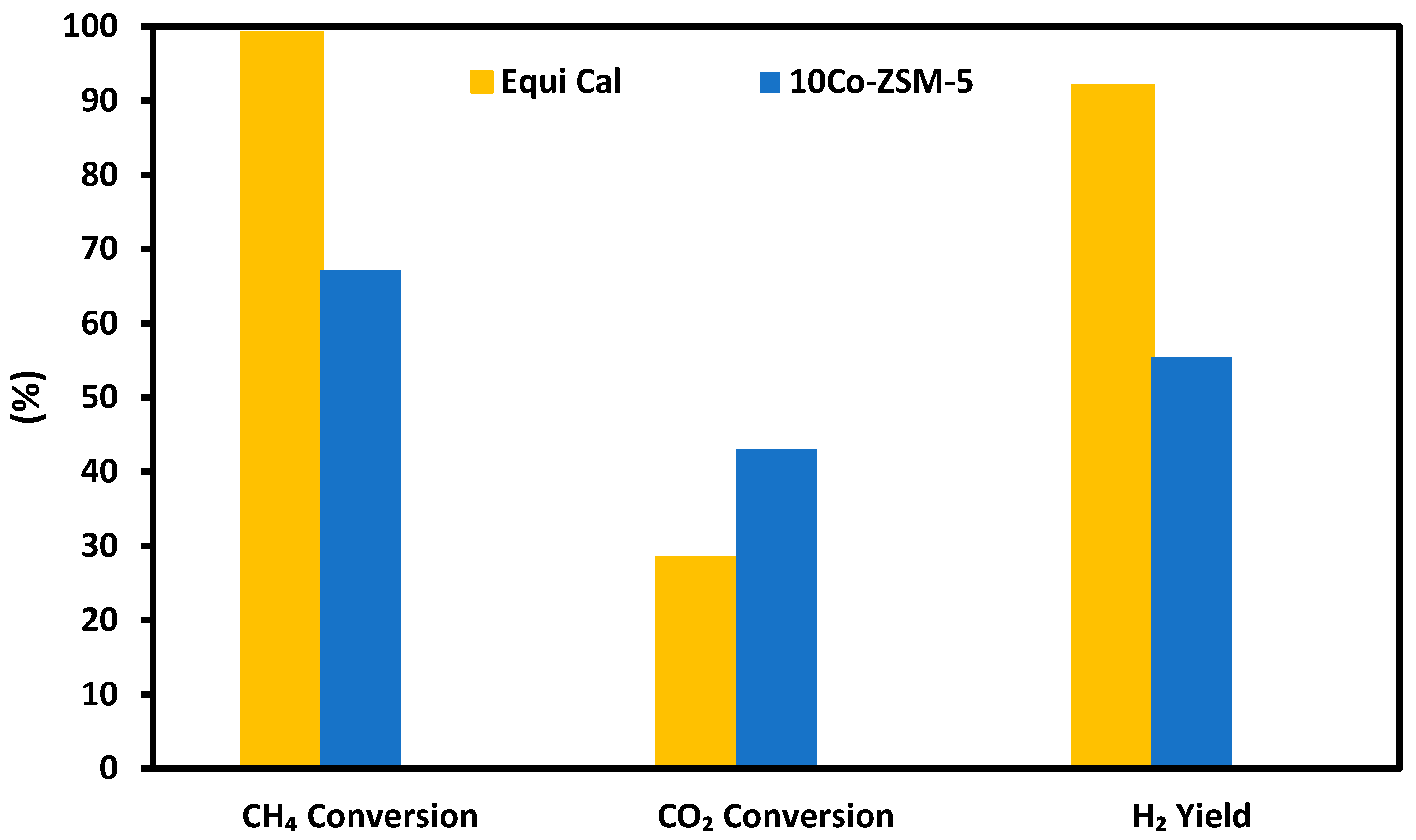
3.3. Performance of Catalyst in the SRM of Simulated Producer Gas
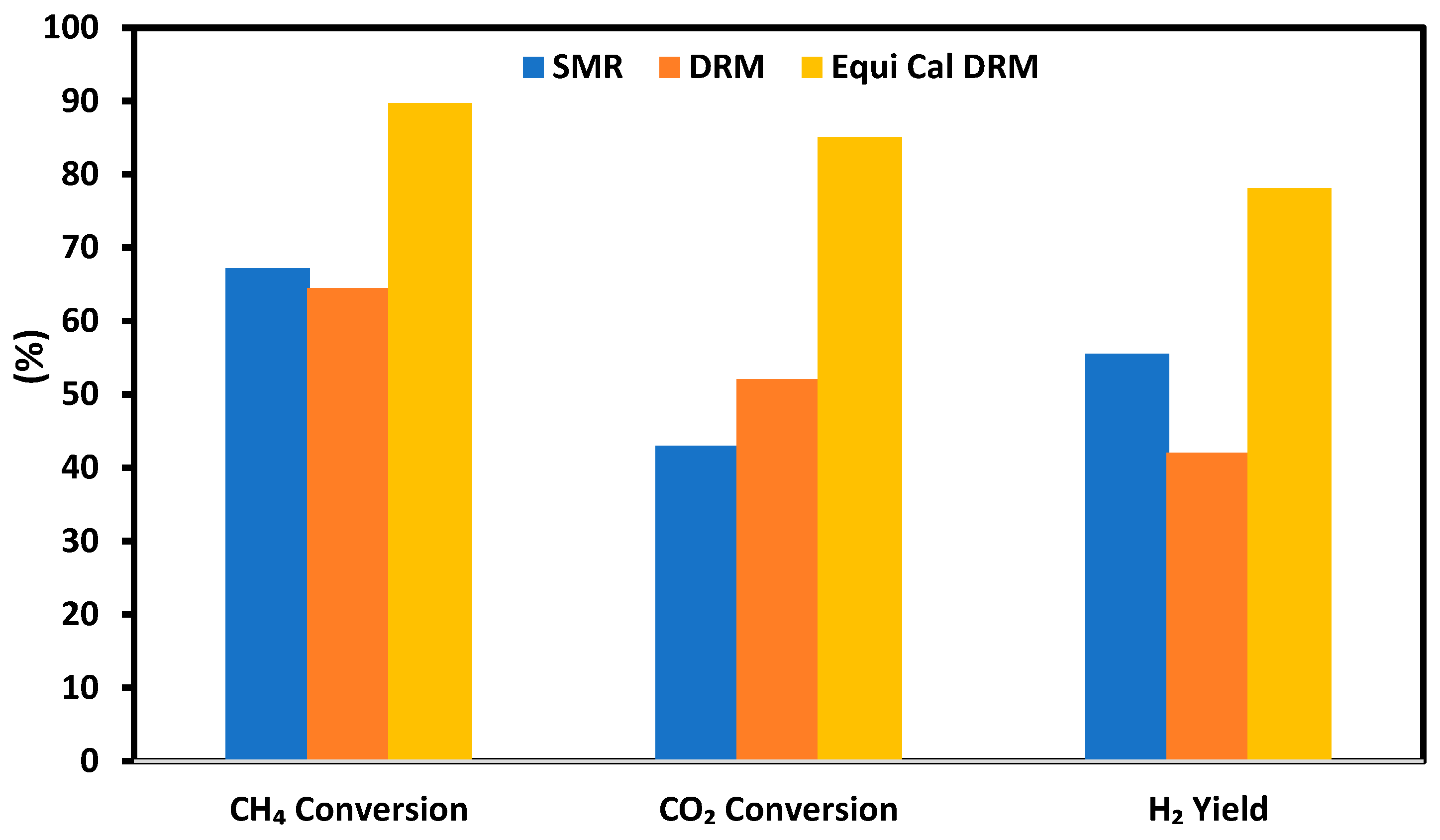
3.4. Effect of Steam Feeding in the SRM
3.5. Effect of Gas Hourly Space Velocity
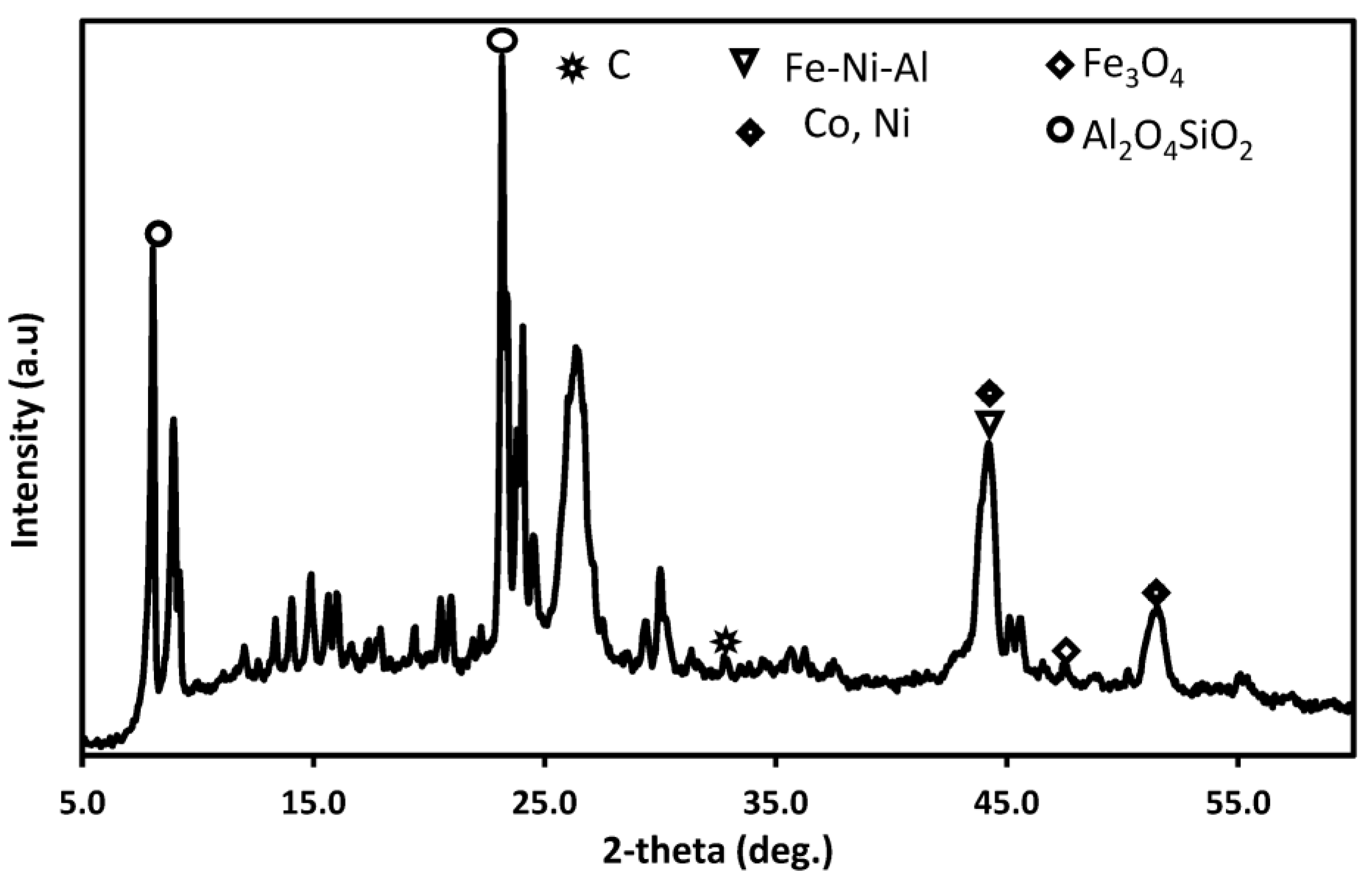
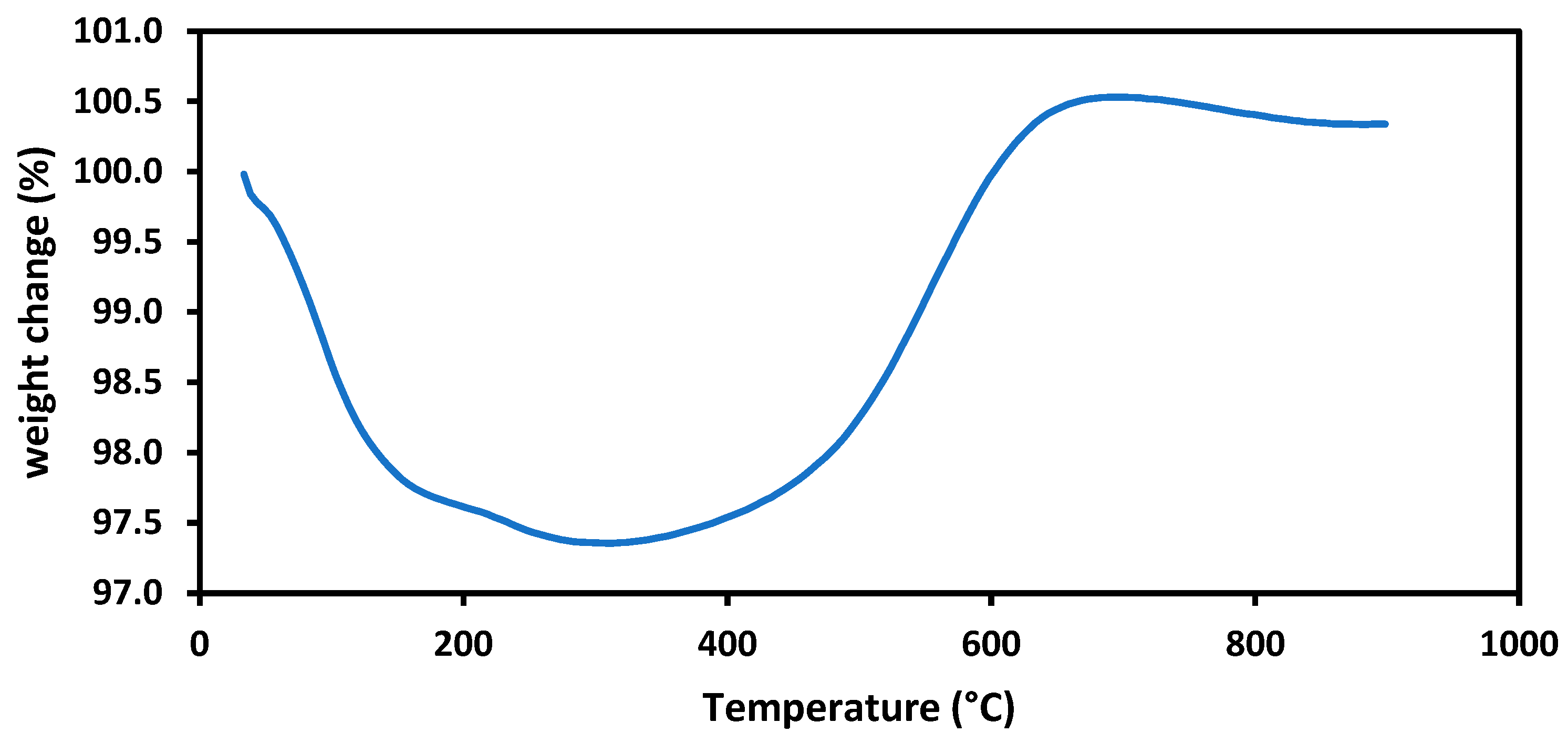
3.6. Changes in Catalyst Characteristics through SRM and DRM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Kim, A.; Lee, B.; Lim, H. Comparative numerical analysis for an efficient hydrogen production via a steam methane reforming with a packed-bed reactor, a membrane reactor, and a sorption-enhanced membrane reactor. Energy Convers. Manag. 2020, 213, 112839. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Hydrogen production from oxygenated hydrocarbons: Review of catalyst development, reaction mechanism and reactor modeling. In Hydrogen Production Technologies; Scrivener Publishing LLC: Beverly, MA, USA, 2017; pp. 1–76. [Google Scholar]
- Peres, A.P.; Lunelli, B.H.; Maciel FIlho, R. Application of biomass to hydrogen and syngas production. Chem. Eng. Trans. 2013, 32, 589–594. [Google Scholar]
- Chiari, L.; Zecca, A. Constraints of fossil fuels depletion on global warming projections. Energy Policy 2011, 39, 5026–5034. [Google Scholar] [CrossRef]
- Lu, B.; Ju, Y.; Abe, T.; Kawamoto, K. Hydrogen-enriched producer gas production and chemical conversion to usable gas product through biomass gasification using NiO nanoparticles dispersed on SBA-15. J. Nanosci. Nanotechnol. 2017, 17, 6190–6197. [Google Scholar] [CrossRef]
- Chang, A.C.; Chang, H.F.; Lin, F.J.; Lin, K.H.; Chen, C.H. Biomass gasification for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Hongrapipat, J. Removal of NH3 and H2S from Biomass Gasification Producer Gas. Ph.D. Thesis, University of Canterbury, Canterbury, New Zealand, 2014. [Google Scholar]
- Speight, J.G. Types of gasifier for synthetic liquid fuel production: Design and technology. In Gasification for Synthetic Fuel Production; Woodhead Publishing: Cambridge, UK, 2015; pp. 29–55. [Google Scholar]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Peiran, Z.; Abbas, T.; Dirk, P. Life cycle Assessment of hydrogen production via natural gas steam reforming vs. biomass gasification. Preprints 2022, 2022010112. [Google Scholar] [CrossRef]
- Torrez-Herrera, J.J.; Korili, S.A.; Gil, A. Recent progress in the application of Ni-based catalysts for the dry reforming of methane. Catal. Rev. 2021, 65, 1300–1357. [Google Scholar] [CrossRef]
- De Llobet, S.; Pinilla, J.L.; Moliner, R.; Suelves, I. Relationship between carbon morphology and catalyst deactivation in the catalytic decomposition of biogas using Ni, Co and Fe based catalysts. Fuel 2015, 139, 71–78. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Rostrupnielsen, J.R.; Hansen, J.B. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Jin, F.; Fu, Y.; Kong, W.; Wang, J.; Cai, F.; Zhang, J.; Xu, J. Dry reforming of methane over trimetallic NiFeCu alloy catalysts. Chem. Phys. Lett. 2020, 750, 137491. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 perovskites as precursors for the preparation of coke-resistant dry reforming catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Sabbe, M.; Poelman, H.; Detavernier, C.; Marin, G.B. Controlling the stability of a Fe–Ni reforming catalyst: Structural organization of the active components. Appl. Catal. B Environ. 2017, 209, 405–416. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Dharanipragada, N.A.; Longo, A.; Meledina, M.; Marin, G.B. Fe-containing magnesium aluminate support for stability and carbon control during methane reforming. ACS Catal. 2018, 8, 5983–5995. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of alloying for steam or dry reforming of methane: A review of recent studies. Catal. Sci. Technol. 2022, 12, 3375–3738. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Kurdymov, S.S.; Konstantinov, G.I.; Murzin, V.Y.; Bukhtenko, O.V.; Maksimov, Y.V. Core-shell bifunctional catalyst for steam methane reforming resistant to H2S: Activity and structure evolution. Int. J. Hydrogen Energy 2015, 40, 2963–2970. [Google Scholar] [CrossRef]
- Djaidja, A.; Messaoudi, H.; Kaddeche, D.; Barama, A. Study of Ni–M/MgO and Ni–M–Mg/Al (M = Fe or Cu) catalysts in the CH4–CO2 and CH4–H2O reforming. Int. J. Hydrogen Energy 2015, 40, 4989–4995. [Google Scholar] [CrossRef]
- Estephane, J.; Aouad, S.; Hany, S.; El Khoury, B.; Gennequin, C.; El Zakhem, H.; El Nakat, J.; Aboukaïs, A.; Abi Aad, E. CO2 reforming of methane over Ni–Co/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrogen Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Siang, T.J.; Singh, S.; Omoregbe, O.; Bach, L.G.; Phuc, N.H.H.; Vo, D.V.N. Hydrogen production from CH4 dry reforming over bimetallic Ni–Co/Al2O3 catalyst. J. Energy Inst. 2018, 91, 683–694. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Yuan, P.; Chen, X. Ni–Co/Al2O3 bimetallic catalysts for CH4 steam reforming: Elucidating the role of Co for improving coke resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, J.; Xiao, D.; Su, D.; Ma, D. Lattice strained Ni-Co alloy as a high-performance catalyst for catalytic dry reforming of methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Crawley, J.W.; Gow, I.E.; Lawes, N.; Kowalec, I.; Kabalan, L.; Catlow, C.R.A.; Logsdail, J.; Taylor, S.H.; Hutchings, G.J. Heterogeneous trimetallic nanoparticles as catalysts. Chem. Rev. 2022, 122, 6795–6849. [Google Scholar] [CrossRef]
- Al-Doghachi, F.A.; Rashid, U.; Taufiq-Yap, Y.H. Investigation of Ce (III) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane. RSC Adv. 2016, 6, 10372–10384. [Google Scholar] [CrossRef]
- Kozonoe, C.E.; Bonfim, R.D.P.F.; Alves, R.M.B.; Schmal, M. The Fe-Co-Cu supported on MWCNT as catalyst for the tri-reforming of methane–investigating the structure changes of the catalysts. Fuel 2019, 256, 115917. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Xie, B.; Xia, S. Theoretical investigation onto the reaction mechanism of dry reforming of methane on core–shell Cu-Ni-Pt ternary alloy clusters. Chem. Phys. Lett. 2021, 781, 138975. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Dalena, F.; Giglio, E.; Marino, A.; Aloise, A.; Giorgianni, G.; Migliori, M.; Giordano, G. Steam reforming of bioethanol using metallic catalysts on zeolitic supports: An overview. Catalysts 2022, 12, 617. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, M.; Quan, C.; Zheng, Y. Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst. Fuel 2020, 273, 117702. [Google Scholar] [CrossRef]
- Ahmed, T.; Xiu, S.; Wang, L.; Shahbazi, A. Investigation of Ni/Fe/Mg zeolite-supported catalysts in steam reforming of tar using simulated-toluene as model compound. Fuel 2018, 211, 566–571. [Google Scholar] [CrossRef]
- Majewska, J.; Michalkiewicz, B. Production of hydrogen and carbon nanomaterials from methane using Co/ZSM-5 catalyst. Int. J. Hydrogen Energy 2016, 41, 8668–8678. [Google Scholar] [CrossRef]
- Pour, A.N.; Mousavi, M. Combined reforming of methane by carbon dioxide and water: Particle size effect of Ni–Mg nanoparticles. Int. J. Hydrogen Energy 2015, 40, 12985–12992. [Google Scholar] [CrossRef]
- Wang, Y. Removal of H2S, NH3 and Tars from the Producer Gas of Biomass Gasification by Secondary Measures. Ph.D. Thesis, University of Canterbury, Canterbury, New Zealand, 2018. [Google Scholar]
- Iminabo, M.; Yip, A.C.K.; Iminabo, J.T.; Pang, S. High-temperature catalytic pyrolysis of radiata pine for production of high-value products. Biomass Convers. Biorefinery 2022, 1–19. [Google Scholar] [CrossRef]
- Khan, W.U.; Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Abasaeed, A.E. La2O3 supported bimetallic catalysts for the production of hydrogen and carbon nanomaterials from methane. Int. J. Hydrogen Energy 2016, 41, 976–983. [Google Scholar] [CrossRef]
- Wang, I.W.; Kutteri, D.A.; Gao, B.; Tian, H.; Hu, J. Methane pyrolysis for carbon nanotubes and COx-free H2 over transition-metal catalysts. Energy Fuels 2018, 33, 197–205. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 2015, 330, 418–430. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane decomposition over Ni–Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrogen Energy 2016, 41, 1574–1584. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and Mesoporous Materials from Natural and Inexpensive Sources. In Handbook of Ecomaterials; Torres Martínez, L.M., Kharisova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2017; Volume 136, pp. 3379–3399. [Google Scholar]
- Maier, L.; Schädel, B.; Herrera Delgado, K.; Tischer, S.; Deutschmann, O. Steam reforming of methane over nickel: Development of a multi-step surface reaction mechanism. Top. Catal. 2011, 54, 845–858. [Google Scholar] [CrossRef]
- Raju, A.S.; Park, C.S.; Norbeck, J.M. Synthesis gas production using steam hydrogasification and steam reforming. Fuel Process. Technol. 2009, 90, 330–336. [Google Scholar] [CrossRef]
- Chibane, L.; Djellouli, B. Methane steam reforming reaction behaviour in a packed bed membrane reactor. Int. J. Chem. Eng. Appl. 2011, 2, 147. [Google Scholar] [CrossRef]
- Jawad, A.; Rezaei, F.; Rownaghi, A.A. Highly efficient Pt/Mo-Fe/Ni-based Al2O3-CeO2 catalysts for dry reforming of methane. Catal. Today 2020, 350, 80–90. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane decomposition over iron catalyst for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. COx-free hydrogen and carbon nanofibers production by methane decomposition over nickel-alumina catalysts. Korean J. Chem. Eng. 2016, 33, 490–499. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K.I. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2005, 232, 268–275. [Google Scholar] [CrossRef]
- Batebi, D.; Abedini, R.; Mosayebi, A. Combined steam and CO2 reforming of methane (CSDRM) over Ni–Pd/Al2O3 catalyst for syngas formation. Int. J. Hydrogen Energy 2020, 45, 14293–14310. [Google Scholar] [CrossRef]
- Danilova, M.M.; Fedorova, Z.A.; Zaikovskii, V.I.; Porsin, A.V.; Kirillov, V.A.; Krieger, T.A. Porous nickel-based catalysts for combined steam and carbon dioxide reforming of methane. Appl. Catal. B Environ. 2014, 147, 858–863. [Google Scholar] [CrossRef]
- Schwengber, C.A.; da Silva, F.A.; Schaffner, R.A.; Fernandes-Machado NR, C.; Ferracin, R.J.; Bach, V.R.; Alves, H.J. Methane dry reforming using Ni/Al2O3 catalysts: Evaluation of the effects of temperature, space velocity and reaction time. J. Environ. Chem. Eng. 2016, 4, 3688–3695. [Google Scholar] [CrossRef]
- Ullah, K.S.; Omer, A.; Rashid, K.; Rehman, N.U.; Rahimipetroudi, I.; Kim, S.D.; Dong, S.K. Modeling and comprehensive analysis of hydrogen production in a newly designed steam methane reformer with membrane system. Comput. Chem. Eng. 2023, 175, 108278. [Google Scholar] [CrossRef]






| S/N | Catalyst | Surface Area (m2/g) | BJH Adsorption Pore Volume (cm3/g) | BJH Adsorption Pore Diameter (nm) |
|---|---|---|---|---|
| 1 | 10Co-ZSM5 | 247 | 0.035 | 3 |
| 2 | 10Fe-ZSM5 | 211 | 0.037 | 3 |
| 3 | 10Ni-ZSM5 | 242 | 0.033 | 3 |
| 4 | ZSM-5 | 417 | 0.051 | 2 |
| 5 | Reduced 10Co-ZSM5 | 229 | 0.028 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iminabo, J.T.; Iminabo, M.; Yip, A.C.K.; Pang, S. Hydrogen-Rich Syngas Production via Dry and Steam Reforming of Methane in Simulated Producer Gas over ZSM-5-Supported Trimetallic Catalysts. Energies 2023, 16, 7518. https://doi.org/10.3390/en16227518
Iminabo JT, Iminabo M, Yip ACK, Pang S. Hydrogen-Rich Syngas Production via Dry and Steam Reforming of Methane in Simulated Producer Gas over ZSM-5-Supported Trimetallic Catalysts. Energies. 2023; 16(22):7518. https://doi.org/10.3390/en16227518
Chicago/Turabian StyleIminabo, John Tamunosaki, Misel Iminabo, Alex C. K. Yip, and Shusheng Pang. 2023. "Hydrogen-Rich Syngas Production via Dry and Steam Reforming of Methane in Simulated Producer Gas over ZSM-5-Supported Trimetallic Catalysts" Energies 16, no. 22: 7518. https://doi.org/10.3390/en16227518






