Numerical Optimization of Triple-Phase Components in Order-Structured Cathode Catalyst Layer of a Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
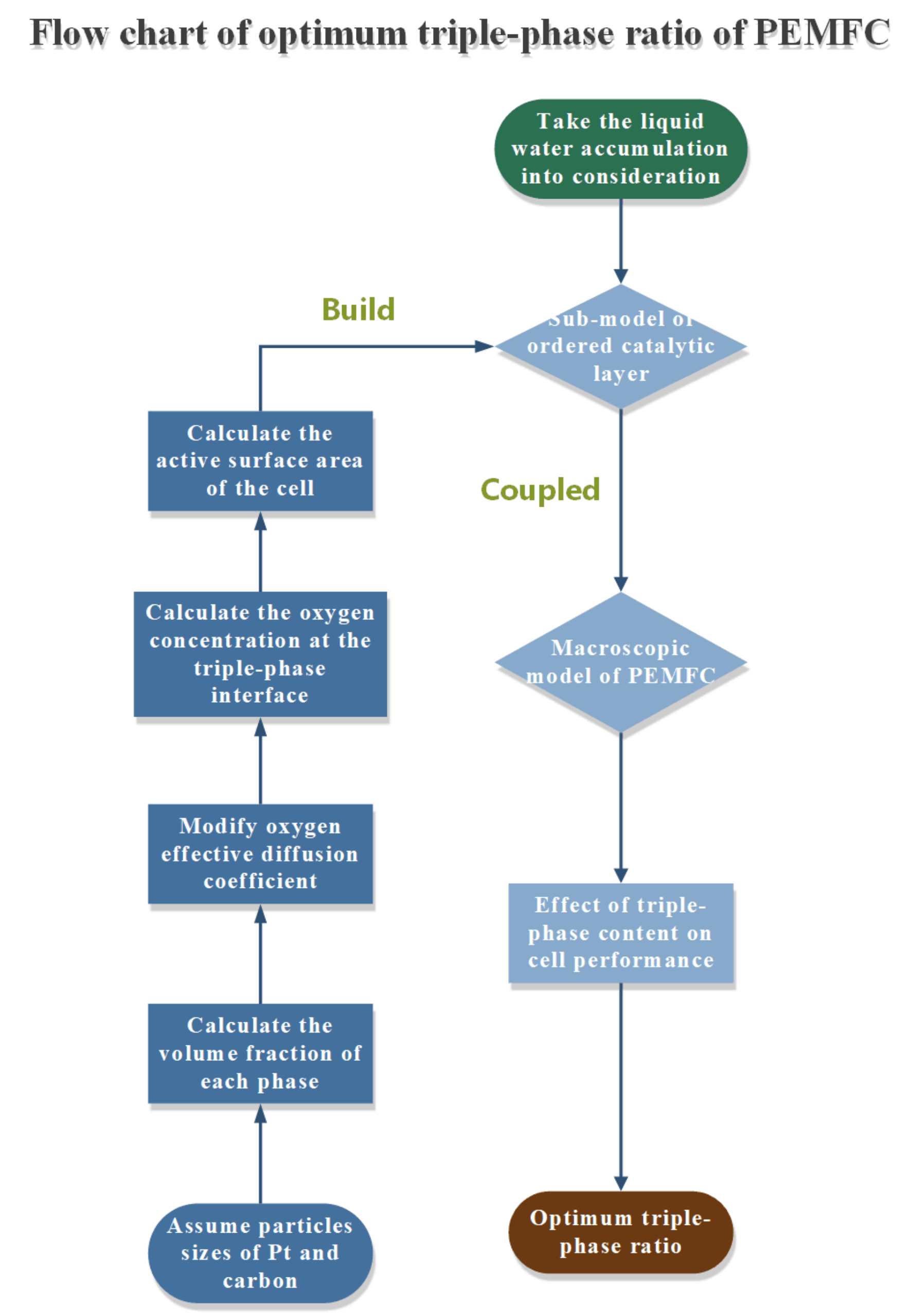
2. Methods
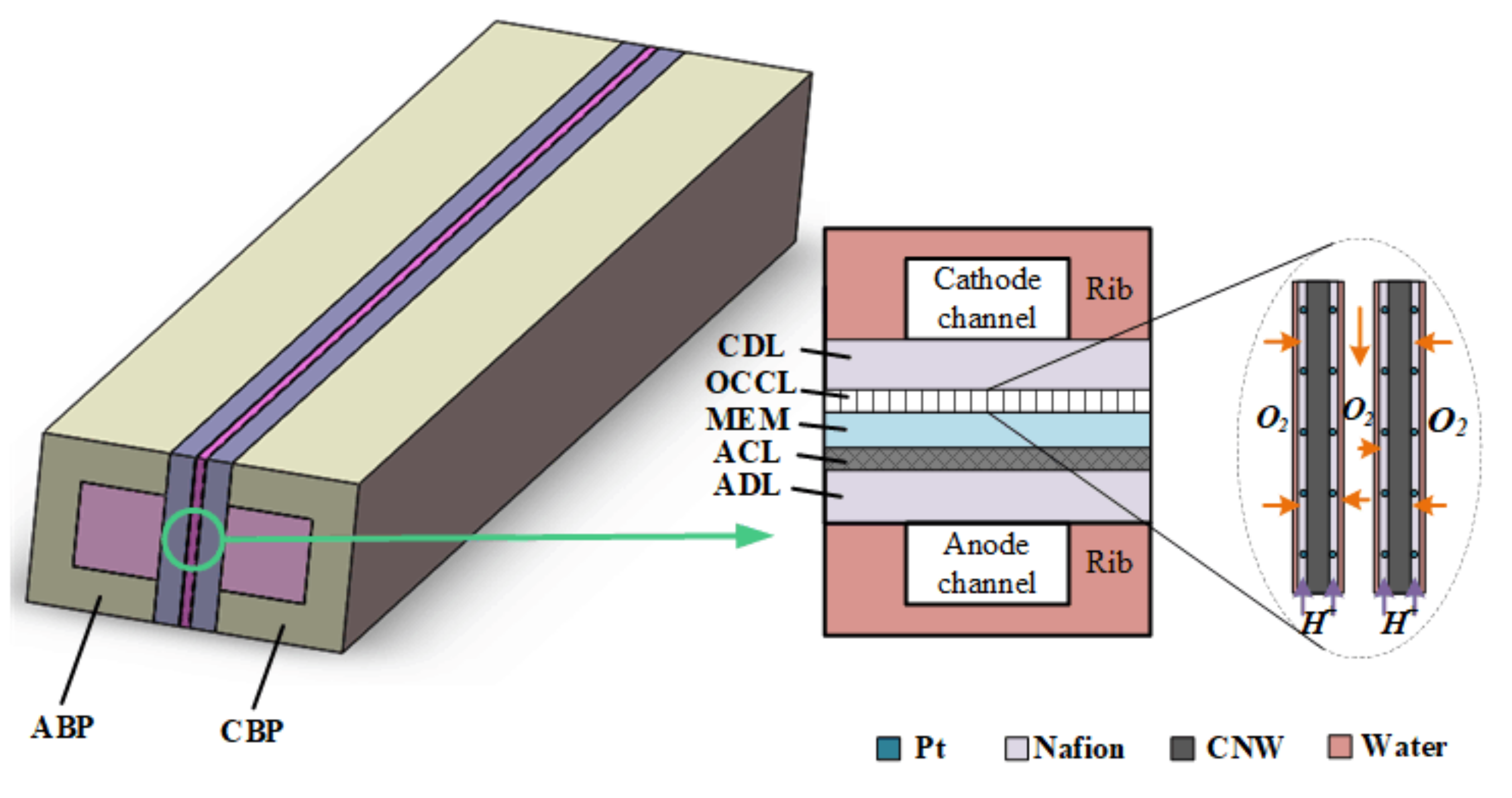
2.1. Computational Domain
2.2. Numerical Model
2.2.1. Governing Equations
- Mass conservation equation
- Momentum conservation equation
- Energy conservation equation
- Component conservation equation
- Charge conservation equation
- Liquid water transport equation
2.2.2. Electrochemical Reaction Equation
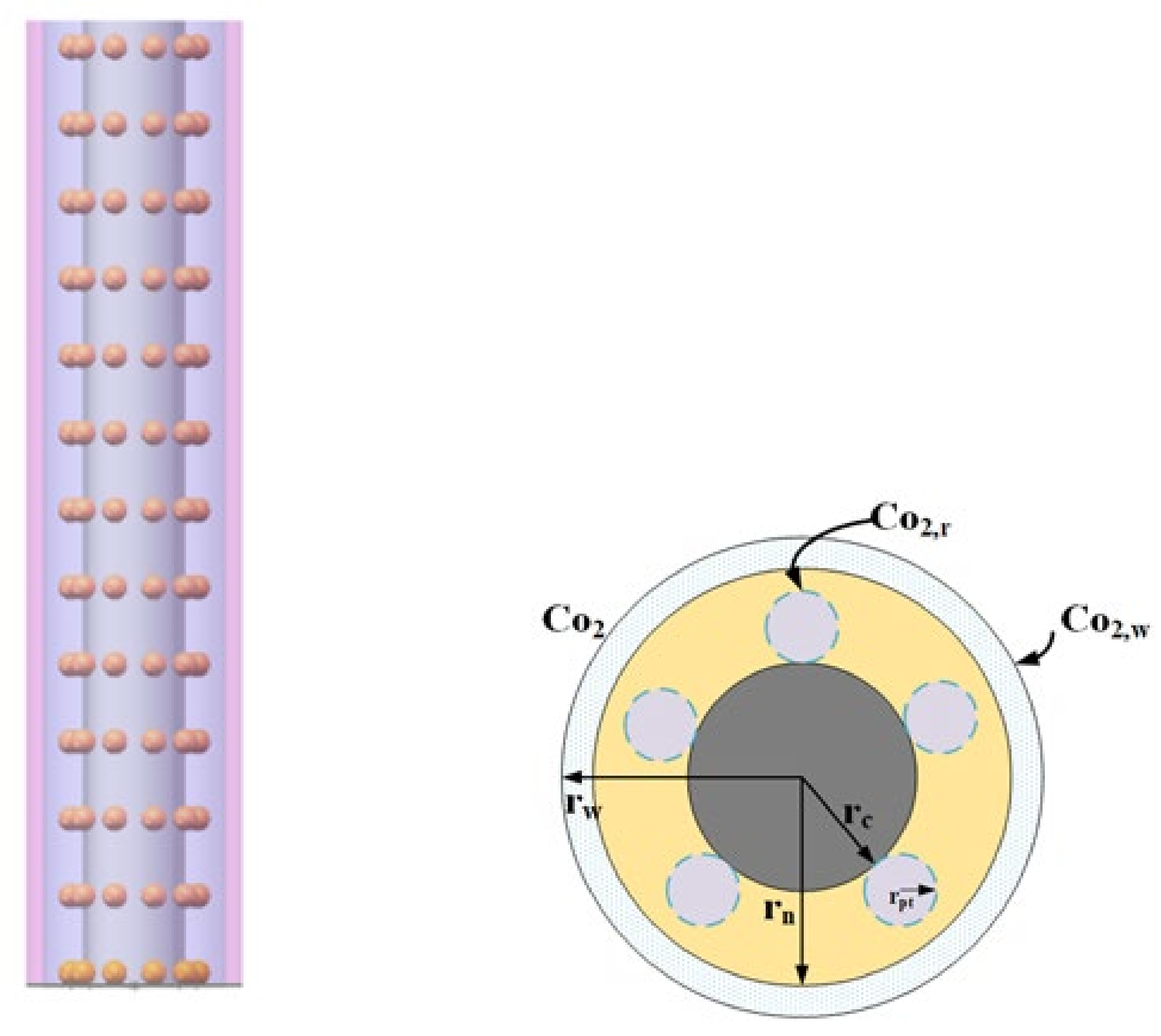
2.2.3. Oxygen Transport in OCCL
2.2.4. Boundary Conditions
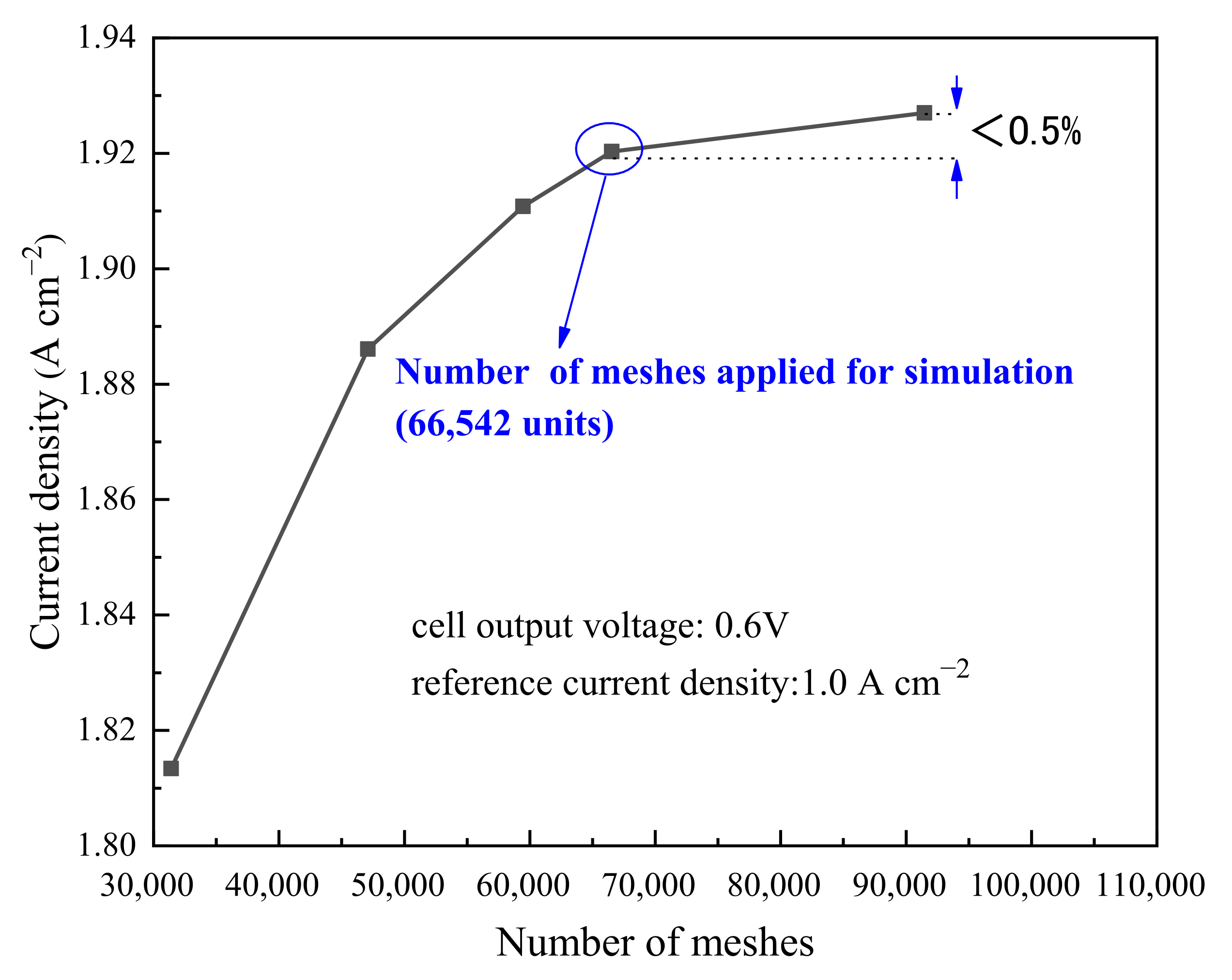
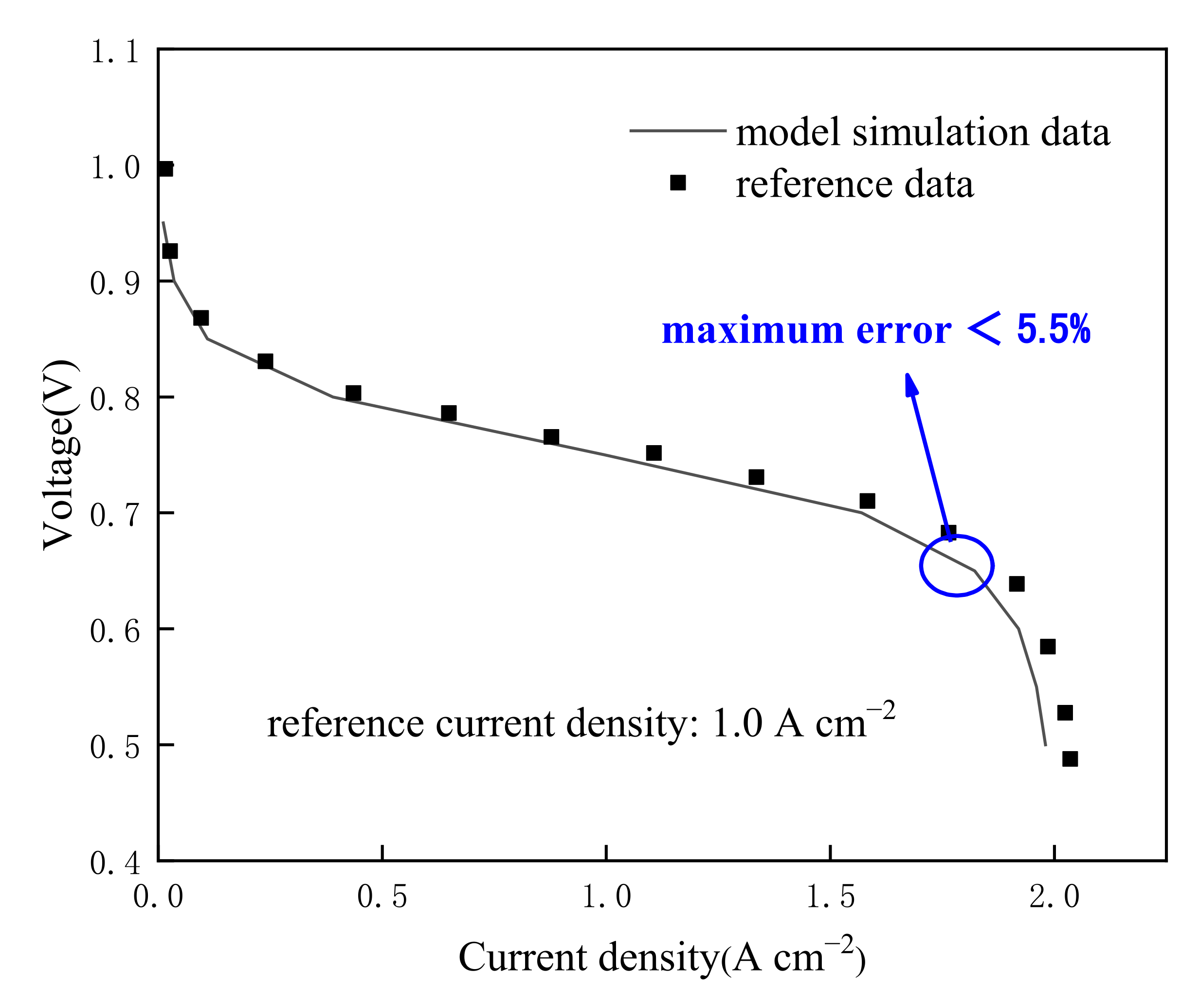
2.3. Model Validation
3. Discussion
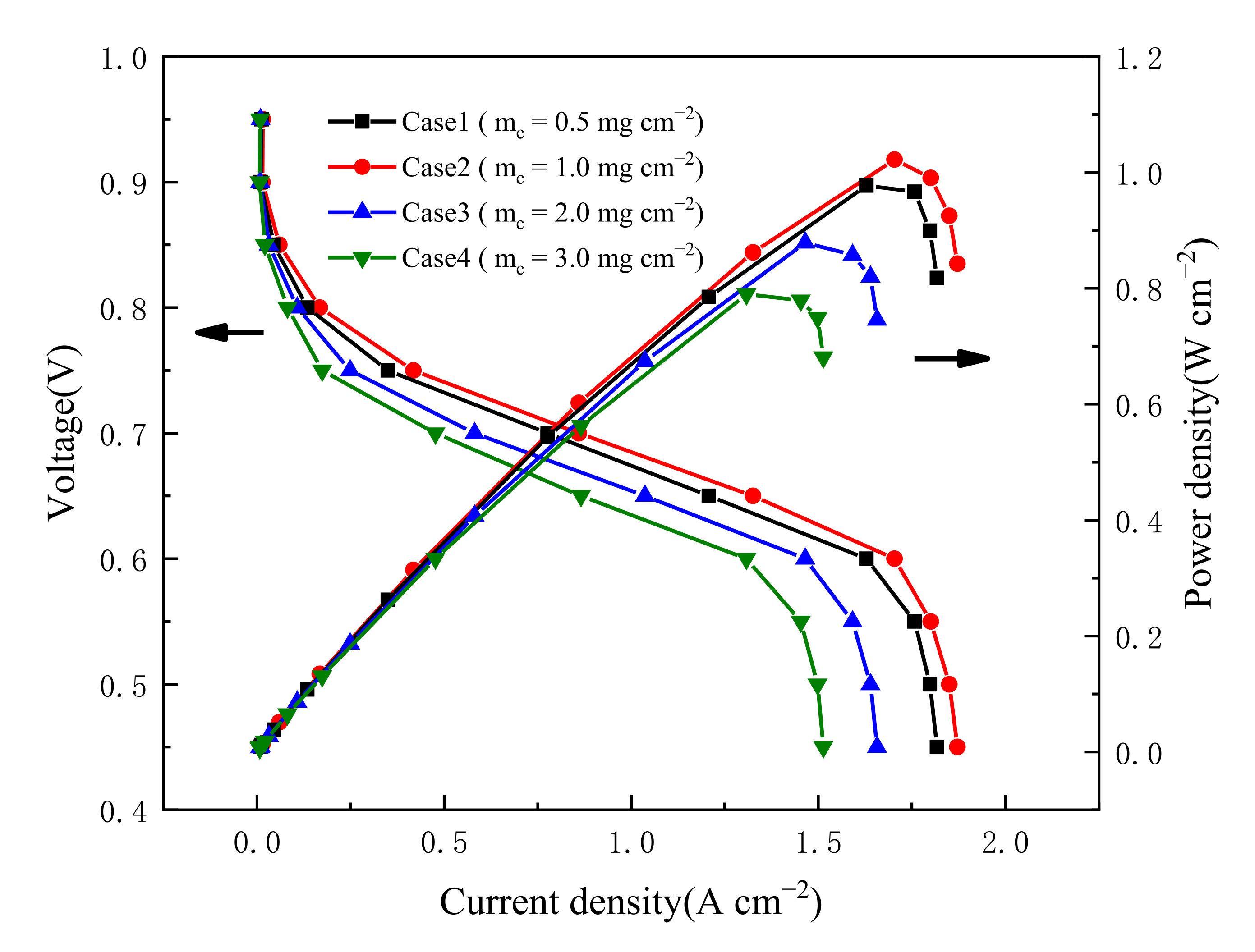
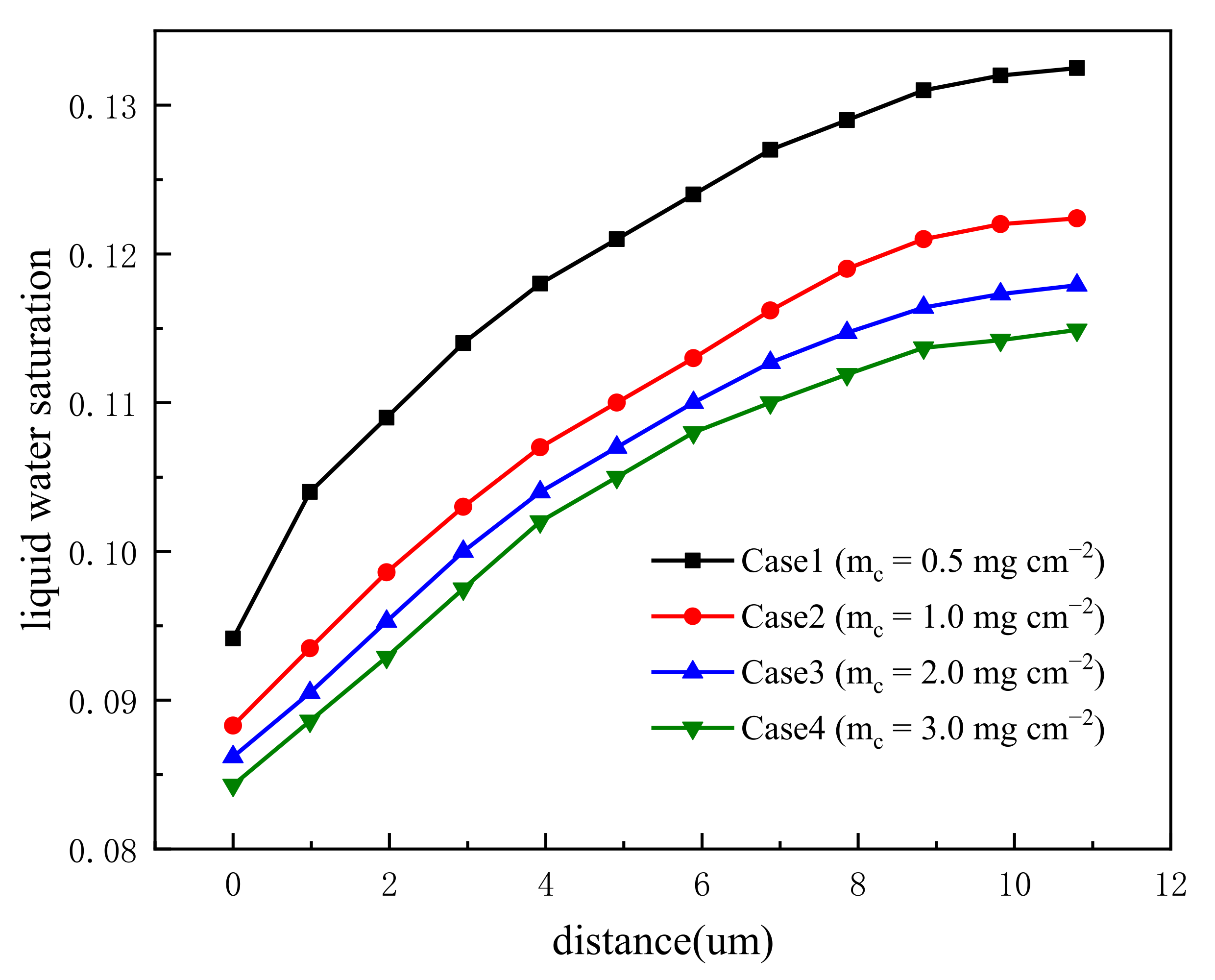
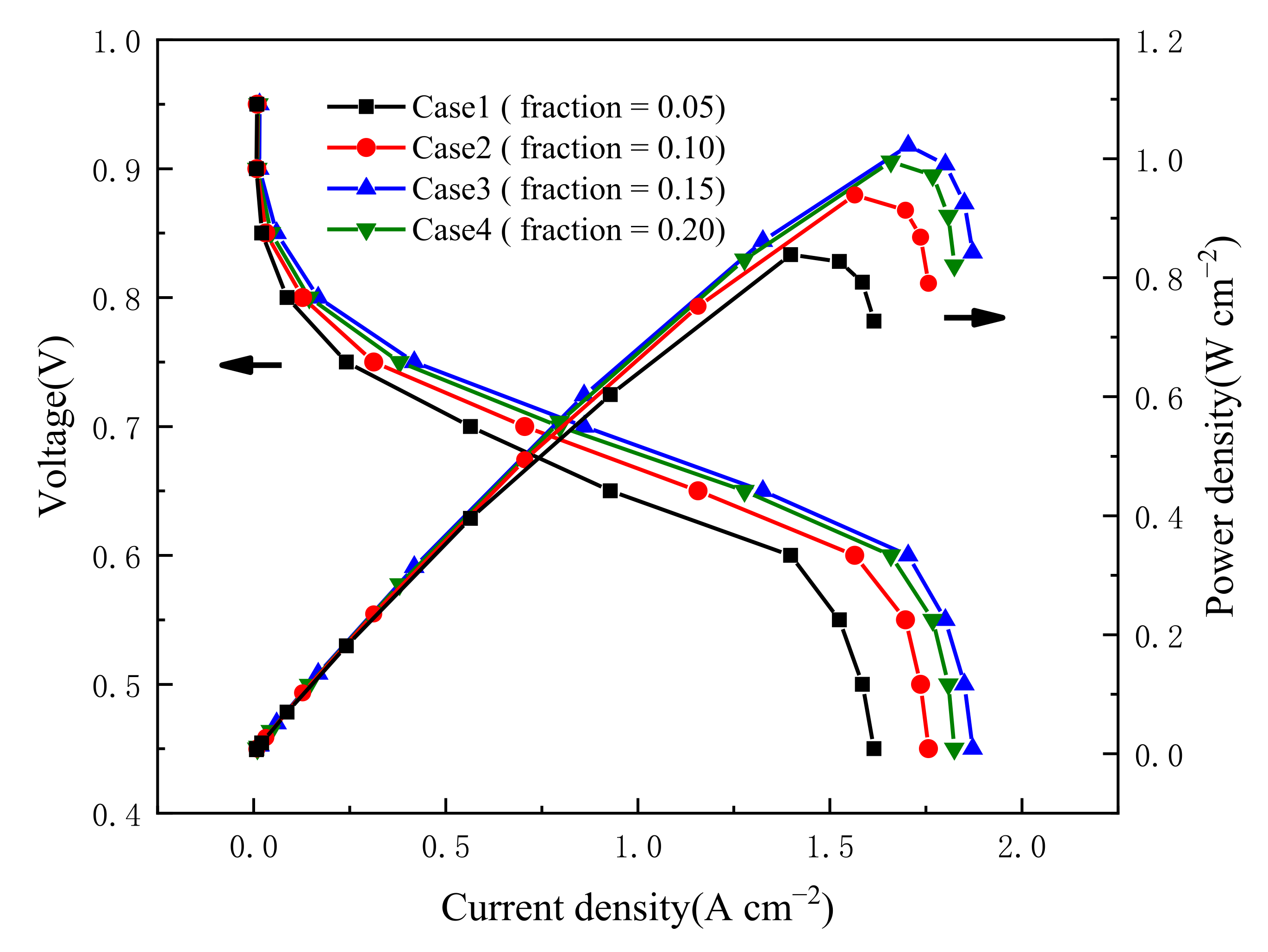
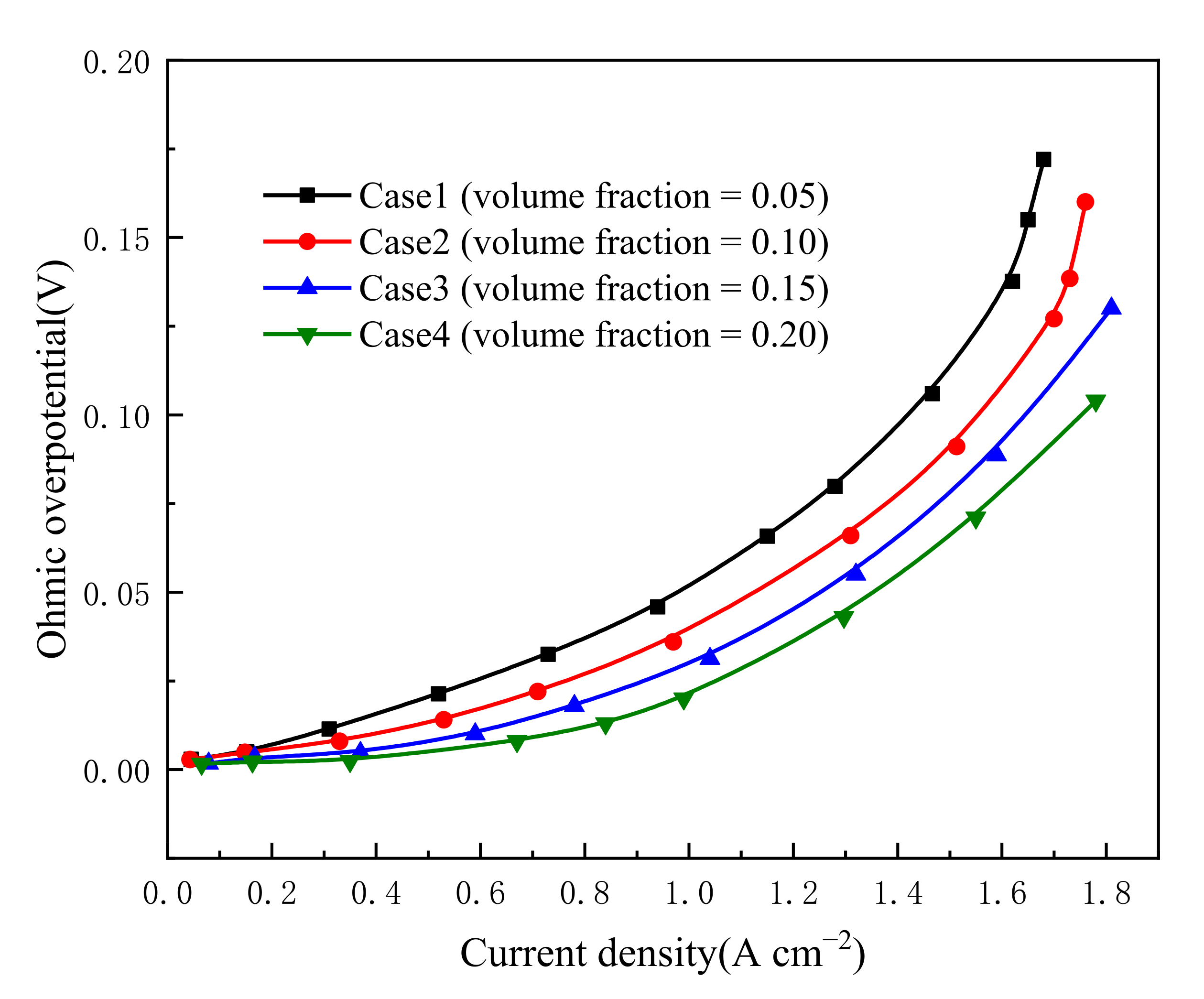
3.1. Effect of Carbon Loading in Cathode
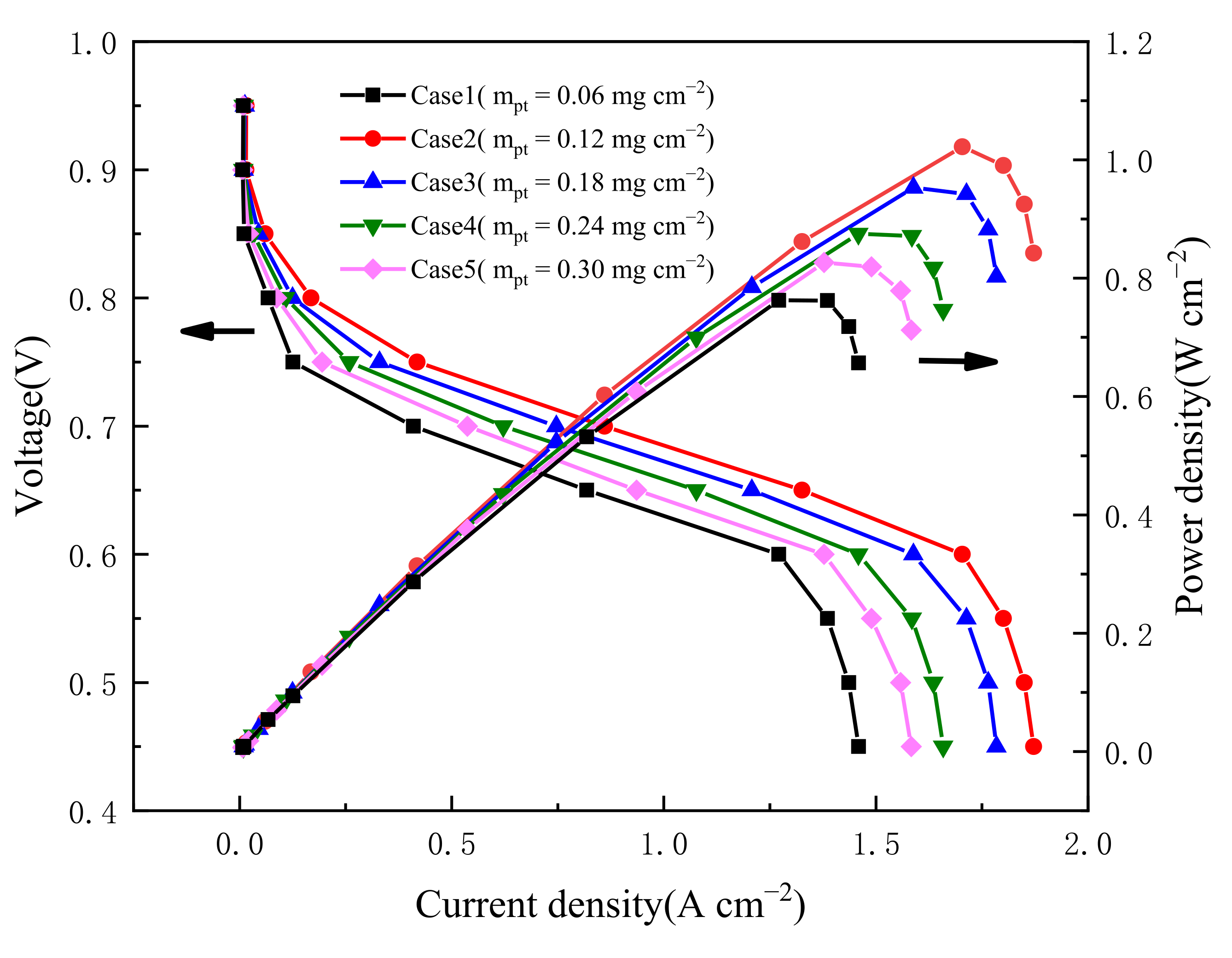
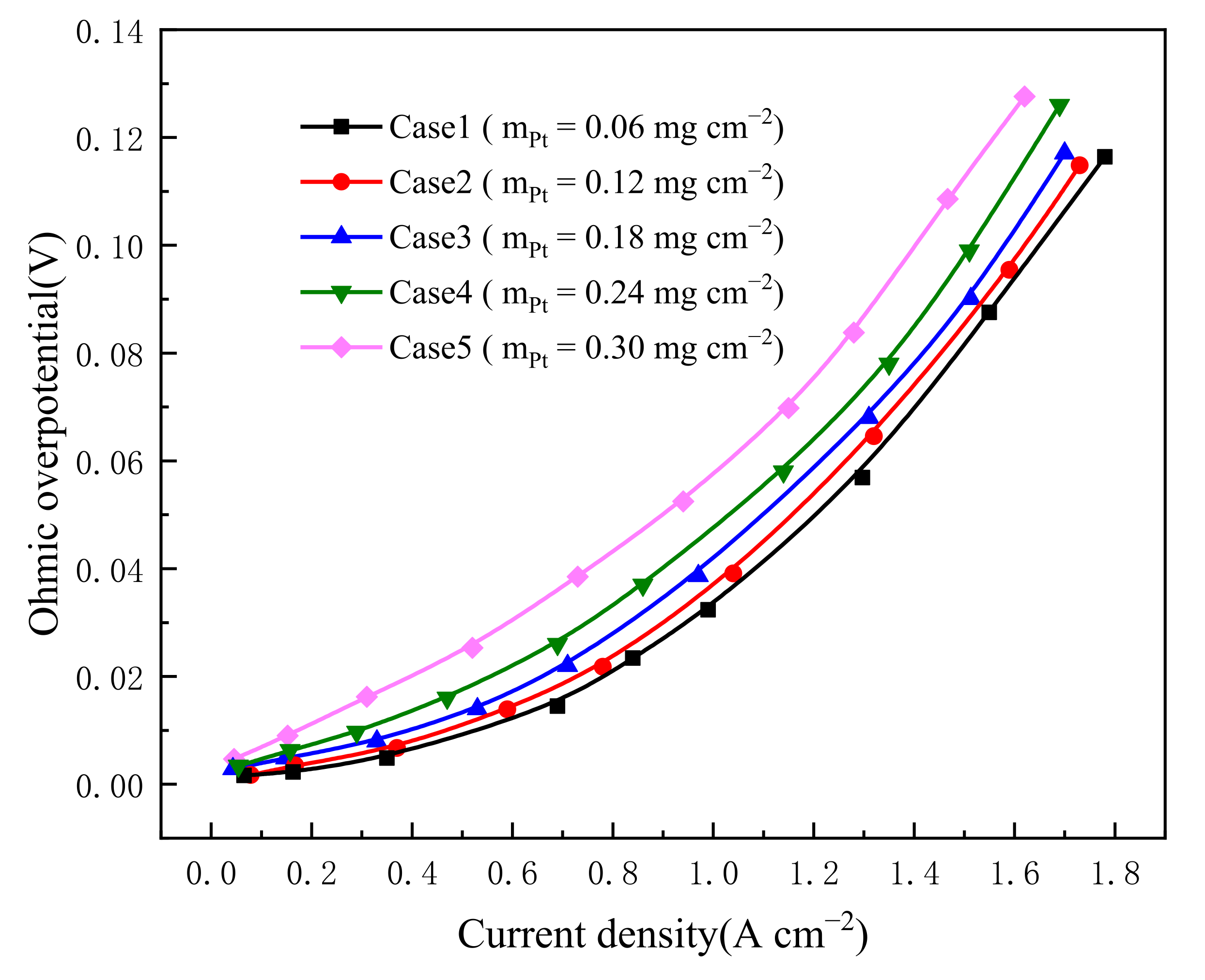
3.2. Effect of Pt Loading in Cathode
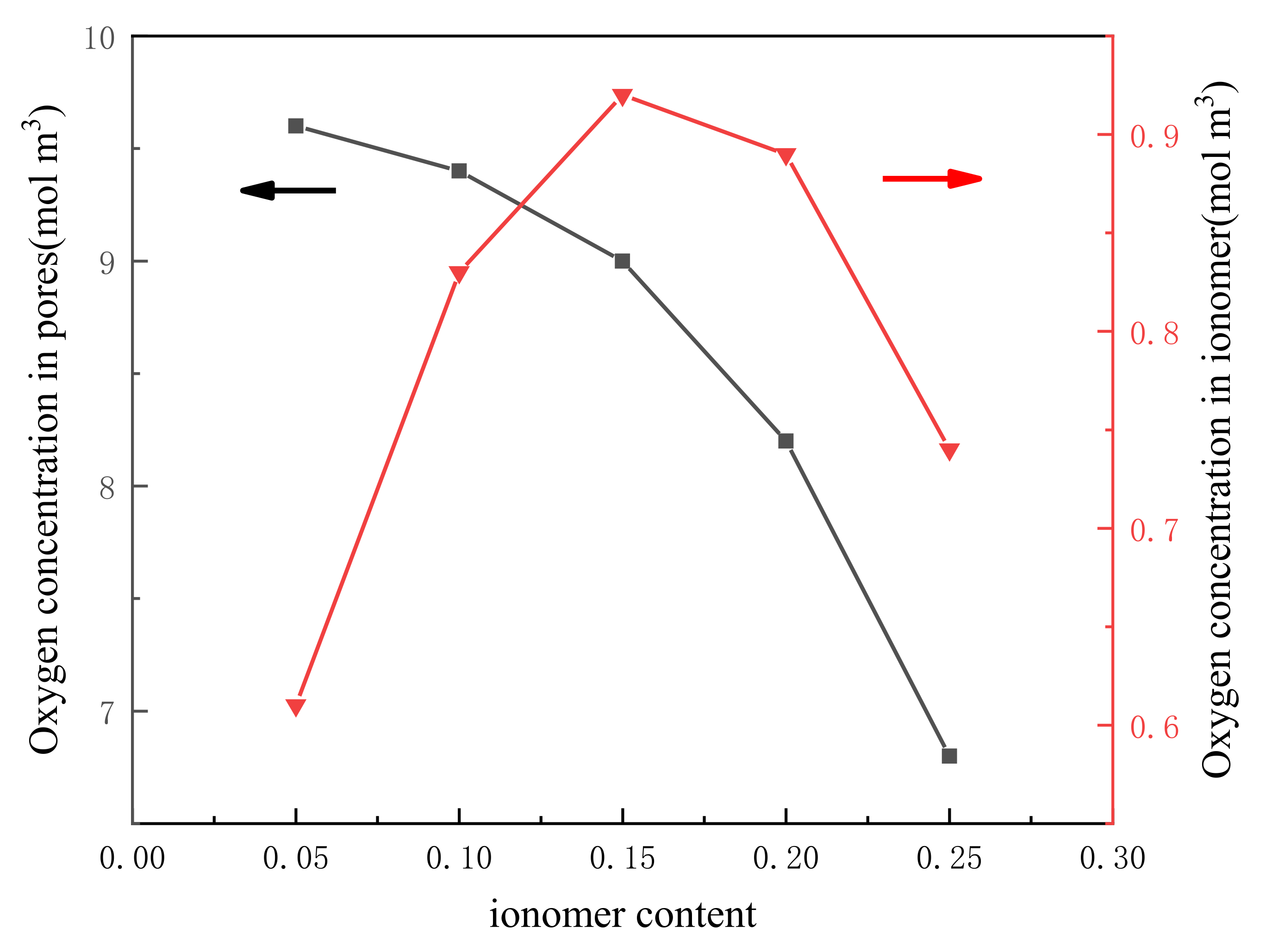
3.3. Effect of Ionomer Loading in Cathode
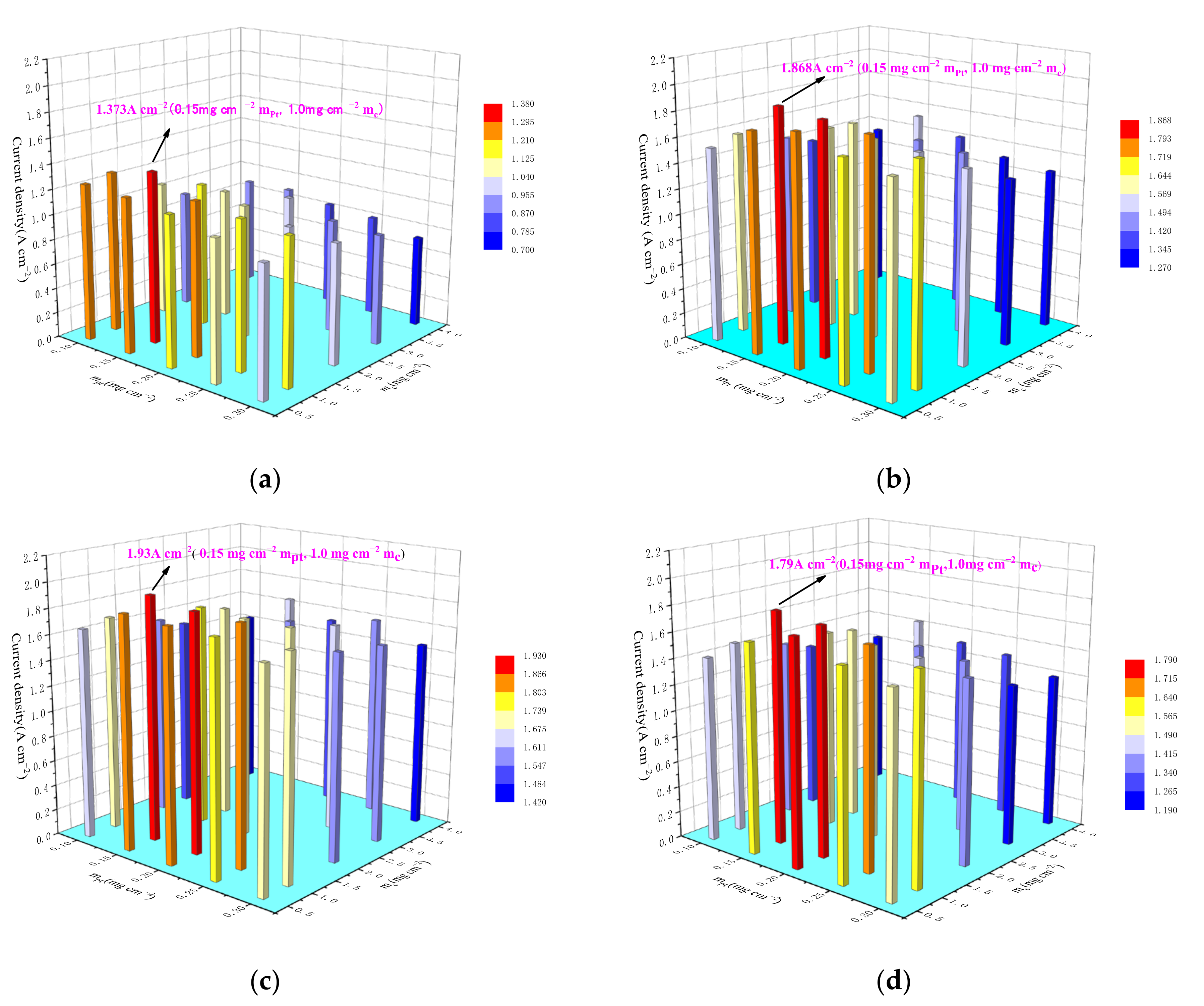
3.4. Optimum Triple-Phase Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | area (cm2) |
| C | component concentration (kmol·m−3) |
| D | effective diffusion coefficient (m2·s−1) |
| F | faraday constant, 96,485 C mol−1 |
| I | cell current density (A m−2) |
| j | current density(A m−3) |
| k | thermal conductivity (W·m−1·K−1), or condensation and evaporation rate (s−1) |
| K | permeability (m2) |
| M | molecular weight (kg kmol−1) |
| p | pressure (Pa·s−1) |
| P | power (W) |
| R | universal gas constant (J·kmol−1k−1) |
| s | water saturation |
| t | time (s) |
| T | temperature (K) |
| U | cell voltage (V) |
| w | quality score |
| Greeks | |
| α | transfer coefficient |
| γ | concentration indices |
| δ | thickness (mm) |
| ζ | stoichiometric number |
| η | overpotential (V) |
| λ | water content |
| µ | viscosity (Pa·s) |
| ρ | density (kg m−3) |
| σ | conductivity (S·m−1), interfacial tension (N m−1) |
| φ | potential (V) |
| Superscripts and subscripts | |
| a | anode |
| c | cathode |
| d | dissolved |
| e | electronic |
| g | gas |
| i | component |
| u | momentum |
| w | water vapor |
| eff | effective value |
| fl | liquid phase |
| in | inlet value |
| ion | proton |
| ref | reference value |
| sat | saturated |
| sl | solid phase |
| wv | vapor |
References
- EAgyekum, B.; Ampah, J.D.; Wilberforce, T.; Afrane, S.; Nutakor, C. Research Progress, Trends, and Current State of Development on PEMFC-New Insights from a Bibliometric Analysis and Characteristics of Two Decades of Research Output. Membranes 2022, 12, 1103. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2020, 33, e1908232. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Lim, K.; Salihi, H.; Ju, H. A parametric study on the performance requirements of key fuel cell components for the real-ization of high-power automotive fuel cells. Int. J. Heat Mass Transf. 2022, 186, 122477. [Google Scholar] [CrossRef]
- Lee, K.A.; Yoon, K.R.; Kwon, S.H.; Lee, K.J.; Jo, S.; Lee, J.S.; Lee, K.Y.; Lee, S.W.; Lee, S.G.; Kim, J.Y. Post-assembly modification of polymeric composite membranes using spin drying for fuel cell applications. J. Mater. Chem. A 2019, 7, 7380–7388. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Kulikovsky, A. The effect of Nafion film on the cathode catalyst layer performance in a low–Pt PEM fuel cell. Electrochem. Commun. 2019, 103, 61–65. [Google Scholar] [CrossRef]
- Wang, G.; Mukherjee, P.P.; Wang, C.Y. Direct numerical simulation (DNS) modeling of PEFC electrodes: Part II. Random microstructure. Electrochim. Acta 2006, 51, 3151–3160. [Google Scholar] [CrossRef]
- Chen, L.; Wu, G.; Holby, E.F.; Zelenay, P.; Tao, W.Q.; Kang, Q. Lattice Boltzmann Pore-Scale Investigation of Coupled Physical-electrochemical Processes in C/Pt and Non-Precious Metal Cathode Catalyst Layers in Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2015, 158, 175–186. [Google Scholar] [CrossRef]
- Vielstich, W.; Gasteiger, H.A. Handbook of Fuel Cells—Fundamentals, Technology and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; p. 603. [Google Scholar]
- Suzuki, T.; Tsushima, S.; Hirai, S. Effects of Nafion ionomer and carbon particles on structure formation in a proton-exchange membrane fuel cell catalyst layer fabricated by the decal-transfer method. Hydrog. Energy 2011, 36, 12361–12369. [Google Scholar] [CrossRef]
- Khajeh-Hosseini-Dalasm, N.; Kermani, M.J.; Moghaddam, D.G.; Stockie, J.M. A parametric study of cathode catalyst layer structural parameters on the performance of a PEM fuel cell. Int. J. Hydrog. Energy 2010, 35, 2417–2427. [Google Scholar] [CrossRef]
- He, P.; Mu, Y.T.; Park, J.W.; Tao, W.Q. Modeling of the effects of cathode catalyst layer design parameters on performance of polymer electrolyte membrane fuel cell. Appl. Energy 2020, 277, 115555. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.L.; Jiang, L.H.; Xia, Z.X.; Sun, H.; Sun, G.Q. High Pt utilization catalyst prepared by ion exchange method for direct methanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 14543–14548. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zhang, X.L.; Nie, L.; Zhang, Y.F.; Liu, X.W. Elimination of water flooding of cathode current collector of micro passive direct methanol fuel cell by super-hydrophilic surface treatment. Appl. Energy 2014, 126, 107–112. [Google Scholar] [CrossRef]
- Middelman, E. Improved PEM fuel cell electrodes by controlled self-assembly. Fuel Cells Bull. 2002, 2002, 9–12. [Google Scholar] [CrossRef]
- Lu, Z.; Yao, S.; Dong, Y.; Wu, D.; Pan, H.; Huang, X.; Wang, T.; Sun, Z.; Chen, X. Earth-abundant coal-derived carbon nanotube/carbon composites as efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. J. Energy Chem. 2021, 56, 87–97. [Google Scholar] [CrossRef]
- Jing, Z.; Yi, X.B.; Liu, S.; Fan, H.L.; Ju, W.; Wang, Q.C.; Ma, J. Vertically aligned carbon nanotubes/carbon fiber paper composite to support Pt nanoparticles for direct methanol fuel cell application. J. Phys. Chem. Solids 2017, 102, 99–104. [Google Scholar]
- Wang, G.; Zou, L.; Huang, Q.; Zou, Z.; Yang, H. Multidimensional nanostructured membrane electrode assemblies for proton exchange membrane fuel cell applications. J. Mater. Chem. 2019, 7, 9447–9477. [Google Scholar] [CrossRef]
- Bonnefont, A.; Ruvinskiy, P.; Rouhet, M.; Orfanidi, A.; Neophytides, S.; Savinova, E. Advanced catalytic layer architectures for polymer electrolyte membrane fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 505–521. [Google Scholar] [CrossRef]
- Yasuda, S.; Furuya, A.; Uchibori, Y.; Kim, J.; Murakoshi, K. Iron–Nitrogen-Doped Vertically Aligned Carbon Nanotube Electrocatalyst for the Oxygen Reduction Reaction. Adv. Funct. Mater. 2016, 26, 738–744. [Google Scholar] [CrossRef]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically aligned carbon nanotube electrodes for high current density operating proton exchange membrane fuel cells. J. Power Sources 2014, 253, 104–113. [Google Scholar] [CrossRef]
- Shin, S.; Liu, J.; Akbar, A.; Um, S. Nanoscale transport characteristics and catalyst utilization of vertically aligned carbon nanotube catalyst layers for fuel cell applications: Comprehensive stochastic modeling of composite morphological structures. J. Catal. 2019, 377, 465–479. [Google Scholar] [CrossRef]
- Huang, Z.H.; Song, Y.; Xu, X.X.; Liu, X.X. Ordered polypyrrole nanowire arrays grown on a carbon cloth substrate for a high-performance pseudocapacitor electrode. ACS Appl. Mater. Interfaces 2015, 7, 25506–25513. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Lim, S.H.; Poh, C.K.; Tang, Z.; Xia, Z.; Luo, Z.; Shen, P.K.; Chua, D.; Feng, Y.P.; Shen, Z.; et al. A highly order-structured membrane electrode assembly with vertically aligned carbon nanotubes for ultra-low Pt loading PEM fuel cells. Adv. Energy Mater. 2011, 1, 1205–1214. [Google Scholar] [CrossRef]
- Meng, X.; Deng, X.; Zhou, L.; Hu, B.; Tan, W.; Zhou, W.; Liu, M.; Shao, Z. A highly ordered hydrophilic–hydrophobic janus Bi-functional layer with ultralow Pt loading and fast gas/water transport for fuel cells. Energy Environ. Mater. 2021, 1, 126–133. [Google Scholar] [CrossRef]
- Deng, R.; Xia, Z.; Sun, R.; Wang, S.; Sun, G. Nanostructured ultrathin catalyst layer with ordered platinum nanotube arrays for polymer electrolyte membrane fuel cells. J. Energy Chem. 2019, 43, 33–39. [Google Scholar] [CrossRef]
- Yao, X.; Su, K.; Sheng, S.; Mao, L.; He, A.; Zhang, J.; Du, S. A novel catalyst layer with carbon matrix for Pt nanowire growth in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2013, 38, 12374–12378. [Google Scholar] [CrossRef]
- Su, K.; Yao, X.; Sui, S.; Wei, Z.; Zhang, J.; Du, S. Matrix Material Study for in situ Grown Pt Nanowire Electrocatalyst Layer in Proton Exchange Membrane Fuel Cells (PEMFCs). Fuel Cells 2015, 15, 449–455. [Google Scholar] [CrossRef]
- Tatyana, R.; Andrei, K. Impedance Spectroscopy Study of the PEM Fuel Cell Cathode with Nonuniform Nafion Loading. J. Electrochem. Soc. 2017, 164, 3016–3021. [Google Scholar]
- Du, C.Y.; Yang, T.; Shi, P.F.; Yin, G.P.; Cheng, X.Q. Performance analysis of the ordered and the conventional catalyst layers in proton exchange membrane fuel cells. Electrochim. Acta 2006, 51, 4934–4941. [Google Scholar] [CrossRef]
- Rao, S.M.; Xing, Y. Simulation of nanostructured electrodes for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 185, 1094–1100. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Three-dimensional multiphase modeling of cold start processes in polymer electrolyte membrane fuel cells. Electrochim. Acta 2009, 54, 6876–6891. [Google Scholar] [CrossRef]
- Yang, W.W.; Zhao, T.S. Two-phase, mass-transport model for direct methanol fuel cells with effect of non-equilibrium evaporation and condensation. Power Sources 2007, 174, 136–147. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, G.; Jiao, K. Characteristics of PEMFC operating at high current density with low external humidification. Energy Convers. Manag. 2017, 150, 763–774. [Google Scholar] [CrossRef]
- Li, W.Z.; Yang, W.W.; Zhang, W.Y.; Qu, Z.G.; He, Y.L. Three-dimensional modeling of a PEMFC with serpentine flow field incorporating the impacts of electrode inhomogeneous compression deformation. Int. J. Hydrogen Energy 2019, 44, 22194–22209. [Google Scholar] [CrossRef]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of operation temperature and reactant gas humidity levels on performance of PEM fuel cells. Renew Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]

| Model | Advantages of the Model Adopted in This Study Compared with Other Model |
|---|---|
| Existing traditional model | Lower oxygen transmission resistance Higher utilization rate of Pt particles Lower production cost |
| Existing ordered catalyst layer model | Consider liquid water and ionomers The ordered submodel of the catalyst layer is coupled to the macroscopic model |
| Parameters | Value | Unit | Reference |
|---|---|---|---|
| Channel width; height | 1.0; 1.0 | mm | |
| Land width | 1.0 | mm | |
| BP length; width; height | 40; 2.0; 1.5 | mm | |
| Thicknesses of GDL; CL; MEM | 0.4; 0.011; 0.108 | mm | |
| Density of MEM | 1980 | kg m−3 | [32] |
| Equivalent weight of MEM | 1.1 | kg mol−1 | [32] |
| Porosity of GDL; CL | 0.6; 0.3 | / | [32] |
| Reference Concentration of anode; cathode | 12; 1.2 | mol m−3 | [30] |
| Diffusivity in water film | 3.032 × 10−9 | m2s−1 | [33] |
| Diffusivity in polymer | 6 × 10−10 | m2s−1 | [30] |
| Diffusivity in pores | 4 × 10−6 | m2s−1 | [30] |
| Henry law constant for oxygen | / | [33] | |
| Concentration Index of anode; cathode | 0.5; 1.0 | / | [34] |
| Evaporation rate constant | 100 | s−1 | [35] |
| Condensation rate constant | 100 | s−1 | [35] |
| Inlet gas relative humidity | 100% | / | [36] |
| Catalyst surface area per unit mass | 112 | m g−1 | [30] |
| Operating temperature | 353 | K | [36] |
| Proton conductivity | 7 | S m−1 | [30] |
| Pt nanoparticle radius | 2 | nm | Assumed |
| Parameters | Expressions |
|---|---|
| Source term in the mass conservation equation | |
| Source term in the momentum conservation equation | |
| Source term in temperature conservation equation | |
| Source term in component conservation equation | |
| Electron generation rate | |
| Proton generation rate | |
| Water vapor condensation rate |
| Title 1 | Title 2 | Title 3 |
|---|---|---|
| mixed density | kg·m−3 | |
| hybrid viscosity | kg·m−1·s−1 | |
| component mass fraction | ||
| effective thermal conductivity | W·m−1·K−1 | |
| effective gas-diffusion coefficient | m2·s−1 | |
| proton phase conductivity | S·m−1 | |
| capillary pressure | Pa | |
| water content | ||
| water activity | ||
| water vapor pressure | Pa | |
| water saturation vapor pressure | Pa | |
| penetration resistance coefficient | ||
| water content diffusion coefficient | m2·s−1 | |
| water back diffusion flux | kg·m−3·s−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Rong, L.; Ma, X.; Yang, W. Numerical Optimization of Triple-Phase Components in Order-Structured Cathode Catalyst Layer of a Proton Exchange Membrane Fuel Cell. Energies 2023, 16, 1623. https://doi.org/10.3390/en16041623
Ye M, Rong L, Ma X, Yang W. Numerical Optimization of Triple-Phase Components in Order-Structured Cathode Catalyst Layer of a Proton Exchange Membrane Fuel Cell. Energies. 2023; 16(4):1623. https://doi.org/10.3390/en16041623
Chicago/Turabian StyleYe, Miao, Long Rong, Xu Ma, and Weiwei Yang. 2023. "Numerical Optimization of Triple-Phase Components in Order-Structured Cathode Catalyst Layer of a Proton Exchange Membrane Fuel Cell" Energies 16, no. 4: 1623. https://doi.org/10.3390/en16041623






