Review of the Integration of Drying and Thermal Treatment Processes for Energy Efficient Reduction of Contaminants and Beneficial Reuse of Wastewater Treatment Plant Biosolids
Abstract
:1. Introduction
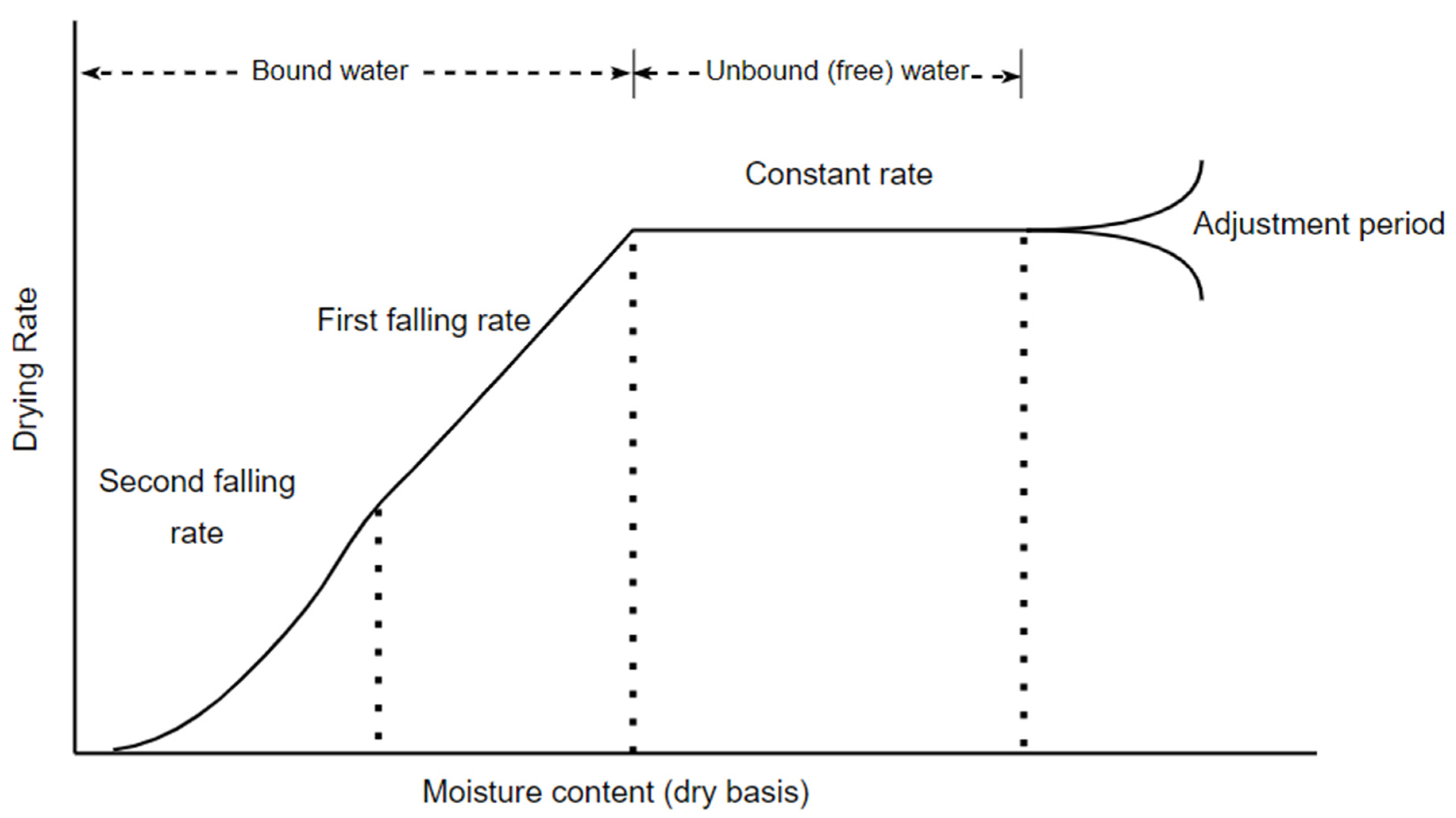
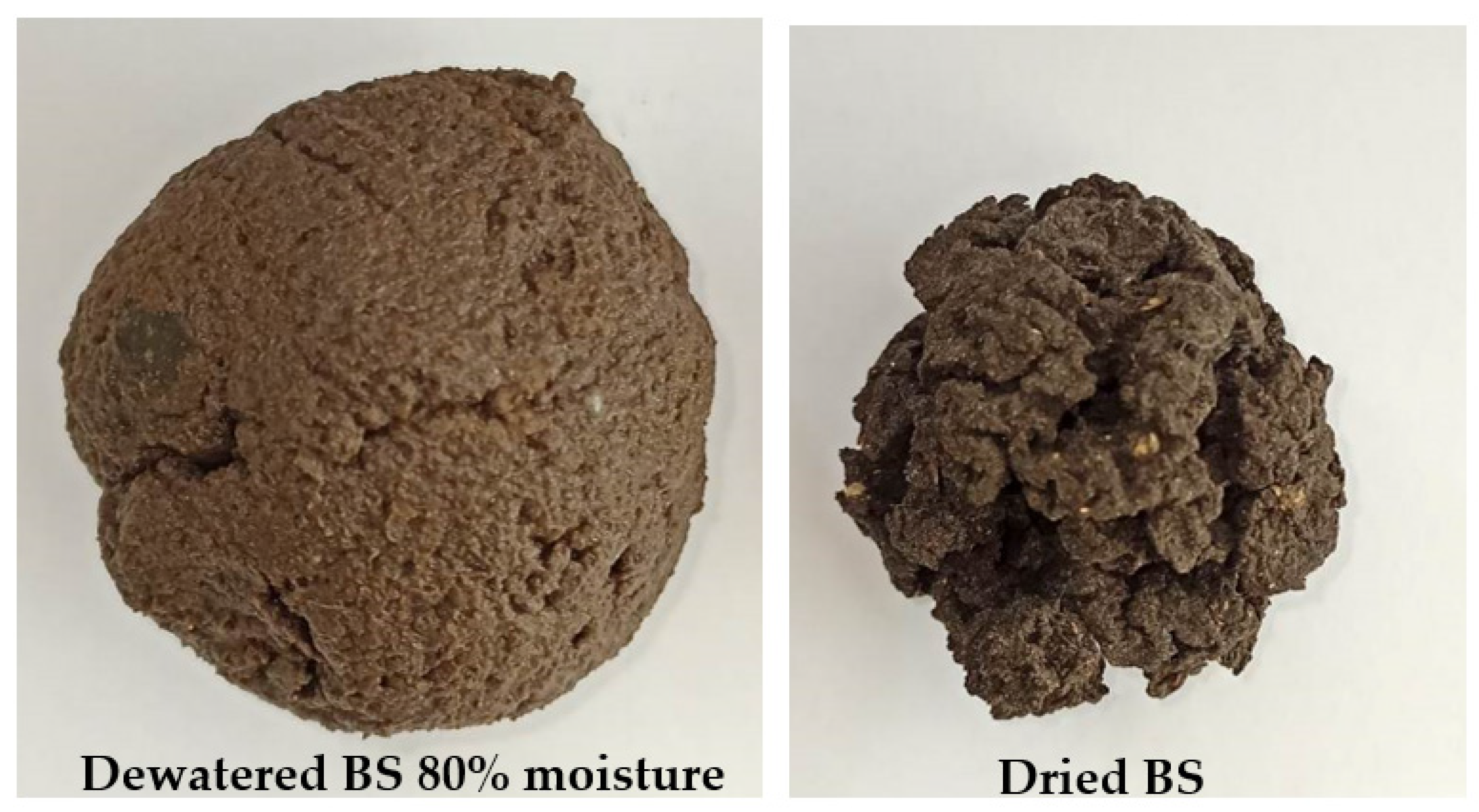
2. Drying of Biosolids
3. Modelling Moisture Removal from Biosolids
4. Thermal Treatment of Biosolids
4.1. Pyrolysis
4.2. Gasification
5. Fate of CECs during Thermal Treatment
6. Influence of Moisture Content
7. Integration of Drying and Thermal Treatment
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronym | Meaning |
| BS | Biosolids |
| CECs | Contaminants of Emerging Concern |
| CE | Circular economies |
| WWTPs | Wastewater treatment plants |
| PCPs | Personal care products |
| PFCs | Perfluorochemicals |
| MC | Moisture content |
| PFAS | Perfluoroalkyl and polyfluoroalkyl substances |
| WtE | Waste-to-energy |
| AD | Anaerobic digestion |
| VS | Volatile solids |
| TS | Total solids |
| LHV | Lower heating value |
References
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant–Challenges and Barriers. Proceedings 2018, 2, 614. [Google Scholar]
- Wijesekara, H.; Bolan, N.S.; Kumarathilaka, P.; Geekiyanage, N.; Kunhikrishnan, A.; Seshadri, B.; Saint, C.; Surapaneni, A.; Vithanage, M. Chapter 3—Biosolids Enhance Mine Site Rehabilitation and Revegetation. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 45–71. [Google Scholar]
- Wang, L.K.; Shammas, N.K.; Hung, Y.T. Biosolids Engineering and Management; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Torri, S.I.; Alberti, C. Characterization of organic compounds from biosolids of Buenos Aires city. J. Soil Sci. Plant Nutr. 2012, 12, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Becerra, F.Y.G.; Acosta, E.J.; Allen, D.G. Alkaline extraction of wastewater activated sludge biosolids. Bioresour. Technol. 2010, 101, 6972–6980. [Google Scholar] [CrossRef]
- Biosolids Snapshot. Available online: https://www.environment.gov.au/system/files/resources/2e8c76c3-0688-47ef-a425-5c89dffc9e04/files/biosolids-snapshot.pdf (accessed on 20 December 2022).
- Joo, S.H.; Monaco, F.D.; Antmann, E.; Chorath, P. Sustainable approaches for minimizing biosolids production and maximizing reuse options in sludge management: A review. J. Environ. Manag. 2015, 158, 133–145. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Canato, M.; Abbà, A.; Miino, M.C. Biosolids: What are the different types of reuse? J. Clean. Prod. 2019, 238, 117844. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B.O.; Smith, S.R. Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- Moodie, D.; Coggan, T.; Berry, K.; Kolobaric, A.; Fernandes, M.; Lee, E.; Reichman, S.; Nugegoda, D.; Clarke, B.O. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in Australian biosolids. Chemosphere 2020, 270, 129143. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Cledón, M.; Brar, S.K.; Surampalli, R.Y. Application of Wastewater and Biosolids in Soil: Occurrence and Fate of Emerging Contaminants. Water Air Soil Pollut. 2016, 227, 77. [Google Scholar] [CrossRef]
- Kuskopf, L.; Sheehan, M.; Whelan, A. Contaminants of Emerging Concern: Developing a Sampling and Analysis Quality Plan for the Cleveland Bay Sewage Treatment Plant. Water e-J. 2020, 5. Available online: https://web.archive.org/web/20201214000856id_/https://watersource.awa.asn.au/wp-content/uploads/2020/12/WEJ_2020_019.pdf (accessed on 18 December 2022). [CrossRef]
- Rahman, S.M.; Eckelman, M.J.; Onnis-Hayden, A.; Gu, A.Z. Comparative Life Cycle Assessment of Advanced Wastewater Treatment Processes for Removal of Chemicals of Emerging Concern. Environ. Sci. Technol. 2018, 52, 11346–11358. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2018, 169, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Gallen, C.; Eaglesham, G.; Drage, D.; Nguyen, T.H.; Mueller, J. A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Roychand, R.; Patel, S.; Halder, P.; Kundu, S.; Hampton, J.; Bergmann, D.; Surapaneni, A.; Shah, K.; Pramanik, B.K. Recycling biosolids as cement composites in raw, pyrolyzed and ashed forms: A waste utilisation approach to support circular economy. J. Build. Eng. 2021, 38, 102199. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total. Environ. 2020, 754, 142122. [Google Scholar] [CrossRef]
- Mohajerani, A.; Karabatak, B. Microplastics and pollutants in biosolids have contaminated agricultural soils: An analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks. Waste Manag. 2020, 107, 252–265. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Silveira, I.T.; Chua, A.; Leusch, F.D. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2020, 263, 128294. [Google Scholar] [CrossRef]
- Mercl, F.; Košnář, Z.; Maršík, P.; Vojtíšek, M.; Dušek, J.; Száková, J.; Tlustoš, P. Pyrolysis of biosolids as an effective tool to reduce the uptake of pharmaceuticals by plants. J. Hazard. Mater. 2021, 405, 124278. [Google Scholar] [CrossRef]
- Sun, C.; Dudley, S.; Trumble, J.; Gan, J. Pharmaceutical and personal care products-induced stress symptoms and detoxification mechanisms in cucumber plants. Environ. Pollut. 2018, 234, 39–47. [Google Scholar] [CrossRef] [Green Version]
- PFAS National Environmental Mangament Plan Version 2.0. Available online: https://www.environment.gov.au/protection/chemicals-management/pfas (accessed on 15 December 2022).
- Smith, S.R.; Cockayne, D.; Kirkland, A.I.; Nellist, P.D.; Bleloch, A. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [Green Version]
- QLD Government. Waste reduction and Recycling Act 2011. End of Waste Code Biosolids (ENEW07359617). 2020. Available online: https://environment.des.qld.gov.au/__data/assets/pdf_file/0029/88724/wr-eowc-approved-biosolids.pdf (accessed on 5 December 2022).
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Ratnnayake, N.; Marzbali, M.H.; Aktar, S.; Selezneva, E.; Paz-Ferreiro, J.; Surapaneni, A.; de Figueiredo, C.C.; et al. A critical literature review on biosolids to biochar: An alternative biosolids management option. Rev. Environ. Sci. Bio/Technol. 2020, 19, 807–841. [Google Scholar] [CrossRef]
- Wang, H.; Brown, S.L.; Magesan, G.N.; Slade, A.H.; Quintern, M.; Clinton, P.W.; Payn, T.W. Technological options for the management of biosolids. Environ. Sci. Pollut. Res. 2008, 15, 308–317. [Google Scholar] [CrossRef]
- EPA. Technical Resources for Biosolids Managers. Available online: https://www.epa.gov/biosolids/technical-resources-biosolids-managers#wastewater (accessed on 18 December 2022).
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Carnevale Miino, M.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G. Sustainable management of biological solids in small treatment plants: Overview of strategies and reuse options for a solar drying facility in Poland. Environ. Sci. Pollut. Res. 2020, 28, 24680–24693. [Google Scholar] [CrossRef]
- Biosolids Technology Fact Sheet. Available online: https://www.epa.gov/sites/production/files/2018-11/documents/heat-drying-factsheet.pdf (accessed on 12 December 2022).
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- McNamara, P.J.; Koch, J.D.; Liu, Z.; Zitomer, D.H. Pyrolysis of Dried Wastewater Biosolids Can Be Energy Positive. Water Environ. Res. 2016, 88, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C. Modelling the Ddrying Kinetics of Fresh-Water and Salt-Water Macroalgae. Ph.D. Thesis, James Cook University, Townsville, Australia, 2020. [Google Scholar]
- Cengel, Heat and Mass Transfer; McGraw-Hill Education: New York, NY, USA, 2011.
- Earle, R.L. Unit Operations in Food Processing; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar] [CrossRef]
- Walker, C.; Sheehan, M. Drying Kinetics of Macroalgae as a Function of Drying Gas Velocity and Material Bulk Density, Including Shrinkage. Clean Technol. 2022, 4, 669–689. [Google Scholar] [CrossRef]
- Bennamoun, L. Solar drying of wastewater sludge: A review. Renew. Sustain. Energy Rev. 2012, 16, 1061–1073. [Google Scholar] [CrossRef]
- Bennamoun, L.; Arlabosse, P.; Léonard, A. Review on fundamental aspect of application of drying process to wastewater sludge. Renew. Sustain. Energy Rev. 2013, 28, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.; Eckholt, M.; Troncoso, F.; Efremov, G. Drying Kinetics of Sludge from a Wastewater Treatment Plant. Dry. Technol. 2004, 22, 2135–2150. [Google Scholar] [CrossRef]
- Bennamoun, L.; Crine, M.; Leonard, A. Convective Drying of Wastewater Sludge: Introduction of Shrinkage Effect in Mathematical Modeling. Dry. Technol. 2013, 31, 643–654. [Google Scholar] [CrossRef]
- Fraikin, L.; Herbreteau, B.; Salmon, T.; Nicol, F.; Crine, M.; Leonard, A. Use of an Experimental Design to Characterize the Convective Drying Behavior of Different Sludges. Dry. Technol. 2015, 33, 1302–1308. [Google Scholar] [CrossRef]
- Font, R.; Gomez-Rico, M.; Fullana, A. Skin effect in the heat and mass transfer model for sewage sludge drying. Sep. Purif. Technol. 2011, 77, 146–161. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Tao, T.; Su, A.; Mujumdar, A.S.; Lee, D.-J. Model for Sludge Cake Drying Accounting for Developing Cracks. Dry. Technol. 2010, 28, 922–926. [Google Scholar] [CrossRef]
- Kiang, Y.-H. Fuel Property Estimation and Combustion Process Characterization; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Perussello, C.A.; Mariani, V.C.; do Amarante, Á.C.C. Numerical and experimental analysis of the heat and mass transfer during okara drying. Appl. Therm. Eng. 2012, 48, 325–331. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, F.; Liang, X.; Yuan, D.; Geng, W.; Wang, L.; Sun, R. Drying Experiment and Drying Model Analysis of Dehydrated Sludge Particles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 768, 022031. [Google Scholar] [CrossRef]
- Ling, W.; Xing, Y.; Hong, C.; Zhang, B.; Hu, J.; Zhao, C.; Wang, Y.; Feng, L. Methods, mechanisms, models and tail gas emissions of convective drying in sludge: A review. Sci. Total. Environ. 2022, 845, 157376. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M.B. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Kundu, S.K.; Patel, S.; Halder, P.; Patel, T.; Marzbali, M.H.; Pramanik, B.K.; Paz-Ferreiro, J.; de Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 2020, 7, 638–649. [Google Scholar] [CrossRef]
- Callegari, A.; Capodaglio, A.G. Properties and Beneficial Uses of (Bio)Chars, with Special Attention to Products from Sewage Sludge Pyrolysis. Resources 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.J.; Zitomer, D.H.; Miller, T.R.; Weirich, C.A.; McNamara, P.J. Emerging investigators series: Pyrolysis removes common microconstituents triclocarban, triclosan, and nonylphenol from biosolids. Environ. Sci. Water Res. Technol. 2015, 2, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. J. A Sustain. Circ. Econ. 2014, 32, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis of Biosolids to Produce Biochars: A Review. Sustainability 2022, 14, 9626. [Google Scholar] [CrossRef]
- Sichone, K.; Lay, M.C.; White, T.; Verbeek, C.J.R.; Kay, H.; van den Berg, L.E. Pilot scale pyrolysis—Determination of critical moisture content for sustainable organic waste pyrolysis. In Proceedings of the Chemeca 2012: Quality of Life through Chemical Engineering, Wellington, New Zealand, 23–26 September 2012; Available online: https://hdl.handle.net/10289/7835 (accessed on 10 December 2022).
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Mohamed, S., Ed.; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 1. [Google Scholar]
- Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Fouche, L.; Halder, P.; Setiawan, A.; Shah, K. Transformation of biosolids to biochar: A case study. Environ. Prog. Sustain. Energy 2018, 38, 13113. [Google Scholar] [CrossRef]
- Xiong, S.; Zhuo, J.; Zhang, B.; Yao, Q. Effect of moisture content on the characterization of products from the pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2013, 104, 632–639. [Google Scholar] [CrossRef]
- Lui, J.; Chen, W.-H.; Tsang, D.C.; You, S. A critical review on the principles, applications, and challenges of waste-to-hydrogen technologies. Renew. Sustain. Energy Rev. 2020, 134, 110365. [Google Scholar] [CrossRef]
- Champion, W.M.; Cooper, C.D.; Mackie, K.R.; Cairney, P. Development of a chemical kinetic model for a biosolids fluidized-bed gasifier and the effects of operating parameters on syngas quality. J. Air Waste Manag. Assoc. 2013, 64, 160–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, T.; Zitomer, D.; McNamara, P. Pyrolysis of wastewater biosolids significantly reduces estrogenicity. J. Hazard. Mater. 2016, 317, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moško, J.; Pohořelý, M.; Cajthaml, T.; Jeremiáš, M.; Robles-Aguilar, A.A.; Skoblia, S.; Beňo, Z.; Innemanová, P.; Linhartová, L.; Michalíková, K.; et al. Effect of pyrolysis temperature on removal of organic pollutants present in anaerobically stabilized sewage sludge. Chemosphere 2020, 265, 129082. [Google Scholar] [CrossRef] [PubMed]
- Per- and polyfluoroalkyl substances (PFAS): Incineration to manage PFAS waste Streams. Available online: https://www.epa.gov/sites/production/files/2019-09/documents/technical_brief_pfas_incineration_ioaa_approved_final_july_2019.pdf (accessed on 2 December 2022).
- Hall, H.; Moodie, D.; Vero, C. PFAS in biosolids: A review of international regulations. Water E-J. 2021, 5, 41. [Google Scholar] [CrossRef]
- Beu, L.S. Reduction of Perfluorocompound (PFC) Emissions: 2005 State-of-the-Technology-Report; SEMATECH: Austin, TX, USA, 2005. [Google Scholar]
- Tsang, W.; Burgess, D.R.; Babushok, V. On the Incinerability of Highly Fluorinated Organic Compounds. Combust. Sci. Technol. 1998, 139, 385–402. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W., Jr.; Bell, K.Y. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2020, 93, 826–843. [Google Scholar] [CrossRef]
- Garg, A.; Shetti, N.P.; Basu, S.; Nadagouda, M.N.; Aminabhavi, T.M. Treatment technologies for removal of per- and polyfluoroalkyl substances (PFAS) in biosolids. Chem. Eng. J. 2023, 453, 139964. [Google Scholar] [CrossRef]
- Mercl, F.; Košnář, Z.; Pierdonà, L.; Ulloa-Murillo, L.M.; Száková, J.; Tlustoš, P. Changes in availability of Ca, K, Mg, P and S in sewage sludge as affected by pyrolysis temperature. Plant Soil Environ. 2020, 66, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total. Environ. 2019, 695, 133846. [Google Scholar] [CrossRef]
- Altarawneh, M. A chemical kinetic model for the decomposition of perfluorinated sulfonic acids. Chemosphere 2020, 263, 128256. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Guo, J.; Feng, Y. Hydrogen-rich gas production from pyrolysis of wet sludge in situ steam agent. Int. J. Hydrog. Energy 2017, 42, 18309–18314. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.; Li, A.; Yao, H. Influence of residual moisture on deep dewatered sludge pyrolysis. Int. J. Hydrog. Energy 2014, 39, 1253–1261. [Google Scholar] [CrossRef]
- Spinosa, L.; Ayol, A.; Baudez, J.-C.; Canziani, R.; Jenicek, P.; Leonard, A.; Rulkens, W.; Xu, G.; Van Dijk, L. Sustainable and Innovative Solutions for Sewage Sludge Management. Water 2011, 3, 702–717. [Google Scholar] [CrossRef] [Green Version]
- Lumley, N.P.; Ramey, D.F.; Prieto, A.L.; Braun, R.J.; Cath, T.Y.; Porter, J.M. Techno-economic analysis of wastewater sludge gasification: A decentralized urban perspective. Bioresour. Technol. 2014, 161, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Lalaguna, N.; Sánchez, J.; Murillo, M.; Atienza-Martínez, M.; Gea, G. Energetic assessment of air-steam gasification of sewage sludge and of the integration of sewage sludge pyrolysis and air-steam gasification of char. Energy 2014, 76, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Tic, W.J.; Guziałowska-Tic, J.; Pawlak-Kruczek, H.; Woźnikowski, E.; Zadorożny, A.; Niedźwiecki, Ł.; Wnukowski, M.; Krochmalny, K.; Czerep, M.; Ostrycharczyk, M.; et al. Novel Concept of an Installation for Sustainable Thermal Utilization of Sewage Sludge. Energies 2018, 11, 748. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, D.; Song, X.; Zhao, L. Study on the combined sewage sludge pyrolysis and gasification process: Mass and energy balance. Environ. Technol. 2012, 33, 2481–2488. [Google Scholar] [CrossRef]
- Li, H.; Feng, K. Life cycle assessment of the environmental impacts and energy efficiency of an integration of sludge anaerobic digestion and pyrolysis. J. Clean. Prod. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Bianchini, A.; Bonfiglioli, L.; Pellegrini, M.; Saccani, C. Sewage sludge drying process integration with a waste-to-energy power plant. Waste Manag. 2015, 42, 159–165. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Lu, C.-H.; Liao, C.-K.; Ger, R.H.-R. Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant. Int. J. Hydrog. Energy 2016, 41, 21641–21648. [Google Scholar] [CrossRef]
- Council, L.C. Technical Report Loganholme Waste-Water Treatment Plant: Biosolids Gasification Demonstration Project (PBE-075). 2021. Available online: https://arena.gov.au/assets/2021/03/loganholme-wastewater-treatment-plant.pdf (accessed on 22 December 2022).

| Drying Type | Advantages | Disadvantages | ||
|---|---|---|---|---|
| Convective | 700–1400 | 0.2–30 |
|
|
| Conductive | 800–900 | 7–35 |
|
|
| Solar | 30–200 | N/A * |
|
|
| Drying Apparatus | Principal Heat Transfer | Gas Velocity [m/s] | Initial Moisture Content | Sample Dimension(s) | Measured Variables | Model(s) Developed | Reference | |
|---|---|---|---|---|---|---|---|---|
| Drying tunnel | Convective | 0.43, 0.65 | 80, 90, 100, 112 | 72.5% wb | 0.1 × 0.1 × 0.005 m, 0.1 × 0.1 × 0.009 m | Mass | Fickian, Modified Quasi-stationary, Two-period | [42] |
| Drying Tunnel | Convective | 1.58–1.82 | 122–158 | 3 kg/kg dm 2.6 kg/kg dm | 12 mm cylinders | Mass | Fickian | [43] |
| Drying tunnel | Convective | 1, 2, 3 | 80, 90, 140, 200 | 86, 88, 6% wb | 15 mm cylinder | Mass, surface temperature | Empirical | [44] |
| Drying tunnel | Convective | 2.4–5.5 | 31–64 | 2.4–4.2 kg/kg dm | 2.5–3 cm spheres, cylindrical tablet 1 cm × 6.6 cm | Mass, internal temperature | Fickian | [45] |
| Drying Tunnel | Convective | 1.00 | 83.7 | 80–82% wb | 26 mm diameter, 10 mm height sludge cylinders | Mass | No drying model developed, cracking model developed | [46] |
| Satorious moisture analyzer | Radiative | N/A | 80, 120, 160 | 80% wb | 2–20 mm spheres | Mass | Fickian | [49] |
| Parameter | Test 1 | Test 2 | Test 3 | Unit |
|---|---|---|---|---|
| Moisture content of the feedstock | 83.9 | 53.47 | 32.13 | % (w.b.) |
| Moisture content of dried Biosolids | 53.47 | 32.13 | 4.02 | % (w.b.) |
| Average consumption of heat energy | 4141 | 6300 | 7203 | kJ/kgH2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nylen, J.; Sheehan, M. Review of the Integration of Drying and Thermal Treatment Processes for Energy Efficient Reduction of Contaminants and Beneficial Reuse of Wastewater Treatment Plant Biosolids. Energies 2023, 16, 1964. https://doi.org/10.3390/en16041964
Nylen J, Sheehan M. Review of the Integration of Drying and Thermal Treatment Processes for Energy Efficient Reduction of Contaminants and Beneficial Reuse of Wastewater Treatment Plant Biosolids. Energies. 2023; 16(4):1964. https://doi.org/10.3390/en16041964
Chicago/Turabian StyleNylen, Julian, and Madoc Sheehan. 2023. "Review of the Integration of Drying and Thermal Treatment Processes for Energy Efficient Reduction of Contaminants and Beneficial Reuse of Wastewater Treatment Plant Biosolids" Energies 16, no. 4: 1964. https://doi.org/10.3390/en16041964









