The Pyrolysis Behaviors of Blended Pellets of Pine Wood and Urea-Formaldehyde Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Elemental Analysis (wt%) | Industrial Analysis (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O1 | N | S | M | V | A | FC | |
| UF resin | 33.65 | 5.46 | 32.23 | 17.50 | 0 | 9.78 | 74.17 | 1.38 | 14.67 |
| PW | 33.25 | 4.52 | 24.27 | 0.90 | 0.29 | 5.28 | 56.84 | 31.49 | 6.39 |
| Chlorella Vulgaris [13] | 49.71 | 7.21 | 24.66 | 8.75 | 0.54 | 1.89 | 75.42 | 9.13 | 13.56 |
| Rice husk [13] | 40.10 | 5.09 | 40.93 | 0.44 | 0 | 11.23 | 66.74 | 13.44 | 8.59 |
| Moso Bamboo [14] | 44.87 | 5.73 | 38.32 | 0.71 | 0.01 | 8.67 | 74.81 | 2.56 | 13.96 |
| Eucalyptus Dunnii [15] | 45.67 | 6.50 | 46.74 | 0.14 | 0.96 | 4.37 | 84.86 | 0.31 | 14.83 |
2.2. Pellet Preparation Experiment
2.3. TG-FTIR
2.4. PY-GC/MS
2.5. The Interaction Effect of Co-Pyrolysis
3. Results and Discussion
3.1. Effect of the Introduction of UF Resin on the Pyrolysis Characteristics of Blended Pellets
3.1.1. Pyrolysis Characteristics of PW Pellets
3.1.2. Pyrolysis Characteristics of UF Resin Pellets
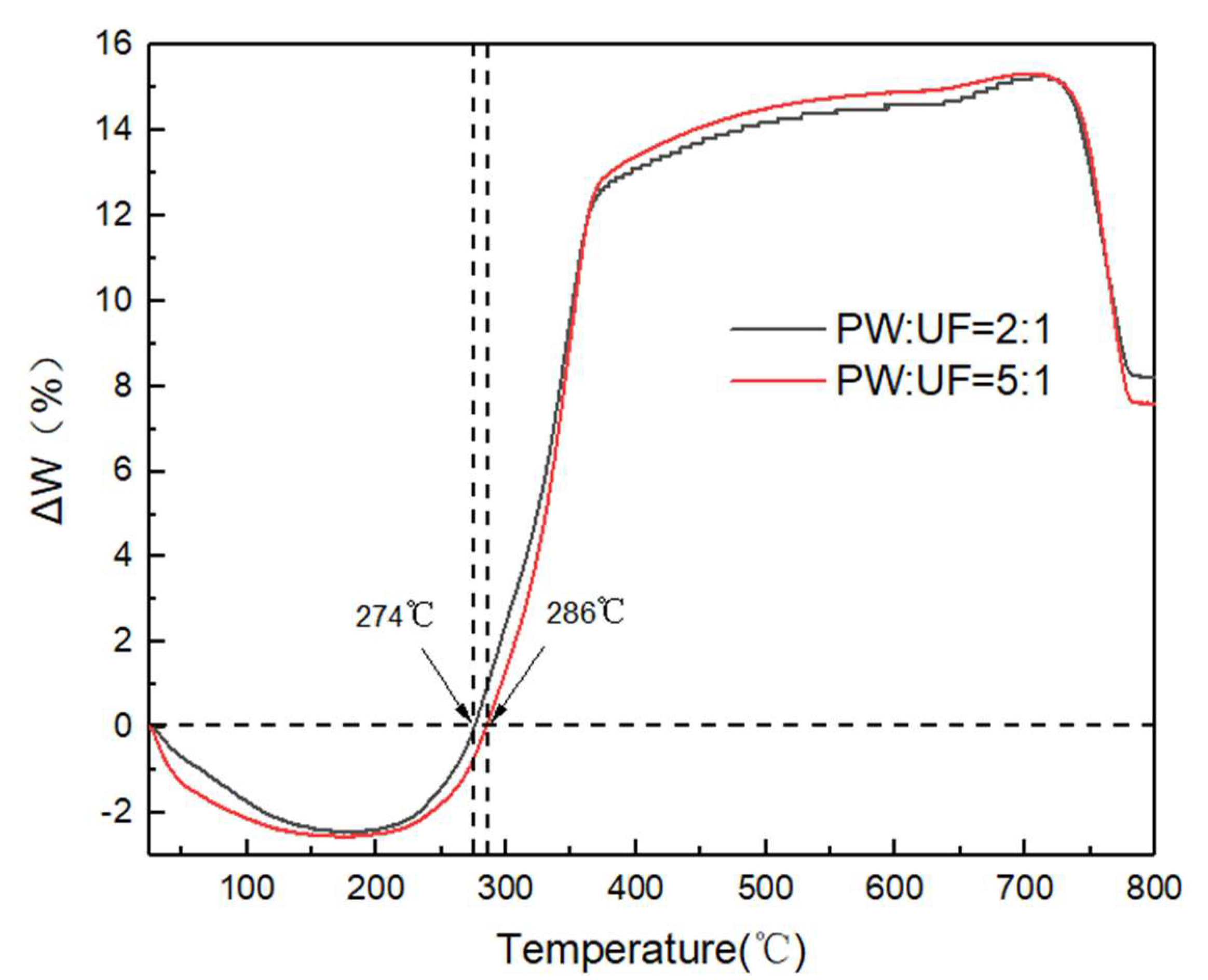
3.1.3. Effect of UF Resin on Pyrolysis Characteristics of Blended Pellets
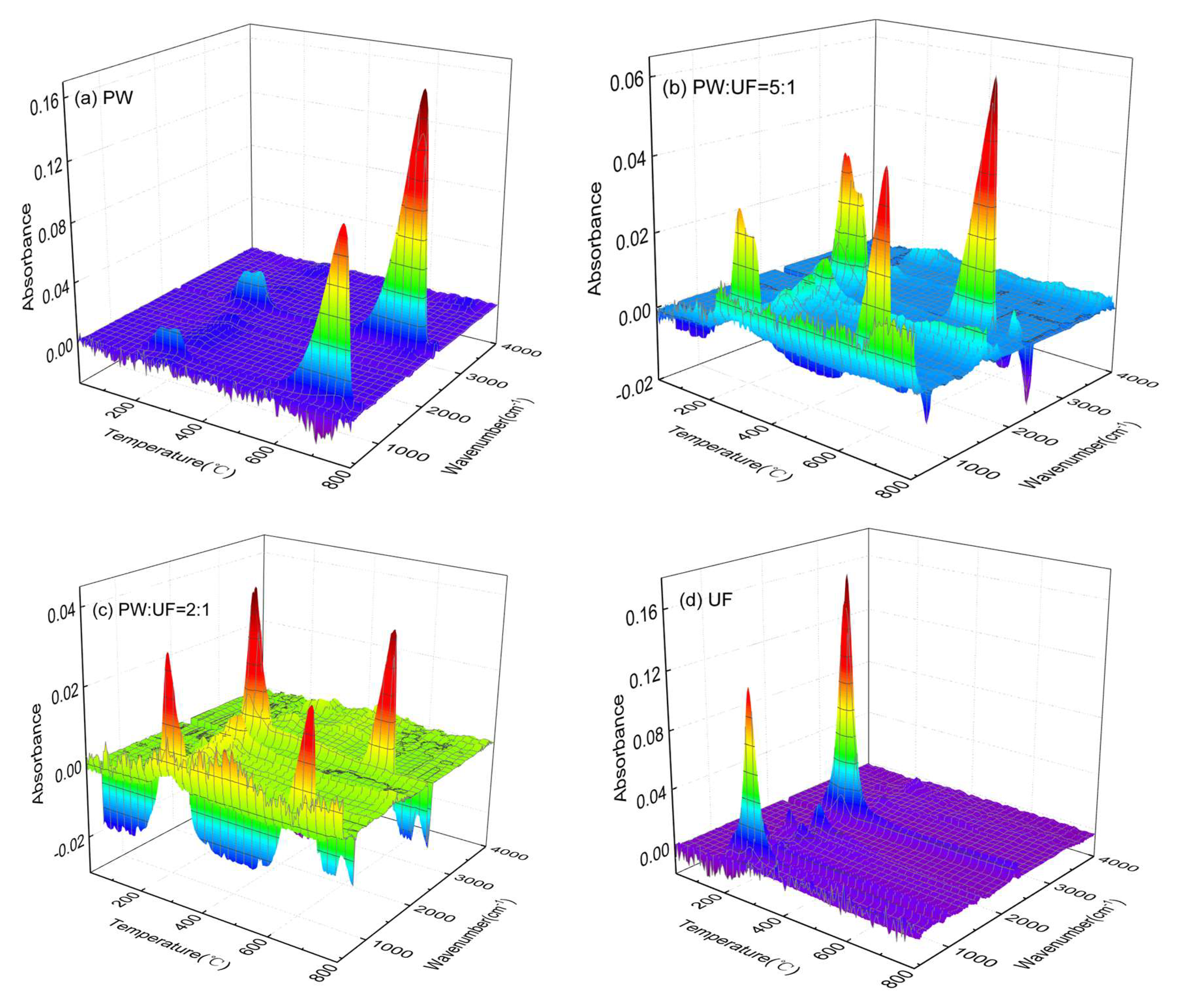
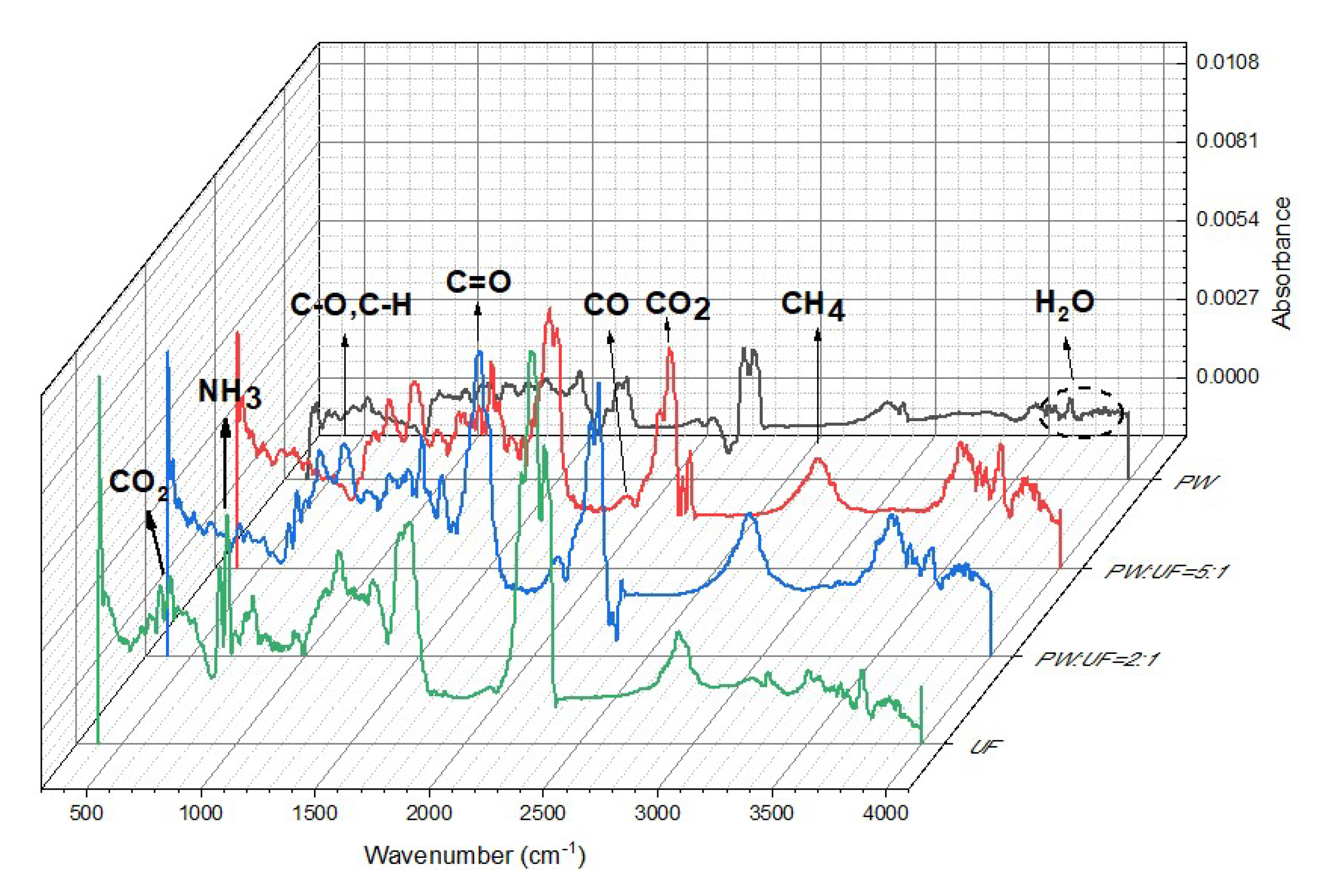
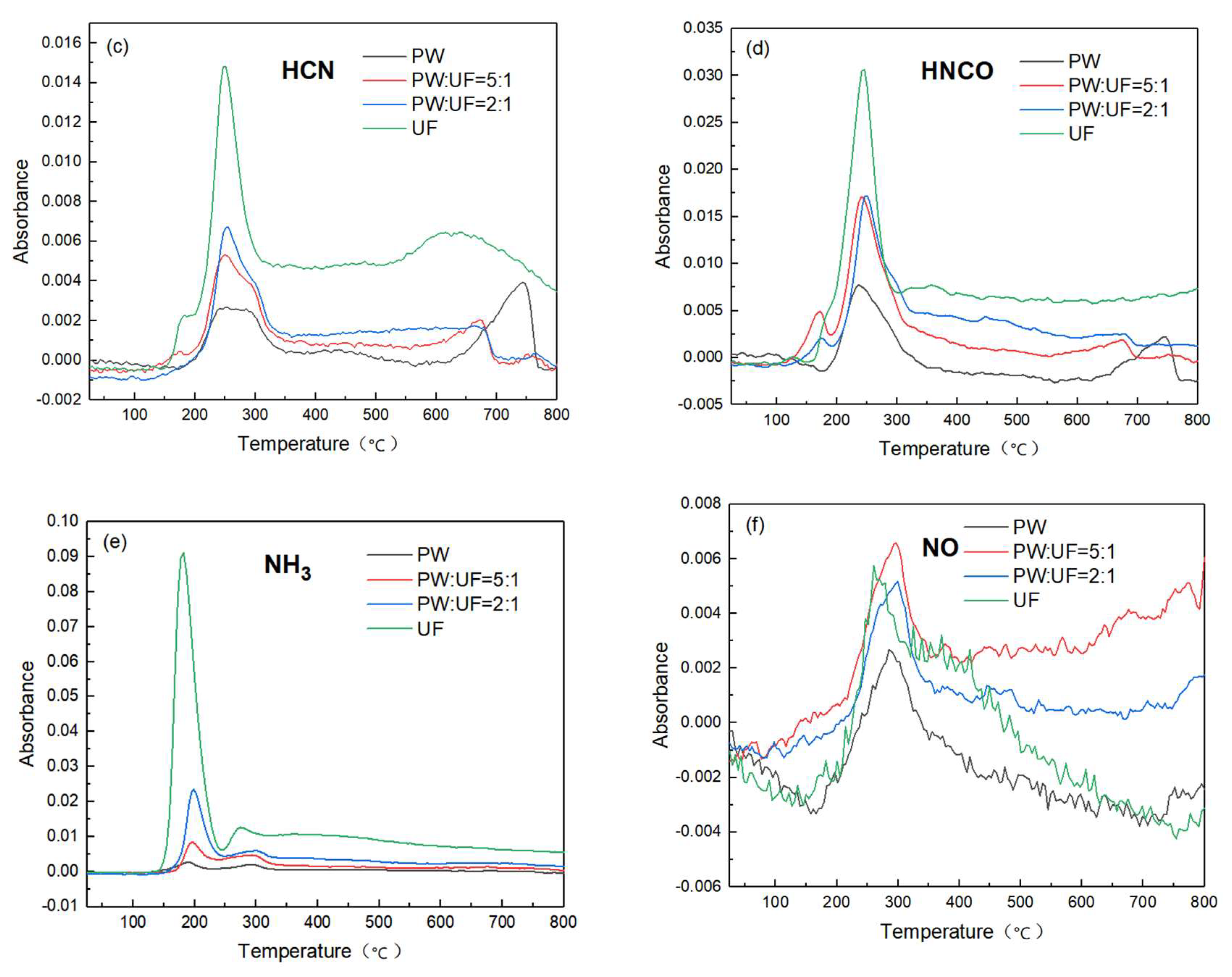
3.2. Gas Phase Product Analysis of Pyrolysis
3.3. Compositional Analysis of Liquid Phase Products of Co-Pyrolysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiaobing, X. Status of Wood-based Panel Industry in China. China Wood Ind. 2016, 30, 11–14+28. [Google Scholar] [CrossRef]
- Renwei, S.; Meifen, T. Application Status of Adhesives in Wood-based Panels. J. Green Sci. Technol. 2020, 139–140. [Google Scholar] [CrossRef]
- Yufeng, M.; Xuanang, G.; Chunpeng, W. Research Progress in Wood Adhesives. Chem. Ind. For. Prod. 2020, 40, 1–15. [Google Scholar] [CrossRef]
- Tianhang, R.; Ming, L.; Yiqiang, W.; Xingong, L.; Yan, Q. Research Progress on the Recycling of Waste Wood-Based Panels. China For. Prod. Ind. 2022, 59, 34–40. [Google Scholar] [CrossRef]
- Lykidis, C.; Grigoriou, A. Hydrothermal recycling of waste and performance of the recycled wooden particleboards. Waste Manag. 2008, 28, 57–63. [Google Scholar] [CrossRef]
- Derong, Z. The Properties of the Regenerated Partcles by Steam Explosion and the Particleboard Manufacture Technology. J. Beijing For. Univ. 2010, 21, 5—7+23. [Google Scholar]
- Meng-meng, S.; Wei-tao, X.; Tong-tong, C.; Hao-yuan, L.; Qi, L. Application of Pretreatment Technology in Biomass Pyrolysis. China For. Prod. Ind. 2019, 46, 33–38. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Yoo, K.-S.; Yim, J.-H.; Sohn, J.M.; Jeong, K.-E.; Jeon, J.-K.; Park, Y.-K. Thermal degradation of plywood with block polypropylene in TG and batch reactor system. J. Ind. Eng. Chem. 2011, 17, 549–553. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Pyrolysis of wood waste containing urea-formaldehyde and melamine-formaldehyde resins. J. Anal. Appl. Pyrolysis 2008, 81, 113–120. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Comparison of gasification and pyrolysis of thermal pre-treated wood board waste. J. Anal. Appl. Pyrolysis 2009, 85, 171–183. [Google Scholar] [CrossRef]
- Jun, L. Thermal Analysis on PF/ Waste Medium Density Fiberboard Composite by TG and DSC. China Wood Ind. 2015, 29, 14–17. [Google Scholar] [CrossRef]
- Fang, H.; Wu, Q.; Jiang, R.; Li, H.; Zheng, Y.; Han, X. Perparation and Carbonization Behavior of Bamboo Fiber Modified by Copolymerized Phenolic Resin. Chin. J. Mater. Res. 2014, 28, 853–857. [Google Scholar]
- Yuqing, X.; Yanfen, L.; Xiaoqian, M.; Na, S. Co-pyrolysis characteristics and kinetics of chlorella vulgaris and rice husk. Renew. Energy Resour. 2021, 39, 1570–1575. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, X.; Liu, L.; Li, P.; Tian, L. Slow pyrolysis characteristics of bamboo subfamily evaluated through kinetics and evolved gases analysis. Bioresour. Technol. 2019, 289. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; Alves, J.L.F.; Galdino, W.V.D.A.; Andersen, S.L.F.; de Sena, R.F. Pyrolysis kinetic evaluation by single-step for waste wood from reforestation. Waste Manag. 2018, 72. [Google Scholar] [CrossRef]
- Li, X.; Bing, H.; Luo, S.; Zhang, W.; Zuo, Z.; Ren, D. Study on the Pyrolysis Behaviors of Urea-Formaldehyde Resin and Rice Straw Mixed Pellets. Front. Energy Res. 2022, 9. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245. [Google Scholar] [CrossRef]
- Chen, H.; Xie, Y.; Chen, W.; Xia, M.; Li, K.; Chen, Z.; Chen, Y.; Yang, H. Investigation on co-pyrolysis of lignocellulosic biomass and amino acids using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 196, 320–329. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Tippayawong, N. Non-isothermal pyrolysis characteristics of giant sensitive plants using thermogravimetric analysis. Bioresour. Technol. 2010, 101, 5638–5644. [Google Scholar] [CrossRef]
- Feng, Y.S.; Huang, Z.Y.; Mu, J. Pyrolysis characteristics of poplar particleboards containing UF resins. J. Beijing For. Univ. 2012, 34, 119–122. [Google Scholar] [CrossRef]
- Hirata, T.; Kawamoto, S.; Okuro, A. Pyrolysis of melamine–formaldehyde and urea–formaldehyde resins. J. Appl. Polym. Sci. 1991, 42. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Pyrolytic kinetics, reaction mechanisms and products of waste tea via TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 184, 436–447. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C. Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis. Energy Convers. Manag. 2016, 120, 370–377. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.; Bej, S.; Thring, R.; Bakhshi, N. Production of H2 and medium Btu gas via pyrolysis of lignins in a fixed-bed reactor. Fuel Process. Technol. 2001, 70, 9–26. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Fang, L.; Liu, L.; Luo, S.; Wang, J.; Zuo, Z.; Peng, B.; Chen, Y.; Yi, C.; Sun, W. Study on the Kinetics and Pyrolysis Behavior of Waste Containing Urea-Formaldehyde Resins. J. Biobased Mater. Bioenergy J. 2020, 14, 444–452. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J. Hazard. Mater. 2010, 173, 205–210. [Google Scholar] [CrossRef]
- Richards, G.N. Glycolaldehyde from pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 1987, 10, 251–255. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, Z.; Liao, H.; Lu, Q. Comparison of Fast Pyrolysis of Poplar and Pine Woods on the Basis of Py-GC-MS Analysis. Chem. Ind. For. Prod. 2013, 33, 41–47. [Google Scholar] [CrossRef]
- Güllü, D.; Demirbaş, A. Biomass to methanol via pyrolysis process. Energy Convers. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Shen, D.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Dong, C.-Q.; Zhang, Z.-F.; Lu, Q.; Yang, Y.-P. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 2012, 57, 49–59. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.-C.; Dong, C.-Q.; Zhang, Z.-F.; Zhang, X.-M.; Zhu, X.-F. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Piskorz, J.; Radlein, D.; Scott, D.S. On the mechanism of the rapid pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 1986, 9, 121–137. [Google Scholar] [CrossRef]
- Azeez, A.M.; Meier, D.; Odermatt, J. Temperature dependence of fast pyrolysis volatile products from European and African biomasses. J. Anal. Appl. Pyrolysis 2011, 90, 81–92. [Google Scholar] [CrossRef]


| Number | m/z | Molecular Formula | Compound Name | Area (%) | |||
|---|---|---|---|---|---|---|---|
| PW | PW:UF = 5:1 | PW:UF = 2:1 | UF resin | ||||
| 1 | 180 | C10H12O3 | Phenol,2,6-dimethoxy-4-vinyl- | 6.83 | 7.56 | 6.87 | - |
| 2 | 180 | C10H12O3 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 6.54 | 4.02 | 3.09 | - |
| 3 | 60 | C2H4O2 | Acetic acid | 4.77 | 5.25 | 3.92 | 0.82 |
| 4 | 154 | C8H10O3 | 2,6-Dimethoxyphenol | 4.71 | 6.02 | 5.33 | - |
| 5 | 89 | C3H7NO2 | Sarcosine | 4.66 | 8.81 | 10.91 | 17.27 |
| 6 | 194 | C11H14O3 | €-2,6-Dimethoxy-4-(prop-1-en-1-yl) phenol | 4.6 | 5.52 | 4.93 | - |
| 7 | 210 | C11H14O4 | trans-Sinapyl alcohol | 4.01 | 1.16 | 0.74 | - |
| 8 | 150 | C9H10O2 | 4-Hydroxy-3-methoxystyrene | 3.24 | 3.72 | 3.26 | - |
| 9 | 74 | C3H6O2 | Hydroxyacetone | 2.95 | 2.03 | 1.62 | - |
| 10 | 86 | C4H6O2 | Butanedial | 2.62 | - | - | - |
| 11 | 113 | C4H7N3O | Creatinine | 2.48 | - | - | - |
| 12 | 98 | C5H6O2 | 1,2-Cyclopentanedione | 2.31 | 2.67 | 1.87 | - |
| 13 | 168 | C9H12O3 | Phenol,2,6-dimethoxy-4-methyl- | 2.24 | 2.51 | 2.01 | - |
| 14 | 124 | C7H8O2 | Guaiacol | 2.14 | 2.64 | 2.1 | - |
| 15 | 164 | C10H12 | €(e)-isoeugenol | 2.09 | 2.64 | 2.27 | - |
| 16 | 72 | C4H8O | Cyclopropyl carbinol | 2.03 | - | 1.86 | - |
| 17 | 94 | C6H6O | Phenol | 1.99 | 2.08 | 2.04 | - |
| 18 | 104 | C4H8O3 | 1,2-Ethanediol,1-acetate | 1.91 | - | - | - |
| 19 | 130 | C4H3FN2O2 | 5-Fluorouracil | 1.62 | 1.06 | 0.77 | - |
| 20 | 138 | C8H10O2 | 2-Methoxy-4-methylphenol | 1.53 | 1.07 | 0.95 | - |
| 21 | 194 | C11H14O3 | Phenol,2,6-dimethoxy-4-(2-propen-1-yl)- | 1.35 | 1.5 | 1.93 | - |
| 22 | 210 | C11H14O4 | 2-Propanone,1-(4-hydroxy-3,5-dimethoxyphenyl)- | 1.14 | 0.76 | 0.74 | - |
| 23 | 317 | C20H15NO3 | Benzoic acid, 2-[([1,1’-biphenyl]-4-ylamino) carbonyl]- | - | - | - | 9.39 |
| 24 | 162 | C6H10O5 | 1,6-anhydro-beta-d-glucopyranos | 5.98 | 1.25 | 2.08 | - |
| 25 | 102 | C4H6O3 | Methyl pyruvate | - | 1.88 | 1.97 | 5.84 |
| 26 | 144 | C6H8O4 | 1,4:3,6-Dianhydro-α- D-glucopyranose | 0.42 | - | - | 3.45 |
| 27 | 98 | C5H6O2 | 3-Furanmethanol | - | - | - | 2.7 |
| 28 | 71 | C4H9N | Cyclobutylamine | - | - | - | 2.58 |
| 29 | 84 | C4H8N2 | N, N-Dimethylglycinonitrile | - | - | - | 2.4 |
| 30 | 128 | C8H16O | 2,2-Dimethylcyclohexanol | - | - | - | 2.33 |
| 31 | 129 | C6H15N3 | hexahydro-1,3,5-trimethyl-s-triazin | - | - | - | 2.17 |
| 32 | 112 | C6H8O2 | Methyl cyclopentenolone | - | - | - | 1.68 |
| 33 | 109 | C6H7NO | 2-Acetyl pyrrole | - | - | - | 1.58 |
| 34 | 134 | C9H10O | Phenylacetone | - | - | - | 1.38 |
| 35 | 256 | C16H32O2 | Palmitic acid | - | 0.41 | 0.49 | 1.35 |
| 36 | 128 | C6H12N2O | 5-Amino-1-methyl-2-piperidinone | - | 0.36 | 0.46 | 1.15 |
| 37 | 88 | C4H12N2 | 1,3-Propanediamine, N1-methyl- | - | 1.93 | - | - |
| 38 | 164 | C10H12O2 | Phenol,2-methoxy-4-(1-propen-1-yl)- | - | 1.65 | - | - |
| 39 | 238 | C15H26O2 | 3-methyl-, 3,7-dimethyl-2,6-octadienyl ester, (E)-Butanoic acid | 0.01 | 1.2 | 0.06 | - |
| 40 | 362 | C18H22N2O4S | Thiazolidine-2,4-dione, 5-(2-methoxybenzylidene]-3-(2,6-dimethylmorpholinomethyl)- | - | - | 3.06 | - |
| 41 | 112 | C6H8O2 | 3-Methyl-1,2-cyclopentanedione | 1.02 | 1.86 | 1.98 | - |
| 42 | 84 | C4H4O2 | 2(5H)-Furanone | 1.15 | 1.55 | 1.73 | 1.97 |
| 43 | 98 | C5H6O2 | Furfuryl alcohol | 0.79 | 1.06 | 1.24 | - |
| 44 | 198 | C8H18N6 | 2,2’-Azobis-2-methylpropanimidamide | 0.08 | 1.28 | 1.21 | 0.08 |
| 45 | 152 | C9H12O2 | 4-Ethyl-2-methoxyphenol | 0.58 | 1.12 | 1.12 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Luo, S.; Zuo, Z.; Zhang, W.; Ren, D. The Pyrolysis Behaviors of Blended Pellets of Pine Wood and Urea-Formaldehyde Resin. Energies 2023, 16, 2049. https://doi.org/10.3390/en16042049
Li X, Luo S, Zuo Z, Zhang W, Ren D. The Pyrolysis Behaviors of Blended Pellets of Pine Wood and Urea-Formaldehyde Resin. Energies. 2023; 16(4):2049. https://doi.org/10.3390/en16042049
Chicago/Turabian StyleLi, Xiaoteng, Siyi Luo, Zongliang Zuo, Weiwei Zhang, and Dongdong Ren. 2023. "The Pyrolysis Behaviors of Blended Pellets of Pine Wood and Urea-Formaldehyde Resin" Energies 16, no. 4: 2049. https://doi.org/10.3390/en16042049





