Co-Pyrolysis of Woody Biomass and Oil Shale in a Batch Reactor in CO2, CO2-H2O, and Ar Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
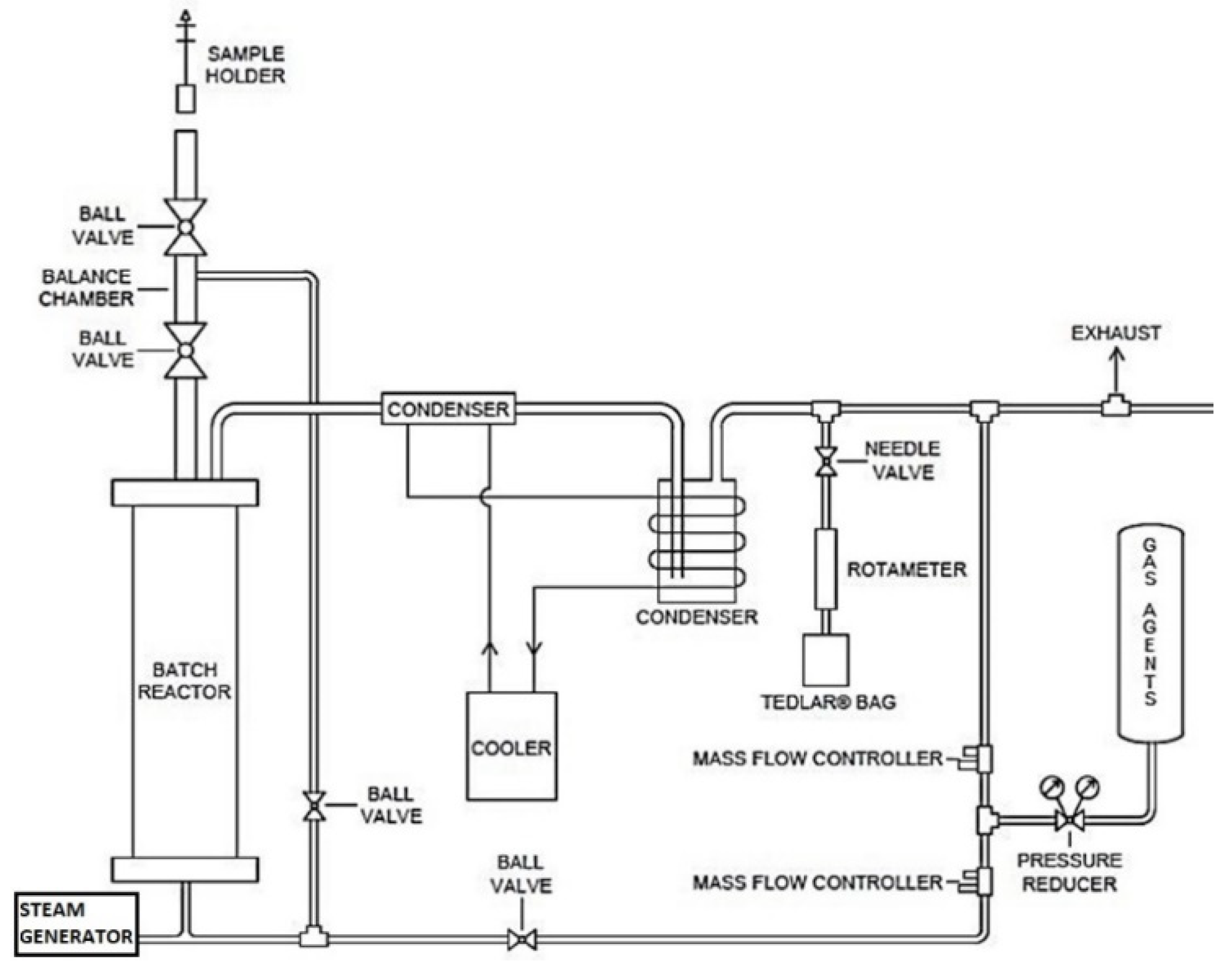
2.2. Experiment Set Up
2.3. Gas Products Analysis
2.4. Solid Product Analysis
3. Results and Discussion
3.1. Feedstock Properties
3.2. Pyrolysis at Different Residence Times
3.3. OS-WB Co-Pyrolysis
3.4. Gas Composition
3.5. Solid Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| BET | Brunauer, Emmett, and Teller surface area |
| CCS | Carbon capture and storage technologies |
| GC-TCD | Gas chromatography with thermal conductivity detector |
| HHV | Gross or higher heating value |
| LHV | Net or lower heating value |
| OS | Oil shale |
| TGA | Thermogravimetric analysis |
| WB | Woody biomass |
Appendix A
Appendix A.1


Appendix A.2

References
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariyam, S.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.R.; McKay, G. A critical review on co-gasification and co-pyrolysis for gas production. Renew. Sustain. Energy Rev. 2022, 161, 112349. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z. Pyrolysis of Biomass; GREEN—Alternative Energy Resources; Walter de Gruyter GmbH: Berlin, Germany, 2017; p. 268. [Google Scholar]
- Akhtar, A.; Krepl, V.; Ivanova, T. A combined overview of combustion, pyrolysis, and gasification of biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Jerzak, W.; Gao, N.; Kalemba-Rec, I.; Magdziarz, A. Catalytic intermediate pyrolysis of post-extraction rapeseed meal by reusing ZSM-5 and Zeolite Y catalysts. Catal. Today 2022, 404, 63–77. [Google Scholar] [CrossRef]
- Ibrahim, M.D.; Abakr, Y.A.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2). Energies 2022, 15, 8421. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Wang, K.; Zhu, Y. Commercialization and challenges for the next generation of biofuels: Biomass fast pyrolysis. In Proceedings of the 2010 APPEEC Conference, Chengdu, China, 28–31 March 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Bai, F.; Sun, Y.; Liu, Y.; Li, Q.; Guo, M. Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy Convers. Manag. 2015, 97, 374–381. [Google Scholar] [CrossRef]
- Raja, M.A.; Zhao, Y.; Zhang, X.; Li, C.; Zhang, S. Practices for modeling oil shale pyrolysis and kinetics. Rev. Chem. Eng. 2017, 34, 21–42. [Google Scholar] [CrossRef]
- Ristic, N.D.; Djokic, M.R.; Konist, A.; Van Geem, K.M.; Marin, G.B. Quantitative compositional analysis of Estonian shale oil using comprehensive two dimensional gas chromatography. Fuel Process. Technol. 2017, 167, 241–249. [Google Scholar] [CrossRef]
- Maaten, B.; Järvik, O.; Pihl, O.; Konist, A.; Siirde, A. Oil shale pyrolysis products and the fate of sulfur. Oil Shale 2020, 37, 51–69. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, X.; Li, S.; Mikulčić, H.; Bešenić, T.; Deng, S.; Vujanović, M.; Tan, H.; Kumfer, B.M. Synergistic effects during co-pyrolysis of biomass and plastic: Gas, tar, soot, char products and thermogravimetric study. J. Energy Inst. 2019, 92, 108–117. [Google Scholar] [CrossRef]
- Ganev, E.; Ivanov, B.; Vaklieva-Bancheva, N.; Kirilova, E.; Dzhelil, Y. A multi-objective approach toward optimal design of sustainable integrated biodiesel/diesel supply chain based on first-and second-generation feedstock with solid waste use. Energies 2021, 14, 2261. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Liu, A.; Wang, L.; Yu, G. Co-pyrolysis characteristic of biomass and bituminous coal. Bioresour. Technol. 2015, 179, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Krerkkaiwan, S.; Fushimi, C.; Tsutsumi, A.; Kuchonthara, P. Synergetic effect during co-pyrolysis/gasification of biomass and sub-bituminous coal. Fuel Process. Technol. 2013, 115, 11–18. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Jin, L.; Hu, H. Pyrolysis behaviors of two coal-related model compounds on a fixed-bed reactor. Fuel Process. Technol. 2015, 129, 113–119. [Google Scholar] [CrossRef]
- Jiang, H.; Deng, S.; Chen, J.; Zhang, L.; Zhang, M.; Li, J.; Li, S.; Li, J. Preliminary study on co-pyrolysis of spent mushroom substrate as biomass and Huadian oil shale. Energy Fuels 2016, 30, 6342–6349. [Google Scholar] [CrossRef]
- Cerón, A.L.; Konist, A.; Lees, H.; Järvik, O. Current status of co-pyrolysis of oil shale and biomass. Oil Shale 2021, 38, 228–263. [Google Scholar] [CrossRef]
- Chen, B.; Han, X.; Mu, M.; Jiang, X. Studies of the co-pyrolysis of oil shale and wheat straw. Energy Fuels 2017, 31, 6941–6950. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Fang, S.; Ma, X. Behaviors, product characteristics and kinetics of catalytic co-pyrolysis spirulina and oil shale. Energy Convers. Manag. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Nowicki, L.; Siuta, D.; Markowski, M. Carbon Dioxide Gasification Kinetics of Char from Rapeseed Oil Press Cake. Energies 2020, 13, 2318. [Google Scholar] [CrossRef]
- Nowicki, L.; Siuta, D.; Markowski, M. Pyrolysis of rapeseed oil press cake and steam gasification of solid residues. Energies 2020, 13, 4472. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, J.; Huo, X.; Gao, Y.; Hao, J.; Song, M. Effect of CO2 atmosphere on biomass pyrolysis and in-line catalytic reforming. Int. J. Energy Res. 2020, 44, 8936–8950. [Google Scholar] [CrossRef]
- Giudicianni, P.; Cardone, G.; Ragucci, R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2013, 100, 213–222. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Production of liquid feedstock from biomass via steam pyrolysis in a fluidized bed reactor. Energy Fuels 2013, 27, 4748–4759. [Google Scholar] [CrossRef]
- Lee, J.; Yang, X.; Cho, S.H.; Kim, J.K.; Lee, S.S.; Tsang, D.C.W.; Ok, Y.S.; Kwon, E.E. Pyrolysis process of agricultural waste using CO2 for waste management, energy recovery, and biochar fabrication. Appl. Energy 2017, 185, 214–222. [Google Scholar] [CrossRef]
- Sulg, M.; Konist, A.; Järvik, O. Characterization of different wood species as potential feedstocks for gasification. Agron. Res. 2021, 19, 276–299. [Google Scholar]
- Chen, B.; Han, X.; Tong, J.; Mu, M.; Jiang, X.; Wang, S.; Shen, J.; Ye, X. Studies of fast co-pyrolysis of oil shale and wood in a bubbling fluidized bed. Energy Convers. Manag. 2020, 205, 112356. [Google Scholar] [CrossRef]
- Cascante Cirici, P. Biomass and Oil Shale Co-Pyrolysis. Master’s Thesis, Tallinn University of Technology, Tallinn, Estonia, 2019. [Google Scholar]
- Cerón, A.L.; Konist, A.; Lees, H.; Järvik, O. Effect of woody biomass gasification process conditions on the composition of the producer gas. Sustainability 2021, 13, 11763. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Wang, X.S.; Shen, J.; Rhodes, M.J.; Tian, F.; Lee, W.J.; Wu, H.; Li, C.Z. Fast pyrolysis of oil mallee woody biomass: Effect of temperature on the yield and quality of pyrolysis products. Ind. Eng. Chem. Res. 2008, 47, 1846–1854. [Google Scholar] [CrossRef]
- Grieco, E.; Baldi, G. Analysis and modelling of wood pyrolysis. Chem. Eng. Sci. 2011, 66, 650–660. [Google Scholar] [CrossRef]
- Syed, S.; Qudaih, R.; Talab, I.; Janajreh, I. Kinetics of pyrolysis and combustion of oil shale sample from thermogravimetric data. Fuel 2011, 90, 1631–1637. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M. Compositional and kinetic analysis of oil shale pyrolysis using TGA-MS. Fuel 2012, 94, 333–341. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M. Detailed kinetic analysis of oil shale pyrolysis TGA data. AIChE J. 2012, 58, 505–515. [Google Scholar] [CrossRef]
- Johannes, I.; Tiikma, L.; Luik, H. Synergy in co-pyrolysis of oil shale and pine sawdust in autoclaves. J. Anal. Appl. Pyrolysis 2013, 104, 341–352. [Google Scholar] [CrossRef]
- Yanik, J.; Secim, P.; Karakaya, S.; Tiikma, L.; Luik, H.; Krasulina, J.; Raik, P.; Palu, V. Low-temperature pyrolysis and co-pyrolysis of Göynük oil shale and terebinth berries (Turkey) in an autoclave. Oil Shale 2011, 28, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Kiliç, M.; Pütün, A.E.; Uzun, B.B.; Pütün, E. Converting of oil shale and biomass into liquid hydrocarbons via pyrolysis. Energy Convers. Manag. 2014, 78, 461–467. [Google Scholar] [CrossRef]
- Tian, B.; Wang, J.; Qiao, Y.; Huang, H.; Xu, L.; Tian, Y. Understanding the pyrolysis synergy of biomass and coal blends based on volatile release, kinetics and char structure. Biomass Bioenergy 2023, 168, 106687. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Kuo, C.-C.; Yang, M.-W.; Zhuang, Z.-Y.; Lin, P.-W.; Chen, Y.-F.; Yang, H.-S.; Chou, C.-T. CO2 capture from flue gas of a coal-fired power plant using three-bed PSA process. Energies 2021, 14, 3582. [Google Scholar] [CrossRef]
- Xie, W. Uncertainty analysis and evaluation of calibration results of meteorological chromatograph. In Proceedings of the 2022 IEEE 10th Joint International Information Technology and Artificial Intelligence Conference (ITAIC), Chongqing, China, 17–19 June 2022; pp. 1158–1161. [Google Scholar]
- Quan, C.; Xu, S.; An, Y.; Liu, X. Co-pyrolysis of biomass and coal blend by TG and in a free fall reactor. J. Therm. Anal. Calorim. 2014, 117, 817–823. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Pikkor, H.; Maaten, B.; Baird, Z.S.; Järvik, O.; Konist, A.; Lees, H. Surface area of oil shale and its solid pyrolysis products depending on the particle size. Chem. Eng. Trans. 2020, 81, 961–966. [Google Scholar]


| WB ** | OS ** | |||||
|---|---|---|---|---|---|---|
| Spruce | Alder | Pine | Birch | |||
| Elemental composition [wt%] | C | 50.3 | 49.9 | 50.1 | 49.3 | 27.2 |
| H | 6.6 | 6.6 | 6.6 | 6.6 | 2.8 | |
| N | 0.1 | 0.2 | 0.19 | 0.08 | <0.1 | |
| S | n.d. | n.d. | n.d. | n.d. | 2.0 | |
| O * | 42.7 | 43.0 | 43.1 | 44.0 | 21.0 | |
| Proximate analysis [wt%] | Ash content | 0.3 | 0.3 | 0.3 | 0.3 | 52.5 |
| Moisture | 6.9 | 7.6 | 8.5 | 7.7 | 0.9 | |
| Fixed carbon | 14.2 | 14.0 | 14.5 | 12.8 | 2.0 | |
| Volatile matter | 85.5 | 85.7 | 85.2 | 86.9 | 45.5 | |
| Heating value [MJ/kg] | LHV | 18.4 | 18.5 | 18.6 | 18.1 | 8.7 |
| HHV | 19.8 | 19.9 | 20.0 | 19.9 | 9.7 | |
| OS:WB Ratio | Gas Atmosphere, 0.3 L/min | Gas Species Concentration, vol% | ||
|---|---|---|---|---|
| H2 | CO | CH4 | ||
| 0:1 | CO2 | 0.08 | 1.19 | 0.15 |
| H2O:CO2 1:1 | 0.06 | 1.01 | 0.14 | |
| Ar | 0.08 | 1.30 | 0.14 | |
| 7:3 | CO2 | 0.05 | 0.56 | 0.06 |
| H2O:CO2 1:1 | 0.07 | 0.87 | 0.04 | |
| Ar | 0.05 | 0.82 | 0.07 | |
| 9:1 | CO2 | 0.05 | 0.31 | 0.08 |
| H2O:CO2 1:1 | 0.05 | 0.37 | 0.04 | |
| Ar | 0.06 | 0.65 | 0.06 | |
| 1:0 | CO2 | 0.06 | 0.42 | 0.06 |
| H2O:CO2 1:1 | 0.1 | 0.75 | 0.06 | |
| Ar | 0.04 | 0.42 | 0.05 | |
| OS:WB Ratio | Gas Atmosphere, 0.3 L/min | C, wt% | H, wt% | N, wt% | S, wt% | BET, m2/g |
|---|---|---|---|---|---|---|
| 0:1 | CO2 | 78.92 | 3.26 | 0.40 | n.d. * | 127.9 |
| H2O:CO2 1:1 | 78.86 | 3.47 | 0.38 | n.d. | 173.8 | |
| Ar | 77.29 | 3.39 | 0.43 | n.d. | 175.9 | |
| 7:3 | CO2 | 15.03 | 0.27 | 0.05 | 0.69 | 17.8 |
| H2O:CO2 1:1 | 15.87 | 0.40 | 0.04 | 0.69 | 19.5 | |
| Ar | 14.39 | 0.27 | 0.05 | 1.09 | 6.1 | |
| 9:1 | CO2 | 12.00 | 0.15 | 0.02 | 0.72 | 19.1 |
| H2O:CO2 1:1 | 12.64 | 0.20 | 0.02 | 0.95 | 29.3 | |
| Ar | 11.77 | 0.19 | 0.02 | 1.87 | 45.7 | |
| 1:0 | CO2 | 11.39 | 0.22 | 0.02 | 1.12 | 11.4 |
| H2O:CO2 1:1 | 11.25 | 0.20 | 0.01 | 0.94 | 28.8 | |
| Ar | 11.55 | 0.20 | 0.01 | 0.90 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyons Cerón, A.; Konist, A. Co-Pyrolysis of Woody Biomass and Oil Shale in a Batch Reactor in CO2, CO2-H2O, and Ar Atmospheres. Energies 2023, 16, 3145. https://doi.org/10.3390/en16073145
Lyons Cerón A, Konist A. Co-Pyrolysis of Woody Biomass and Oil Shale in a Batch Reactor in CO2, CO2-H2O, and Ar Atmospheres. Energies. 2023; 16(7):3145. https://doi.org/10.3390/en16073145
Chicago/Turabian StyleLyons Cerón, Alejandro, and Alar Konist. 2023. "Co-Pyrolysis of Woody Biomass and Oil Shale in a Batch Reactor in CO2, CO2-H2O, and Ar Atmospheres" Energies 16, no. 7: 3145. https://doi.org/10.3390/en16073145







