A Minimum Entropy Production Approach to Optimization of Tubular Chemical Reactors with Nature-Inspired Design
Abstract
:1. Introduction
2. Design Optimization in Chemical Engineering
2.1. Mathematical Formulations
2.2. Minimum Entropy Production Approach
2.3. Shape Optimization and Nature-Inspired Solutions
3. Problem Formulation
4. Numerical Method
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup; McGraw Hill: New York, NY, USA, 2002. [Google Scholar]
- Singh, L.; Mahapatra, D.M. (Eds.) Bioreactors; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Li, Y.-H.; Kao, H.-H.; Wang, Y.-R.; Wan, J.; Manatura, K. Performance optimizing and entropy generation analysis of a platinum–stainless-steel segmented microreactor. Chem. Eng. J. 2023, 457, 141151. [Google Scholar] [CrossRef]
- Salmi, T.O.; Mikkola, J.-P.; Wärnå, J.P. Chemical Reaction Engineering and Reactor Technology, 2nd ed.; CRC Press: London, UK, 2019. [Google Scholar]
- Kizilova, N.; Rokicki, J. 3D Bioreactors for cell culture: Fluid dynamics aspects. In Biomechanics in Medicine, Sport and Biology; Hadamus, A., Piszczatowsky, S., Syczewska, M., Blazkiewicz, M., Eds.; Springer Series Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2022; Volume 328, pp. 80–99. [Google Scholar]
- Liu, S. Bioprocess Engineering. Kinetics, Sustainability, and Reactor Design, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Bilous, O.; Amundson, N.R. Optimum temperature gradients in tubular reactors—I. Chem. Eng. Sci. 1956, 5, 81–92. [Google Scholar] [CrossRef]
- Bilous, O.; Amundson, N.R. Optimum temperature gradients in tubular reactors—II. Chem. Eng. Sci. 1956, 5, 115–126. [Google Scholar] [CrossRef]
- Buzatu, P.; Lavric, V. Submerged membrane bioreactors for wastewater treatment: Multi-objective optimization. Chem. Eng. Trans. 2011, 25, 267–272. [Google Scholar]
- Pal, P. Industrial Water Treatment Process Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mierdel, K.; Jess, A.; Gerdes, T.; Schmidt, A.; Hintzer, K. Energy and resource efficient production of fluoroalkenes in high temperature microreactors. ChemEngineering 2019, 3, 77. [Google Scholar] [CrossRef]
- Kudiiarov, V.; Elman, R.; Pushilina, N.; Kurdyumov, N. State of the art in development of heat exchanger geometry optimization and different storage bed designs of a metal hydride reactor. Materials 2023, 16, 4891. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Enders, T.; Delgado, A. Dynamic neural networks as a tool for the online optimization of industrial fermentation. Bioprocess Biosyst. Eng. 2002, 24, 347–354. [Google Scholar]
- Chen, L.Z.; Nguang, S.K.; Chen, X.D. Modelling and Optimization of Biotechnological Processes: Artificial Intelligence Approaches; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Trogadas, P.; Coppens, M.-O. Nature-inspired electrocatalysts and devices for energy conversion. Chem. Soc. Rev. 2000, 49, 3107–3141. [Google Scholar] [CrossRef]
- Trogadas, P.; Xu, L.; Coppens, M.-O. From biomimicking to bioinspired design of electrocatalysts for CO2 reduction to C1 products. Angew. Chem. Int. Ed. 2023, 63, e202314446. [Google Scholar] [CrossRef]
- Solberg, S.B.B.; Kjelstrup, S.; Magnanelli, E.; Kizilova, N.; Barroso, I.L.C.; Acquarone, M.; Folkow, L. Energy-efficiency of respiration in mature and newborn reindeer. J. Comp. Physiol. Ser. B 2020, 190, 509–520. [Google Scholar] [CrossRef]
- Kizilova, N. Construction principles and control over transport systems organization in biological tissues. In Physics and Control; Fradkov, A.L., Churilov, A.N., Eds.; IEEE Computer Society: Washington, DC, USA, 2003; Volume 1, pp. 303–308. [Google Scholar]
- Kizilova, N. Long-distance liquid transport in plants. Proc. Est. Acad. Sci. Ser. Phys. Math. 2008, 57, 179–203. [Google Scholar] [CrossRef]
- Andriesse, C.; Hollestelle, M. Minimum entropy production in photosynthesis. Biophys. Chem. 2001, 90, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D. Scoping biology-inspired chemical engineering. Chin. J. Chem. Eng. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Coppens, M.-O. Learning from nature in building sustainable chemical technology. WIT Trans. Ecol. Environ. 2004, 73, 349–358. [Google Scholar]
- Coppens, M.-O. A nature-inspired approach to reactor and catalysis engineering. Curr. Opin. Chem. Eng. 2012, 1, 281–289. [Google Scholar] [CrossRef]
- Miguel, A.F. A study of entropy generation in tree-shaped flow structures. Int. J. Heat Mass Transf. 2016, 92, 349–359. [Google Scholar] [CrossRef]
- Coppens, M.-O. Multiscale nature inspired chemical engineering. In Multiscale Methods—Bridging the Scales in Science and Engineering; Fish, J., Ed.; Oxford University Press: Oxford, UK, 2009; pp. 536–560. [Google Scholar]
- Raja, V.; Prakash, A.; Kumar, A.; Pacheco, D.A.d.J. Multi-disciplinary engineering design of a high-speed nature-inspired unmanned aquatic vehicle. Ocean. Eng. 2023, 270, 113455. [Google Scholar] [CrossRef]
- Gerbaud, V.; Shcherbakova, N.; Da Cunh, S. A nonequilibrium thermodynamics perspective on nature-inspired chemical engineering processes. Chem. Eng. Res. Des. 2020, 154, 316–330. [Google Scholar] [CrossRef]
- Kjelstrup, S.; Bedeaux, D.; Johannessen, E.; Gross, J. Non-Equilibrium Thermodynamics for Engineers; World Scientific: Singapore, 2017; pp. 180–181. [Google Scholar]
- Johannessen, E.; Kjelstrup, S. A highway in state space for reactors with minimum entropy production. Chem. Eng. Sci. 2005, 60, 3347–3361. [Google Scholar] [CrossRef]
- Bejan, A. Shape and Structure, from Engineering to Nature; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- You, J.; Feng, H.J.; Chen, L.G.; Xie, Z.H. Constructal design of nonuniform heat generation area based on triangular elements: A case of entropy generation minimization. Int. J. Therm. Sci. 2019, 139, 403–412. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Feng, H.J.; Chen, L.G.; You, J.; Xie, Z.J. Constructal design of an arrow-shaped high thermal conductivity channel in a square heat generation body. Entropy 2020, 22, 475. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, Ø.; Johannessen, E.; Kjelstrup, S. Energy efficient reactor design simplified by second law analysis. Int. J. Hydrogen Energy 2010, 35, 13219–13231. [Google Scholar] [CrossRef]
- Nummedal, L.; Røsjorde, A.; Johannessen, E.; Kjelstrup, S. Second law optimization of a tubular steam reformer. Chem. Eng. Process Intensif. 2005, 44, 429–440. [Google Scholar] [CrossRef]
- Bertola, V.; Cafaro, E. A critical analysis of the minimum entropy production theorem and its application to heat and fluid flow. Int. J. Heat Mass Transf. 2008, 51, 1907–1912. [Google Scholar] [CrossRef]
- Korpyś, M.; Gancarczyk, A.; Iwaniszyn, M.; Sindera, K.; Jodłowski, P.J.; Kołodziej, A. Analysis of entropy production in structured chemical reactors: Optimization for catalytic combustion of air pollutants. Entropy 2020, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Pereira Rosinha Grundtvig, I.; Daugaard, A.E.; Woodley, J.M.; Gernaey, K.V.; Krühne, U. Shape optimization as a tool to design biocatalytic microreactors. Chem. Eng. J. 2017, 322, 215–223. [Google Scholar] [CrossRef]
- Edgar, T.F.; Himmelblau, D.M.; Lasdon, L.S. Optimization of Chemical Processes, 2nd ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Awad, M.M. A review of entropy generation in microchannels. Adv. Mech. Eng. 2015, 7, 168781401559029. [Google Scholar] [CrossRef]
- Rashidi, M.M.; Nasiri, M.; Shadloo, M.S.; Yang, Z. Entropy generation in a circular tube heat exchanger using nanofluids: Effects of different modeling approaches. Heat Transf. Eng. 2017, 38, 853–866. [Google Scholar] [CrossRef]
- Alsarraf, J.; Shahsavar, A.; Babaei Mahani, R.; Talebizadehsardari, P. Turbulent forced convection and entropy production of a nanofluid in a solar collector considering various shapes for nanoparticles. Int. Commun. Heat Mass Transf. 2020, 117, 104804. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Production through Heat and Fluid Flow; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Stadler, W. Multicriteria Optimization in Engineering and in the Sciences; Mathematical Concepts and Methods in Science and Engineering; Springer Science & Business Media: New York, NY, USA, 1988; Volume 37. [Google Scholar]
- Nummedal, L. Entropy Production Minimization of Chemical Reactors and Heat Exchangers. Ph.D. Thesis, Department of Chemistry, Norwegian University of Science and Technology, Trondheim, Norway, 2001. [Google Scholar]
- Wechsatol, W.; Lorente, S.; Bejan, A. Tree-shaped flow structures: Are both thermal-resistance and flow-resistance minimisations necessary? Int. J. Exergy 2004, 1, 2–17. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Production Minimization: The Method of Thermodynamic Optimization of Finite-Size Systems and Finite-Time Processes; Advanced Topics in Mechanical Engineering; Kulacki, F.A., Ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Zhou, L.; Hang, J.; Bai, L.; Krzemianowski, Z.; El-Emam, M.A.; Yasser, E.; Agarwal, R. Application of entropy production theory for energy losses and other investigation in pumps and turbines: A review. Appl. Energy 2022, 318, 119211. [Google Scholar] [CrossRef]
- Haseli, Y. The equivalence of minimum entropy production and maximum thermal efficiency in endoreversible heat engines. Heliyon 2016, 2, e00113. [Google Scholar] [CrossRef] [PubMed]
- Bispo, H.; Silva, N.; Brito, R.; Manzi, J. On the equivalence between the minimum entropy generation rate and the maximum conversion rate for a reactive system. Energy Convers. Manag. 2013, 76, 26–31. [Google Scholar] [CrossRef]
- Krishna, R. Resolving steady-state multiplicities for diffusion with surface chemical reaction by invoking the Prigogine principle of minimum entropy production. Chem. Eng. Res. Des. 2017, 128, 231–239. [Google Scholar] [CrossRef]
- Salamon, P.; Hoffmann, K.H.; Schubert, S.; Berry, R.S.; Andresen, B. What conditions make minimum entropy production equivalent to maximum power production? J. Non-Equilib. Thermodyn. 2001, 26, 73–81. [Google Scholar] [CrossRef]
- Dechant, A. Minimum entropy production, detailed balance and Wasserstein distance for continuous-time Markov processes. J. Phys. A Math. Theor. 2022, 55, 094001. [Google Scholar] [CrossRef]
- Li, P.L.; Chen, L.G.; Xia, S.J.; Zhang, L. Entropy production rate minimization for methanol synthesis via a CO2 hydrogenation reactor. Entropy 2019, 21, 174. [Google Scholar] [CrossRef]
- Nummedal, L.; Kjelstrup, S.; Costea, M. Minimizing the entropy production rate of an exothermic reactor with a constant heat-transfer coefficient: The ammonia reaction. Ind. Eng. Chem. Res. 2003, 42, 1044–1056. [Google Scholar] [CrossRef]
- Chen, Q.X.; Xia, S.J.; Wang, W.H.; Chen, L.G. Entropy production rate minimization of steam methane reforming reactor with Dulong-Petit heat transfer law. Energy Conserv. 2018, 37, 31–40. [Google Scholar]
- Li, P.; Chen, L.; Xia, S.; Zhang, L.; Kong, R.; Ge, Y.; Feng, H. Entropy production rate minimization for steam methane reforming reactor heated by molten salt. Energy Rep. 2020, 6, 685–697. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.G.; Xia, S.J.; Ge, Y.L.; Wang, C.; Feng, H.J. Multi-objective optimization for helium-heated reverse water gas shift reactor by using NSGA-II. Int. J. Heat Mass Transf. 2020, 148, 119025. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.G.; Xia, S.J.; Wang, C.; Sun, F.R. Entropy production minimization for reverse water gas shift (RWGS) reactor. Entropy 2018, 20, 415. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.; Razzitte, A.C. Entropy production minimization in dimethylether synthesis: A case study. J. Non-Equilib. Thermodyn. 2018, 43, 111–120. [Google Scholar] [CrossRef]
- Rangel-Hernandez, V.H.; Damian-Ascencio, C.; Juarez-Robles, D.; Gallegos-Muñoz, A.; Zaleta-Aguilar, A.; Plascencia-Mora, H. Entropy generation analysis of a proton exchange membrane fuel cell (PEMFC) with a fermat spiral as a flow distributor. Energy 2011, 36, 4864–4870. [Google Scholar] [CrossRef]
- Sauermoser, M.; Kizilova, N.; Pollet, B.G.; Kjelstrup, S. Flow field patterns for proton exchange membrane fuel cells. Front. Energy Res. 2020, 8, 13. [Google Scholar] [CrossRef]
- Sauermoser, M.; Kjelstrup, S.; Kizilova, N.; Pollet, B.G.; Flekkoy, E.G. Seeking minimum entropy production for a tree-like flow-field in a fuel cell. Phys. Chem. Chem. Phys. 2020, 22, 6993–7003. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V. Entropy generation analysis for the design optimization of solid oxide fuel cells. Int. J. Numer. Methods Heat Fluid Flow 2011, 21, 535–558. [Google Scholar] [CrossRef]
- Rong, H.; Zhao, D.; Becker, S.; Liu, X. Entropy production and thermodynamics exergy investigation on an ammonia-methane fueled micro-combustor with porous medium for thermophotovoltaic applications. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Zhang, L.; Xia, S.; Chen, L.; Ge, Y.; Wang, C.; Feng, H. Entropy generation rate minimization for hydrocarbon synthesis reactor from carbon dioxide and hydrogen. Int. J. Heat Mass Transf. 2019, 137, 1112–1123. [Google Scholar] [CrossRef]
- Chen, L.; Xia, C.; Sun, F. Entropy generation minimization for isothermal crystallization processes with a generalized mass diffusion law. Int. J. Heat Mass Transf. 2018, 116, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Xia, S.; Sun, F. Entropy generation minimization for CO2 hydrogenation to light olefins. Energy 2018, 147, 187–196. [Google Scholar] [CrossRef]
- Chen, Z.; Weng, Z.; Mével, R. Entropy and nitrogen oxides production in steady detonation wave propagating in hydrogen-air mixtures. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Johannessen, E.; Røsjorde, A. Equipartition of entropy production as an approximation to the state of minimum entropy production in diabatic distillation. Energy 2007, 32, 467–473. [Google Scholar] [CrossRef]
- Kong, R.; Chen, L.; Xia, S.; Li, P.; Ge, Y. Minimization of entropy production rate in hydrogen iodide decomposition reactor heated by high-temperature helium. Entropy 2021, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.; Razzitte, A.C. Entropy production in chemical reactors. J. Non-Equilib. Thermodyn. 2017, 42, 265–276. [Google Scholar] [CrossRef]
- Vargas-Almeida, A.; Olivares-Robles, M.A. Geometric conditions for minimizing entropy production in thermocouple design. Results Phys. 2022, 41, 105893. [Google Scholar] [CrossRef]
- Samal, B.; Barik, A.K.; Awad, M.M. Thermo-fluid and entropy generation analysis of newly designed loops for constructal cooling of a square plate. Appl. Therm. Eng. 2019, 156, 250–262. [Google Scholar] [CrossRef]
- Ahmadi, P.; Hajabdollahi, H.; Dincer, I. Cost and entropy generation minimization of a cross-flow plate fin heat exchanger using multi-objective genetic algorithm. J. Heat Transf. 2011, 133, 021801. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Kim, Y.D. A second law analysis and entropy generation minimization of an absorption chiller. Appl. Therm. Eng. 2011, 31, 2405–2413. [Google Scholar] [CrossRef]
- Pazarlıoğlu, H.R.; Ekiciler, R.; Arslan, K.; Mohammed, N.A.M. Exergetic, energetic, and entropy production evaluations of parabolic trough collector retrofitted with elliptical dimpled receiver tube filled with hybrid nanofluid. Appl. Therm. Eng. 2023, 223, 120004. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V.; Sciubba, E. Entropy generation analysis as a design tool—A review. Renew. Sustain. Energy Rev. 2015, 43, 1167–1181. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, X. Analyses of entropy generation and heat entransy loss in heat transfer and heat-work conversion. Int. J. Heat Mass Transf. 2013, 64, 903–909. [Google Scholar] [CrossRef]
- Sun, M.; Xia, S.J.; Chen, L.G.; Wang, C.; Tang, C.Q. Minimum entropy generation rate and maximum yield optimization of sulfuric acid decomposition process using NSGA-II. Entropy 2020, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.H. Minimal entropy production rate of interacting systems. New J. Phys. 2020, 22, 113013. [Google Scholar] [CrossRef]
- Suzuki, M. Irreversibility and entropy production in transport phenomena I. Phys. A Stat. Mech. Its Appl. 2011, 390, 1904–1916. [Google Scholar] [CrossRef]
- Suzuki, M. Irreversibility and entropy production in transport phenomena, III—Principle of minimum integrated entropy production including nonlinear responses. Phys. A Stat. Mech. Appl. 2013, 392, 314–325. [Google Scholar] [CrossRef]
- Maes, C.; Netočný, K. Minimum entropy production principle from a dynamical fluctuation law. J. Math. Phys. 2007, 48, 053306. [Google Scholar] [CrossRef]
- Salamon, P.; Nitzan, A.; Andresen, B.; Berry, R.S. Minimum entropy production and the optimization of heat engines. Phys. Rev. A 1980, 21, 2115. [Google Scholar] [CrossRef]
- Magnanelli, E.; Wilhelmsen, Ø.; Johannessen, E.; Kjelstrup, S. Energy efficient design of membrane processes by use of entropy production minimization. Comput. Chem. Eng. 2018, 117, 105–116. [Google Scholar] [CrossRef]
- Góes, P.; Rosa, D.; Manzi, J. A mathematical approach of the entropic index applied to chemical systems. Can. J. Chem. Eng. 2021, 99, 1011–1019. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, L.; Yang, C.T. Derivation and verification of minimum energy dissipation rate principle of fluid based on minimum entropy production rate principle. Int. J. Sediment Res. 2016, 31, 16–24. [Google Scholar] [CrossRef]
- Camacho-Medina, P.; Olivares-Robles, M.A.; Vargas-Almeida, A.; Solorio-Ordaz, F. Maximum power of thermally and electrically coupled thermoelectric generators. Entropy 2014, 16, 2890–2903. [Google Scholar] [CrossRef]
- Tondeur, D.; Kvaalen, E. Equipartition of entropy production. An optimality criterion for transfer and separation processes. Ind. Eng. Chem. Res. 1987, 26, 50–56. [Google Scholar]
- Sauar, E.; Siragusa, G.; Andresen, B. Equal thermodynamic distance and equipartition of forces principles applied to binary distillation. J. Phys. Chem. A 2001, 105, 2312–2320. [Google Scholar] [CrossRef]
- Bejan, A.; Tondeur, D. Equipartition, optimal allocation, and the constructal approach to predicting organization in nature. Rev. Gén. Therm. 1998, 37, 165–180. [Google Scholar] [CrossRef]
- Kjelstrup, S.; Sauar, E.; Liens, K.M. Equipartition of forces: Review of a new principle for process design and optimization. Period. Polytech. Chem. Eng. 1998, 42, 103–114. [Google Scholar]
- Tondeur, D. Equipartition of entropy production: A design and optimization criterion in chemical engineering. In Finite-Time Thermodynamics and Thermoeconomics; Taylor & Francis: New York, NY, USA, 1990; pp. 175–208. [Google Scholar]
- Kjelstrup, S.; Coppens, M.-O.; Pharoah, J.G.; Pfeifer, P. Nature-inspired energy- and material-efficient design of a polymer electrolyte membrane fuel cell. Energy Fuels 2010, 24, 5097–5108. [Google Scholar] [CrossRef]
- Kizilova, N.; Sauermoser, M.; Kjelstrup, S.; Pollet, B.G. Fractal-like flow-fields with minimum entropy production for polymer electrolyte membrane fuel cells. Entropy 2020, 22, 176. [Google Scholar] [CrossRef]
- Rosa, D.; Goes, P.; Manzi, J. Minimum entropy based plug flow reactor analysis. In Proceedings of the 28th European Symposium on Computer Aided Process Engineering, Graz, Austria, 10–13 June 2018; Elsevier: Amsterdam, The Netherlands, 2018; pp. 507–512. [Google Scholar]
- Magnanelli, E.; Johannessen, E.; Kjelstrup, S. Entropy production minimization as design principle for membrane systems: Comparing equipartition results to numerical optima. Ind. Eng. Chem. Res. 2017, 56, 4856–4866. [Google Scholar] [CrossRef]
- Hossain, S.; Husain, A.; Kim, K.-Y. Shape optimization of a micromixer with staggered-herringbone grooves patterned on opposite walls. Chem. Eng. J. 2010, 162, 730–737. [Google Scholar] [CrossRef]
- Afzal, A.; Kim, K.-Y. Optimization of pulsatile flow and geometry of a convergent–divergent micromixer. Chem. Eng. J. 2015, 281, 134–143. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Huang, Z.; Yang, F.; Wu, Z.; Zheng, L.; Wu, L.; Zhang, Z. Design optimization and sensitivity analysis of the radiation mini-channel metal hydride reactor. Energy 2019, 173, 443–456. [Google Scholar] [CrossRef]
- Magnanelli, E.; Solberg, S.B.B.; Kjelstrup, S. Nature-inspired geometrical design of a chemical reactor. Chem. Eng. Res. Des. 2019, 152, 20–29. [Google Scholar] [CrossRef]
- Alarcón, A.; Busqué, R.; Andreu, T.; Guilera, J. Design of a multi-tubular catalytic reactor assisted by CFD based on free-convection heat-management for decentralized synthetic methane production. Catalysts 2022, 12, 1053. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Garimella, S.; Li, D.; Colin, S.; King, M.R. Heat Transfer and Fluid Flow in Minichannels and Microchannels; Elsevier: Singapore, 2006. [Google Scholar]
- White, F.M. Fluid Mechanics, 7th ed.; McGraw-Hill: New York, NY, USA, 2011; pp. 384–385. [Google Scholar]
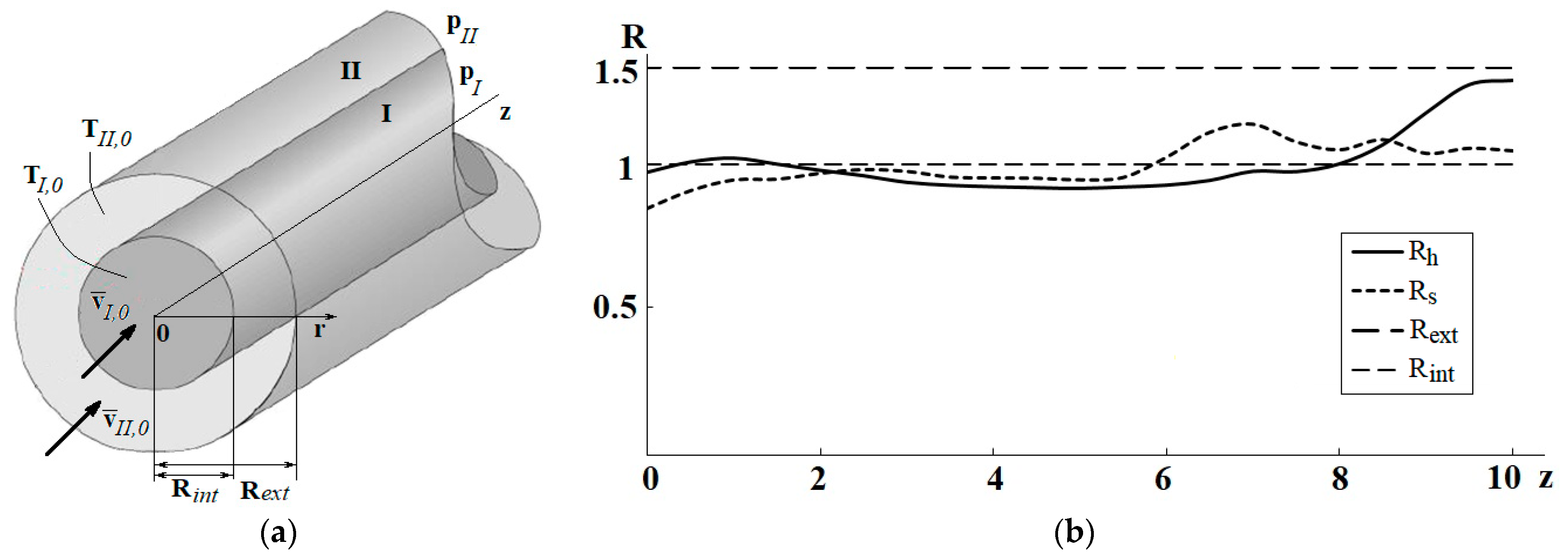
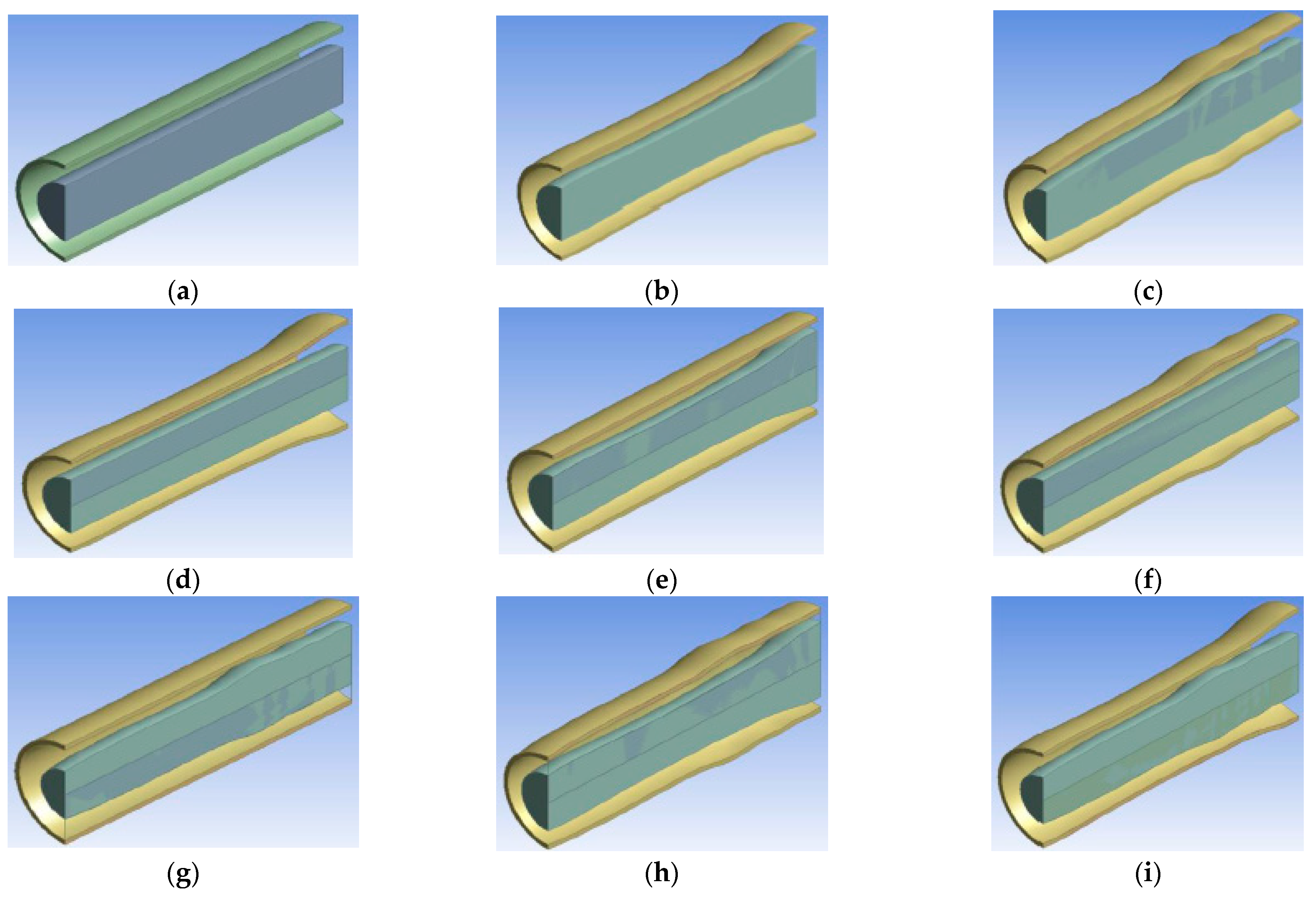
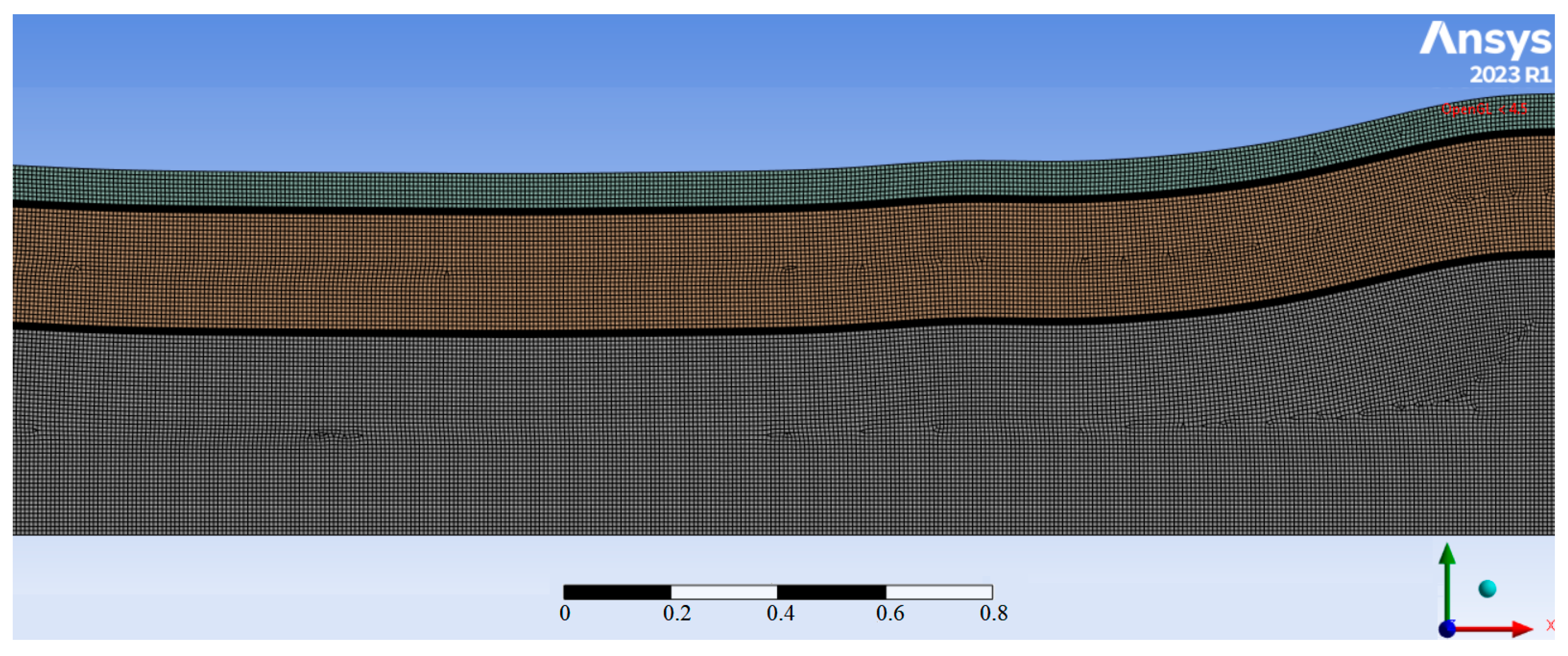
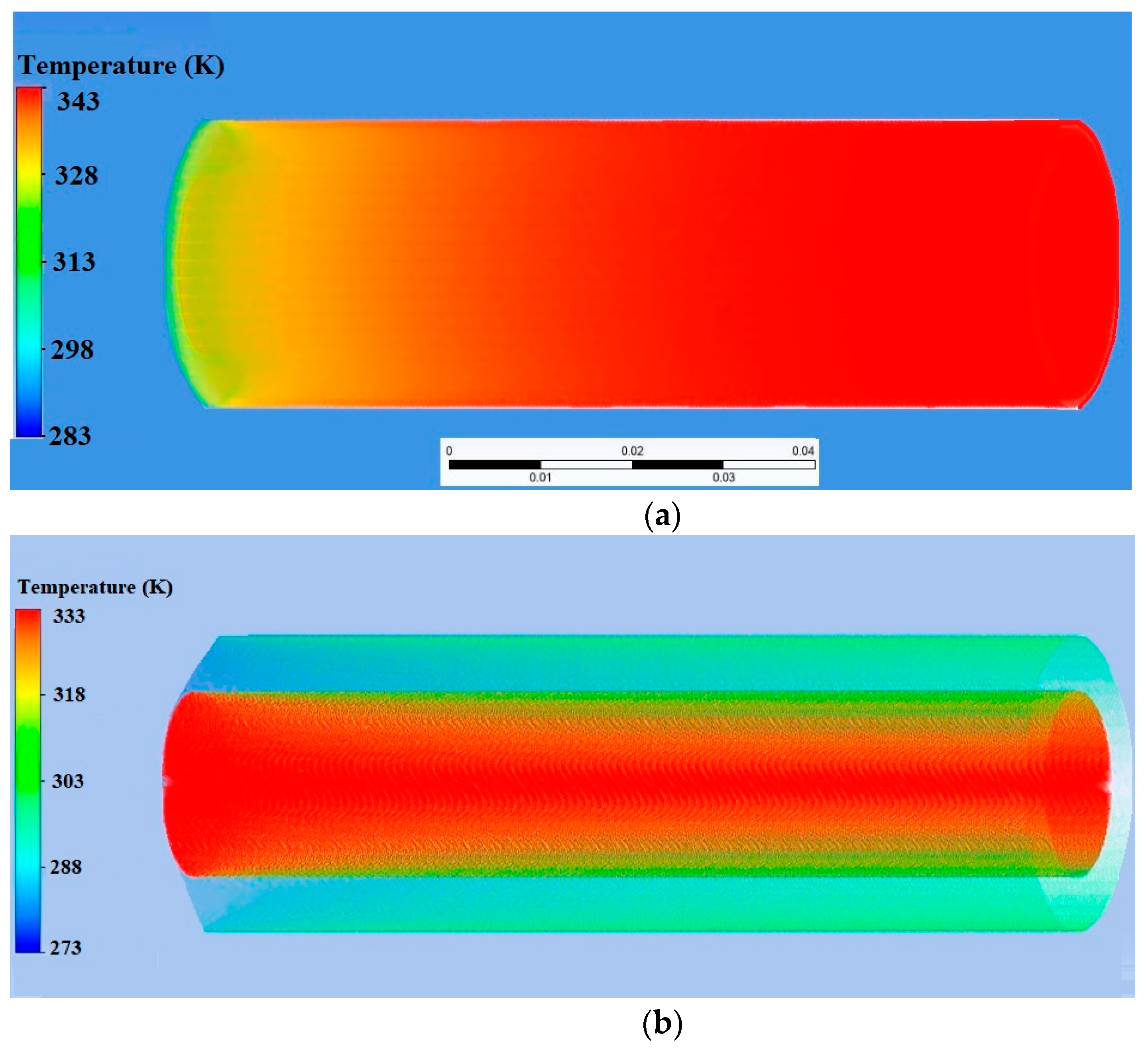

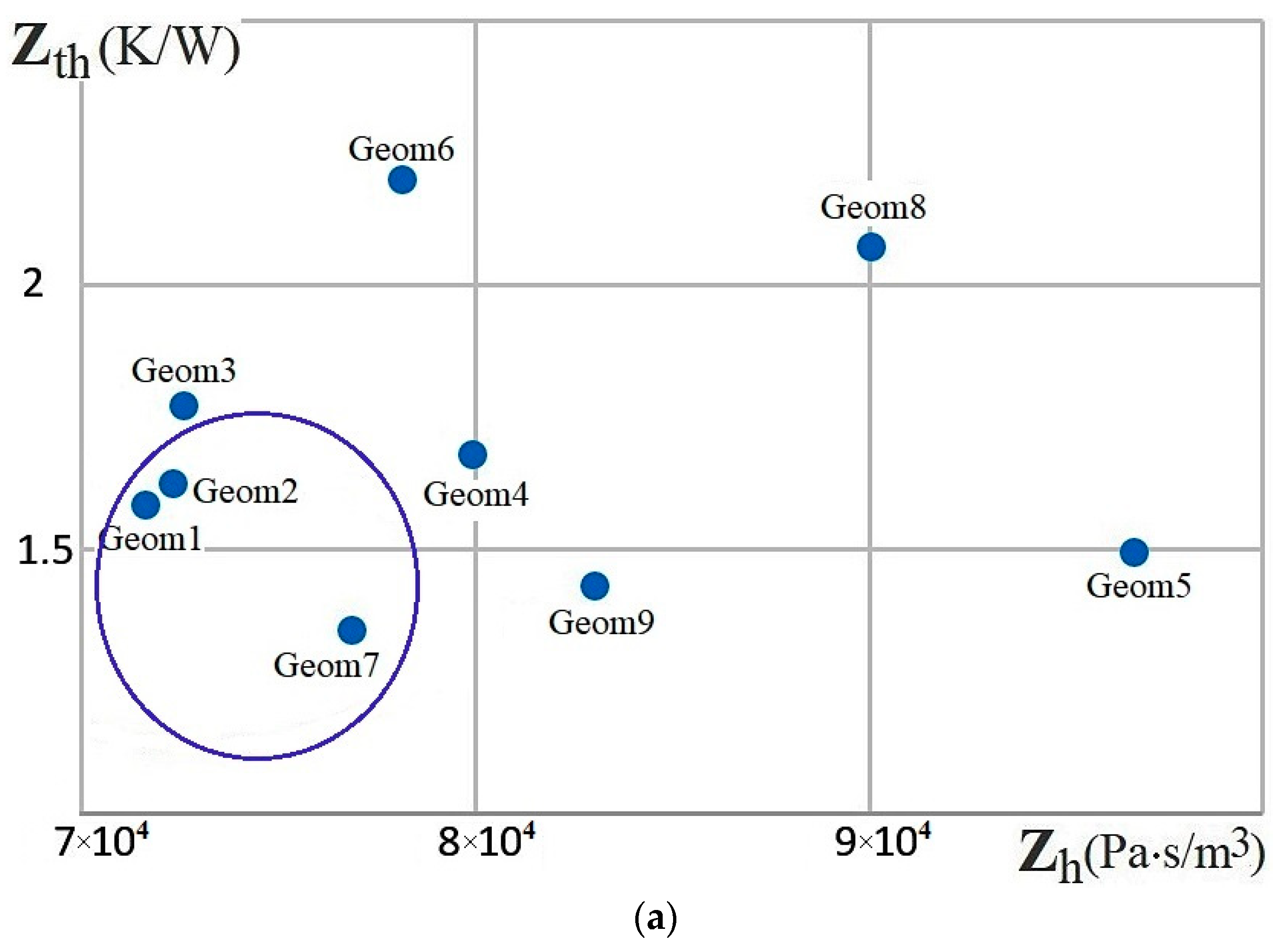
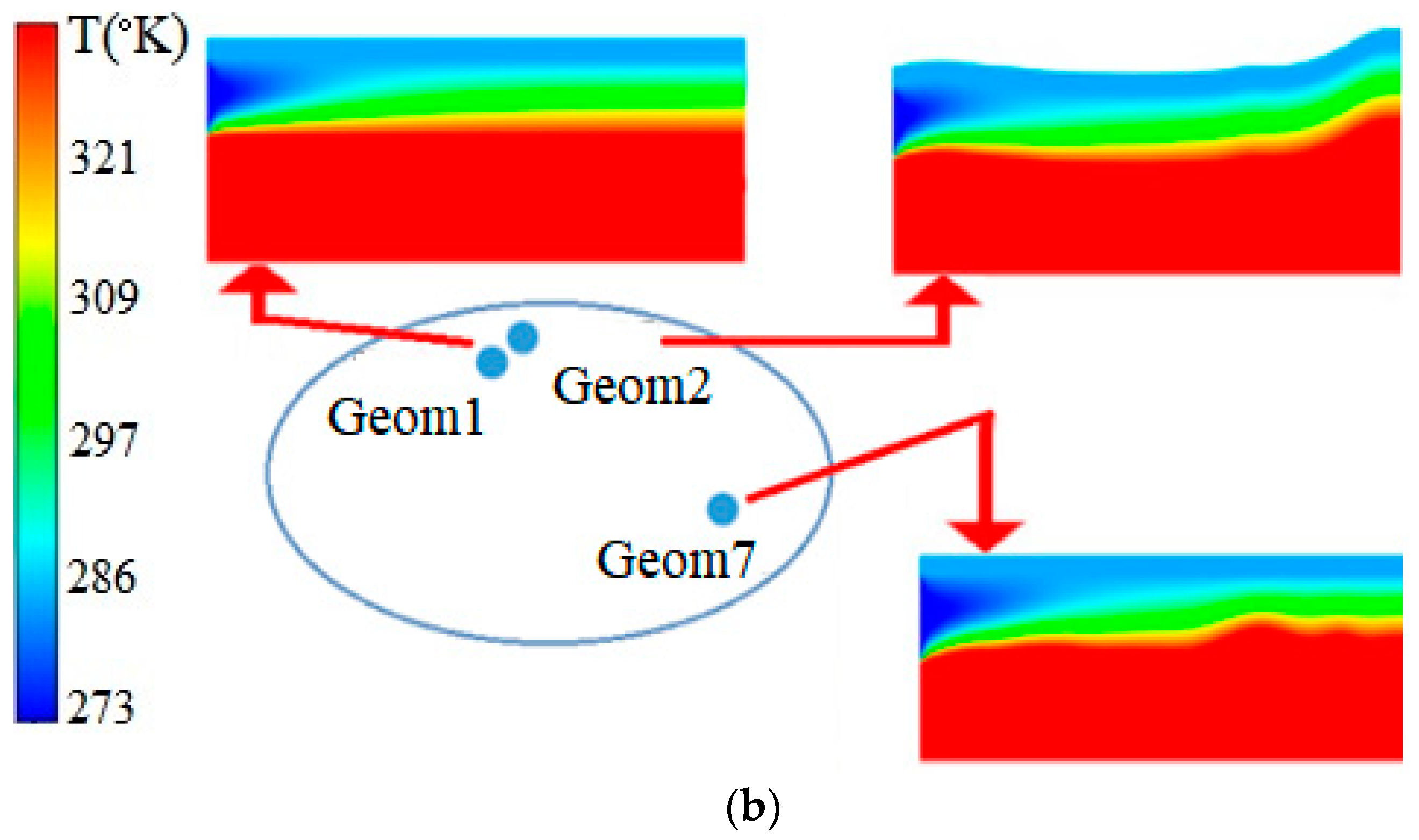
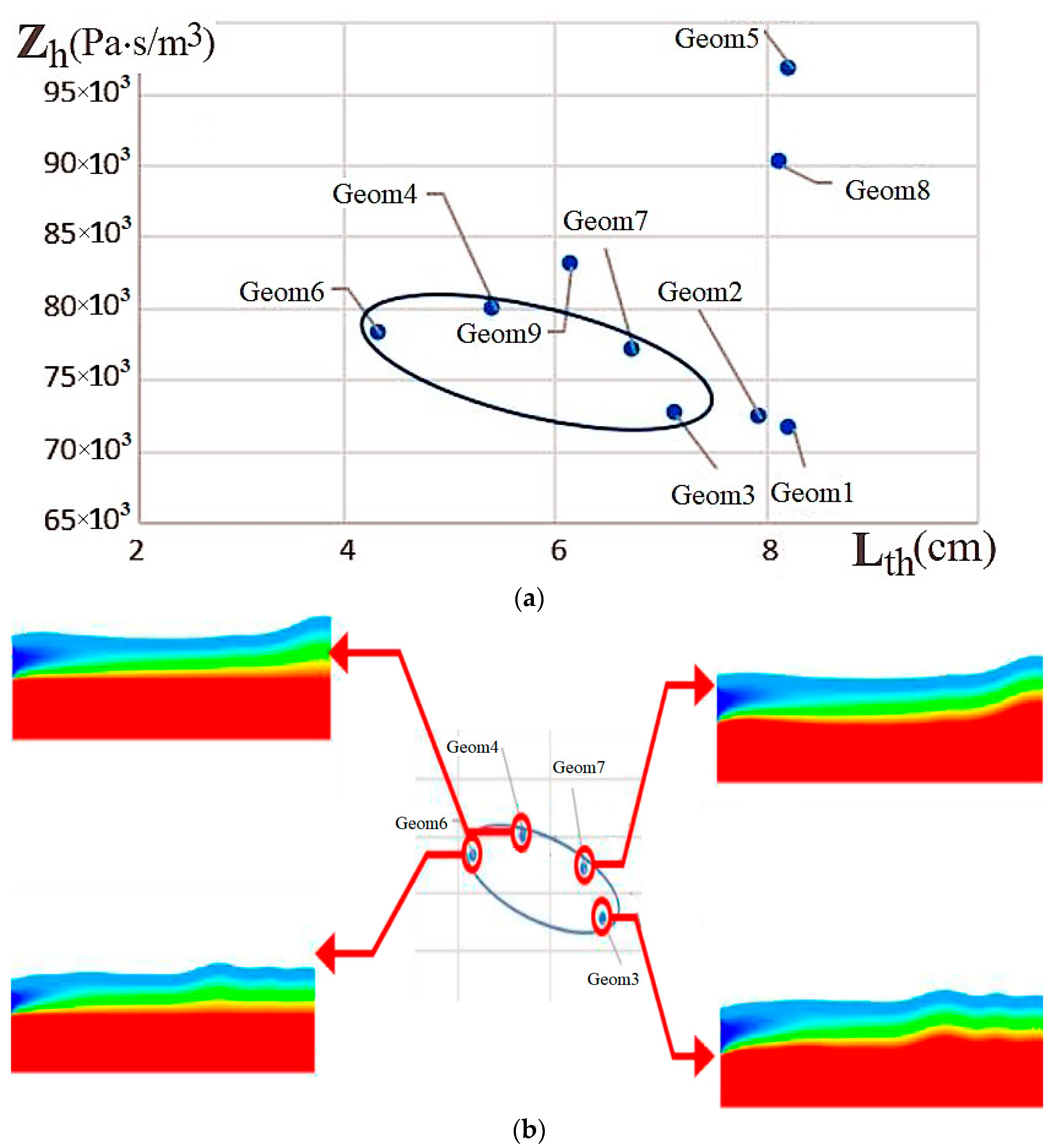
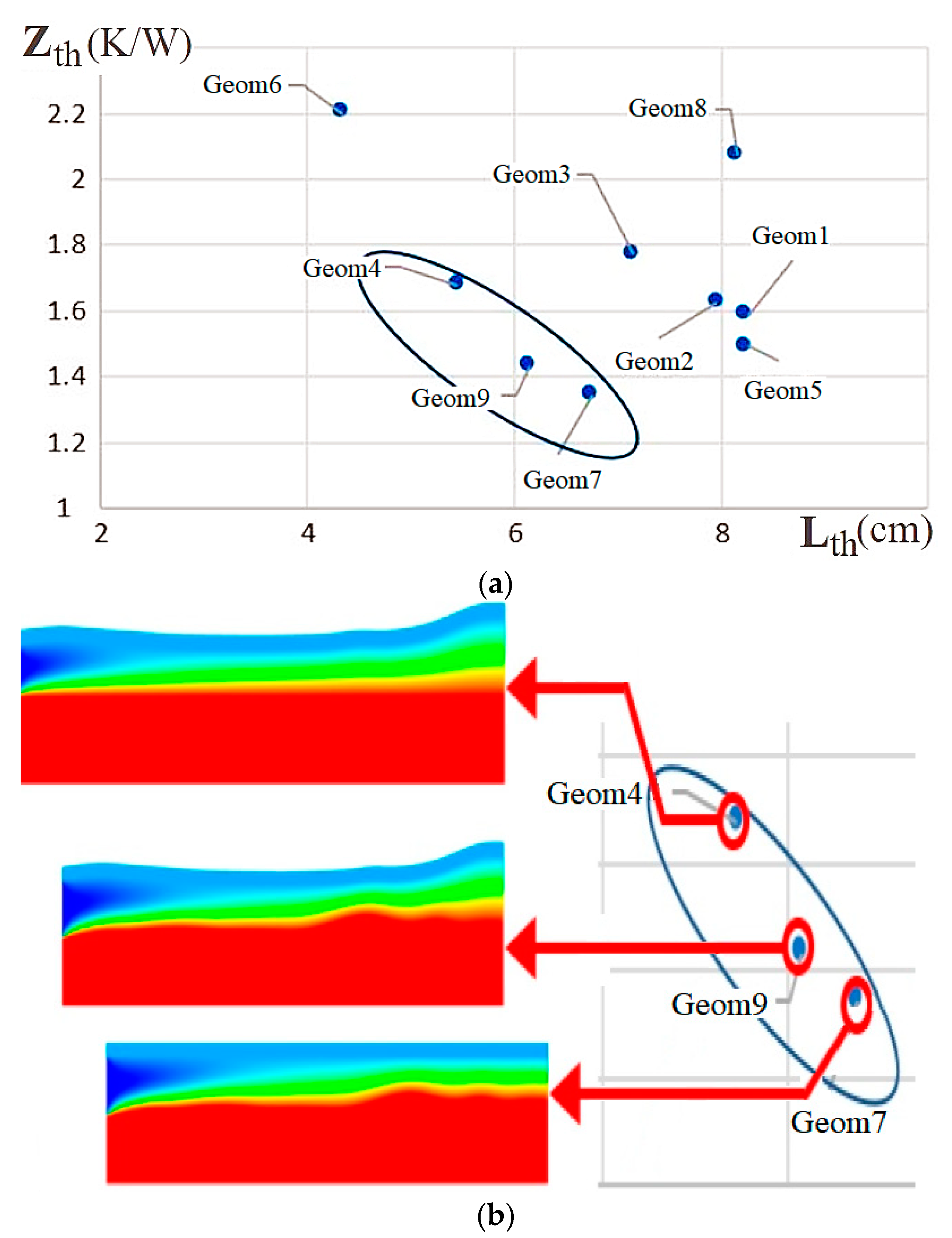
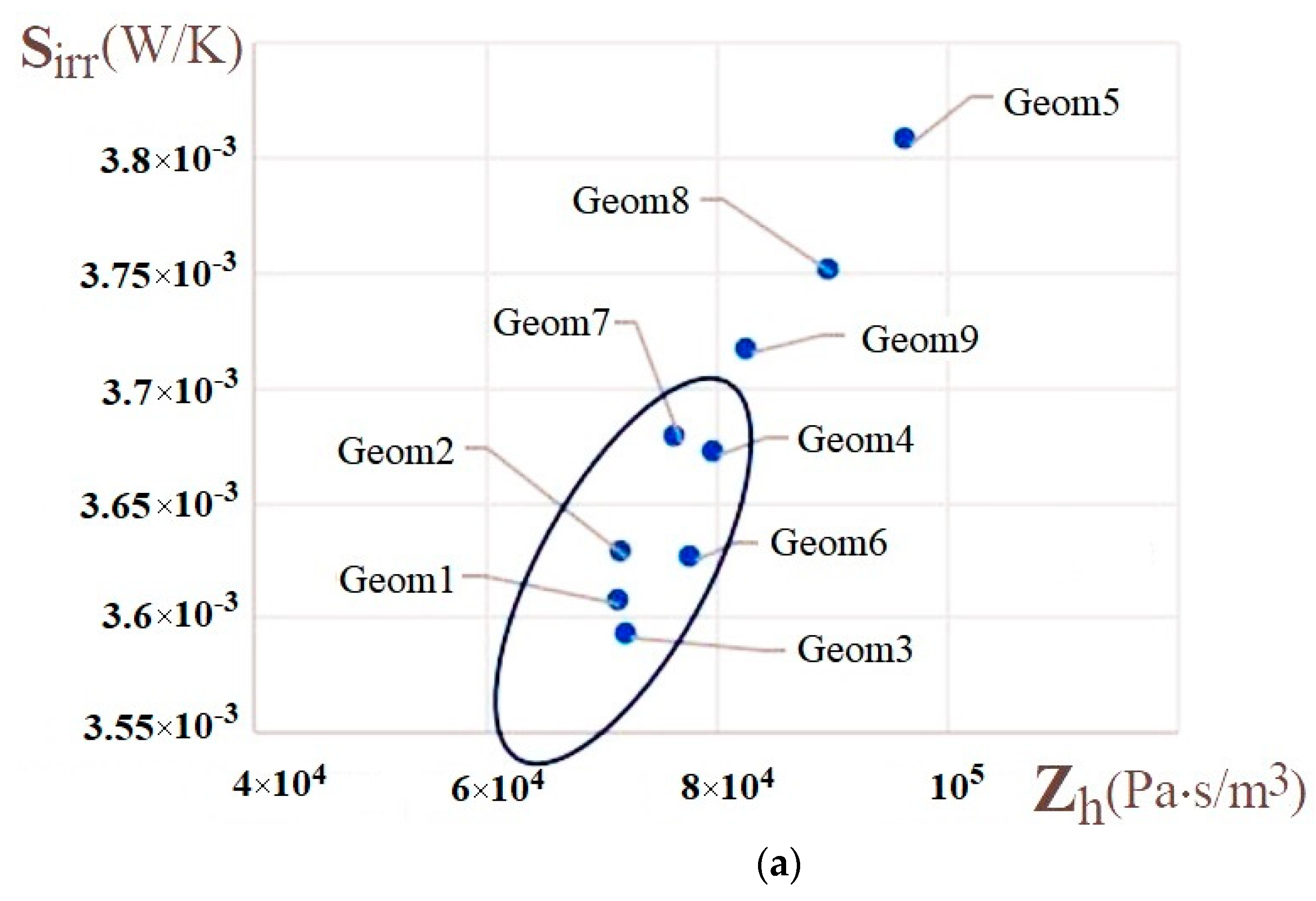
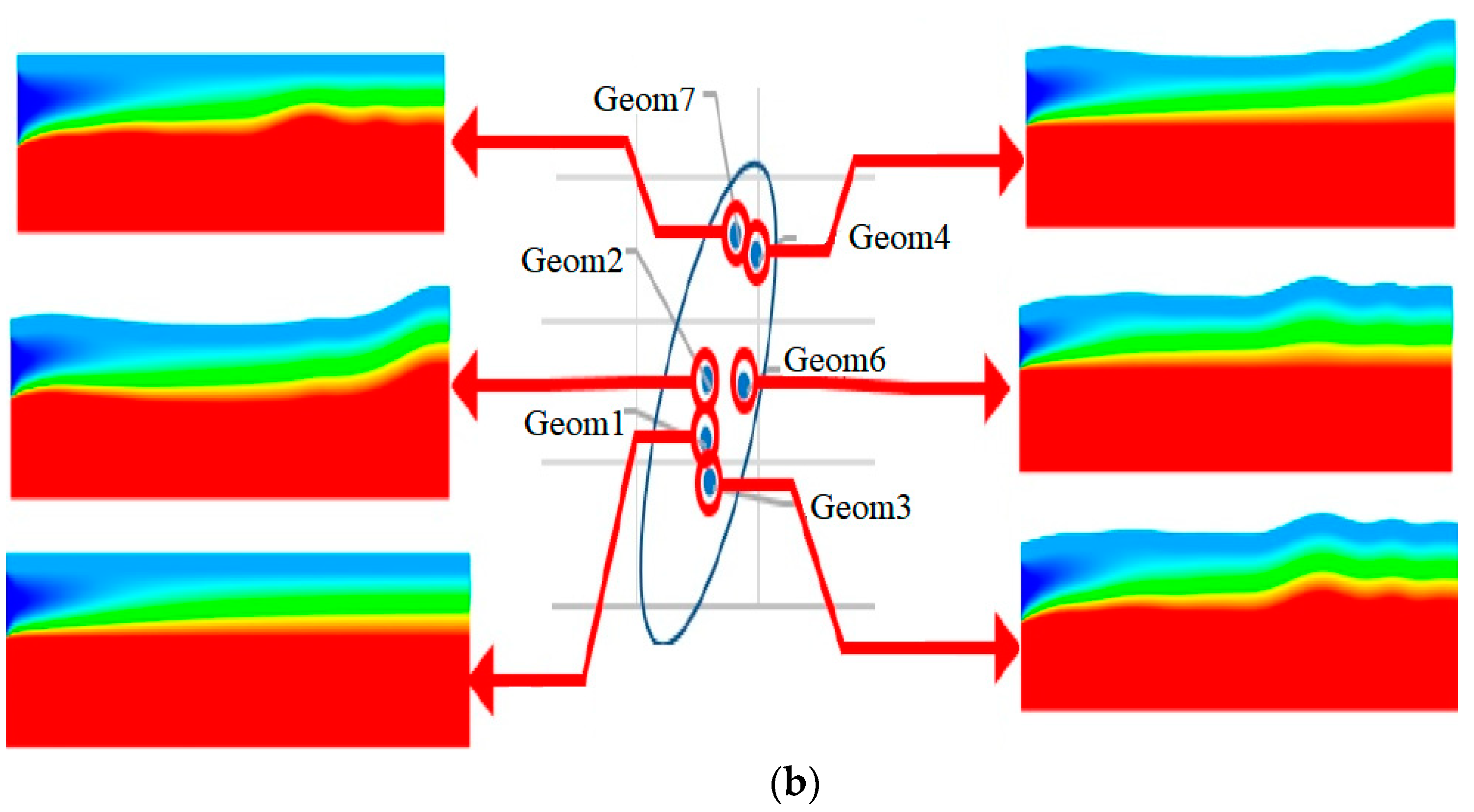
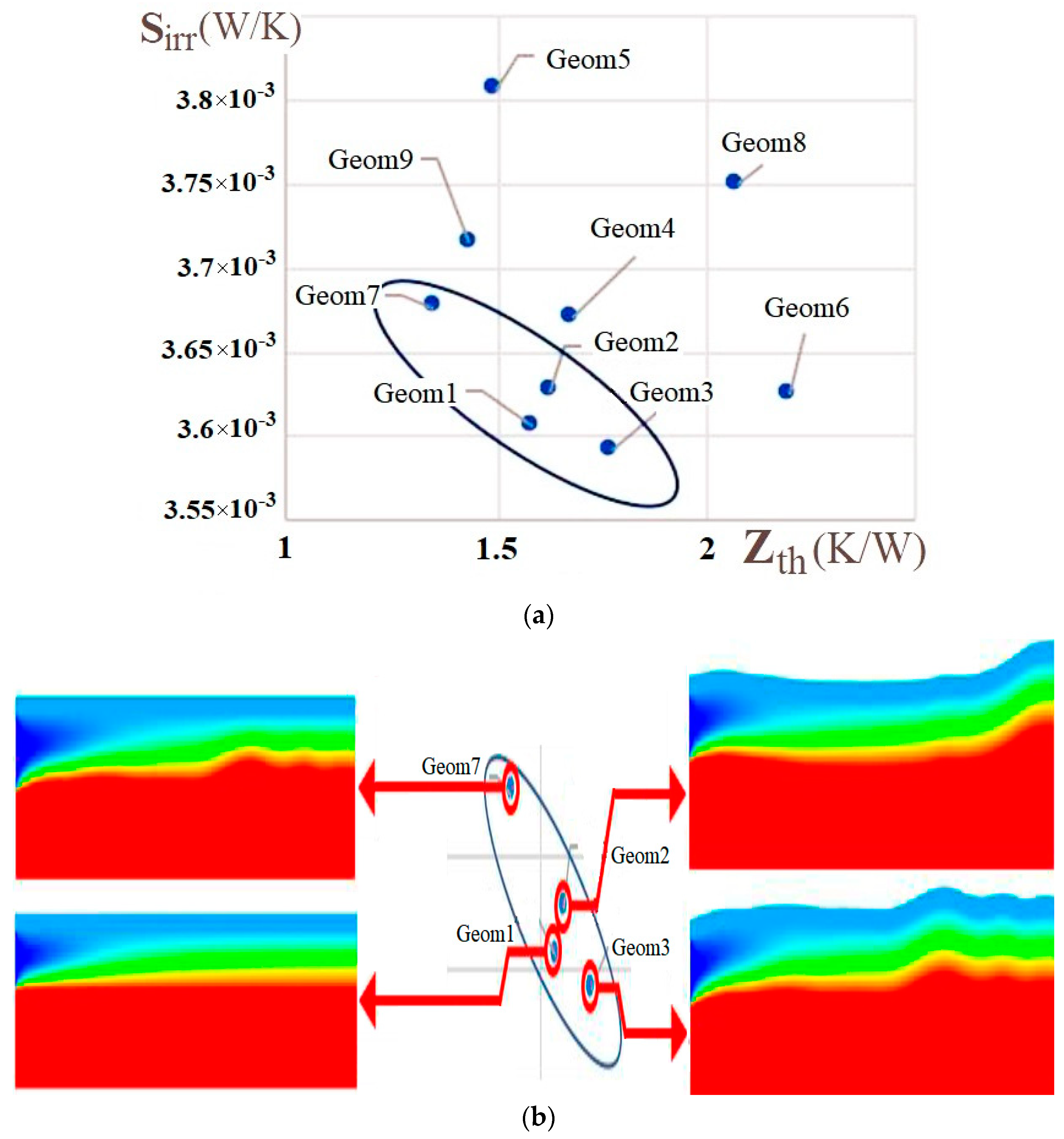

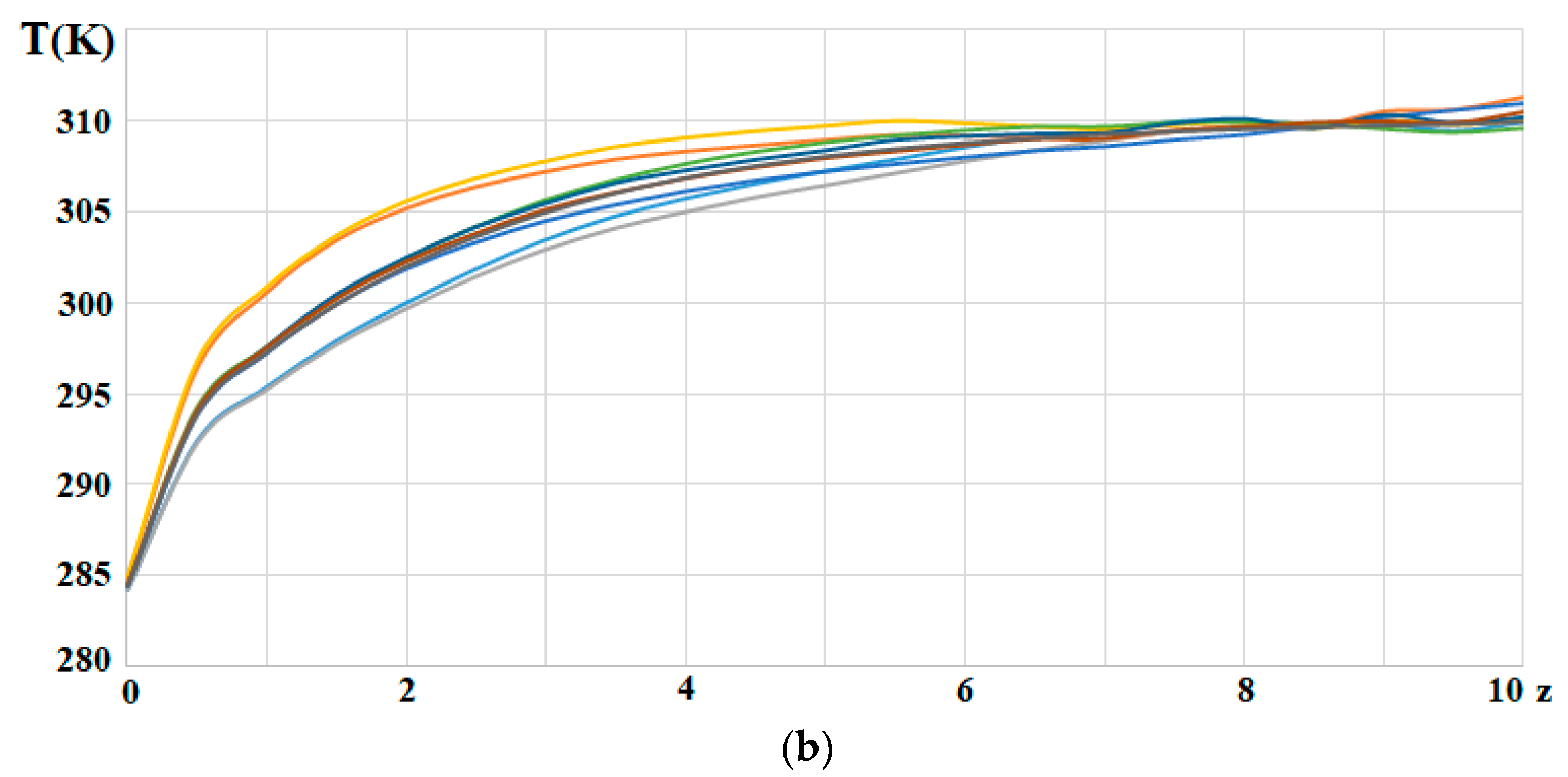
| Name | Rint | Rext | Image |
|---|---|---|---|
| Geom1 (base case reactor) | 1 | 1.5 | Figure 2a |
| Geom2 | Rh(z) scaled to 1 | Rh(z) scaled to 1.5 | Figure 2b |
| Geom3 | Rs(z) scaled to 1 | Rs(z) scaled to 1.5 | Figure 2c |
| Geom4 | 1 | Rh(z) scaled to 1.5 | Figure 2d |
| Geom5 | Rh(z) scaled to 1 | 1.5 | Figure 2e |
| Geom6 | 1 | Rs(z) scaled to 1.5 | Figure 2f |
| Geom7 | Rs(z) scaled to 1 | 1.5 | Figure 2g |
| Geom8 | Rh(z) scaled to 1 | Rs(z) scaled to 1.5 | Figure 2h |
| Geom9 | Rs(z) scaled to 1 | Rh(z) scaled to 1.5 | Figure 2i |
| - | 1, 2, 7 | 3, 4, 6, 7 | 1, 3 | |
| - | 4, 7, 9 | 1, 2, 3, 7 | ||
| - | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kizilova, N.; Shankar, A.; Kjelstrup, S. A Minimum Entropy Production Approach to Optimization of Tubular Chemical Reactors with Nature-Inspired Design. Energies 2024, 17, 432. https://doi.org/10.3390/en17020432
Kizilova N, Shankar A, Kjelstrup S. A Minimum Entropy Production Approach to Optimization of Tubular Chemical Reactors with Nature-Inspired Design. Energies. 2024; 17(2):432. https://doi.org/10.3390/en17020432
Chicago/Turabian StyleKizilova, Natalya, Akash Shankar, and Signe Kjelstrup. 2024. "A Minimum Entropy Production Approach to Optimization of Tubular Chemical Reactors with Nature-Inspired Design" Energies 17, no. 2: 432. https://doi.org/10.3390/en17020432





