Studying the Improvement of Solar Collector Mechanism with Phase Change Materials
Abstract
:1. Introduction
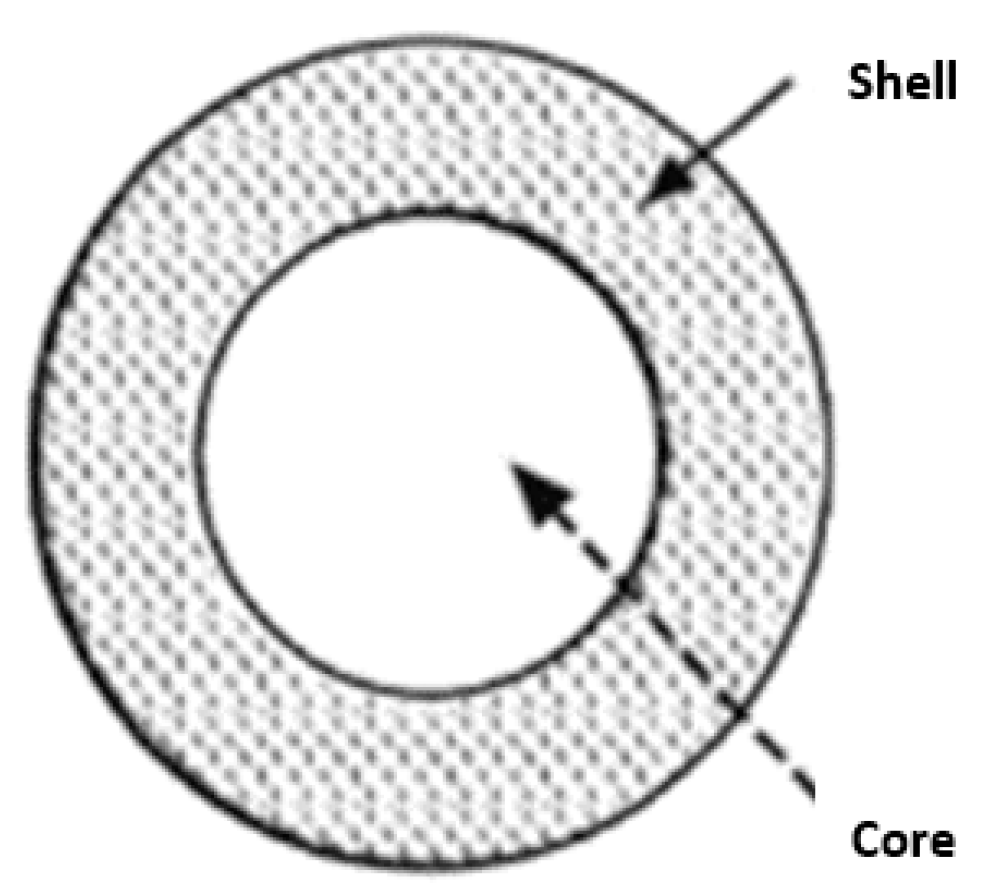
2. Nano and Microencapsulation
2.1. Description of the Solar Collector
2.2. NEPCM Material Properties
2.3. Description of the Solar Collector
2.3.1. Governing Equations for the Water Chamber
2.3.2. Governing Equations for PCM Housing
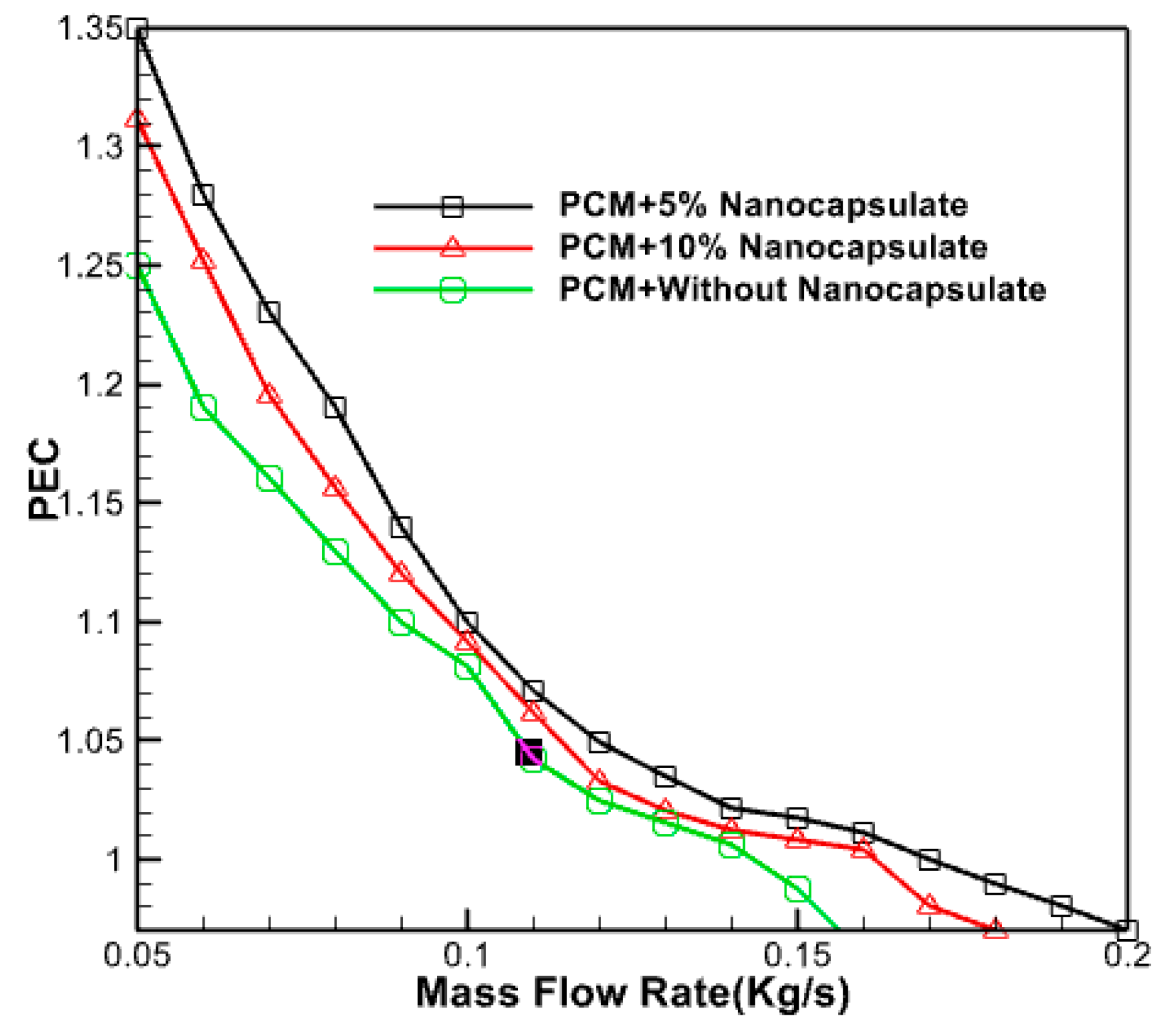
2.3.3. Performance Evaluation Criteria
2.3.4. Geometric Modeling and Boundary Conditions
3. Results
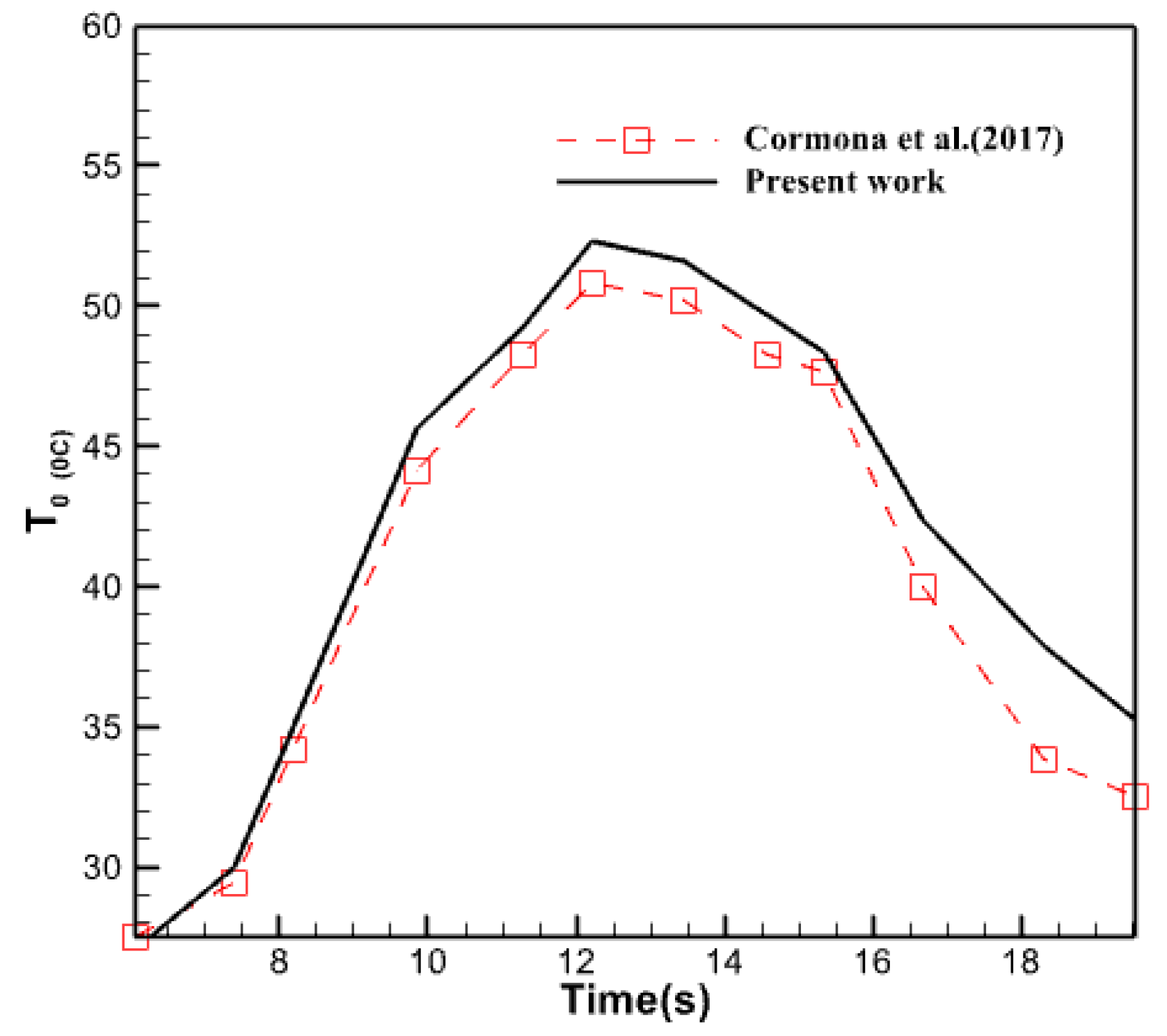
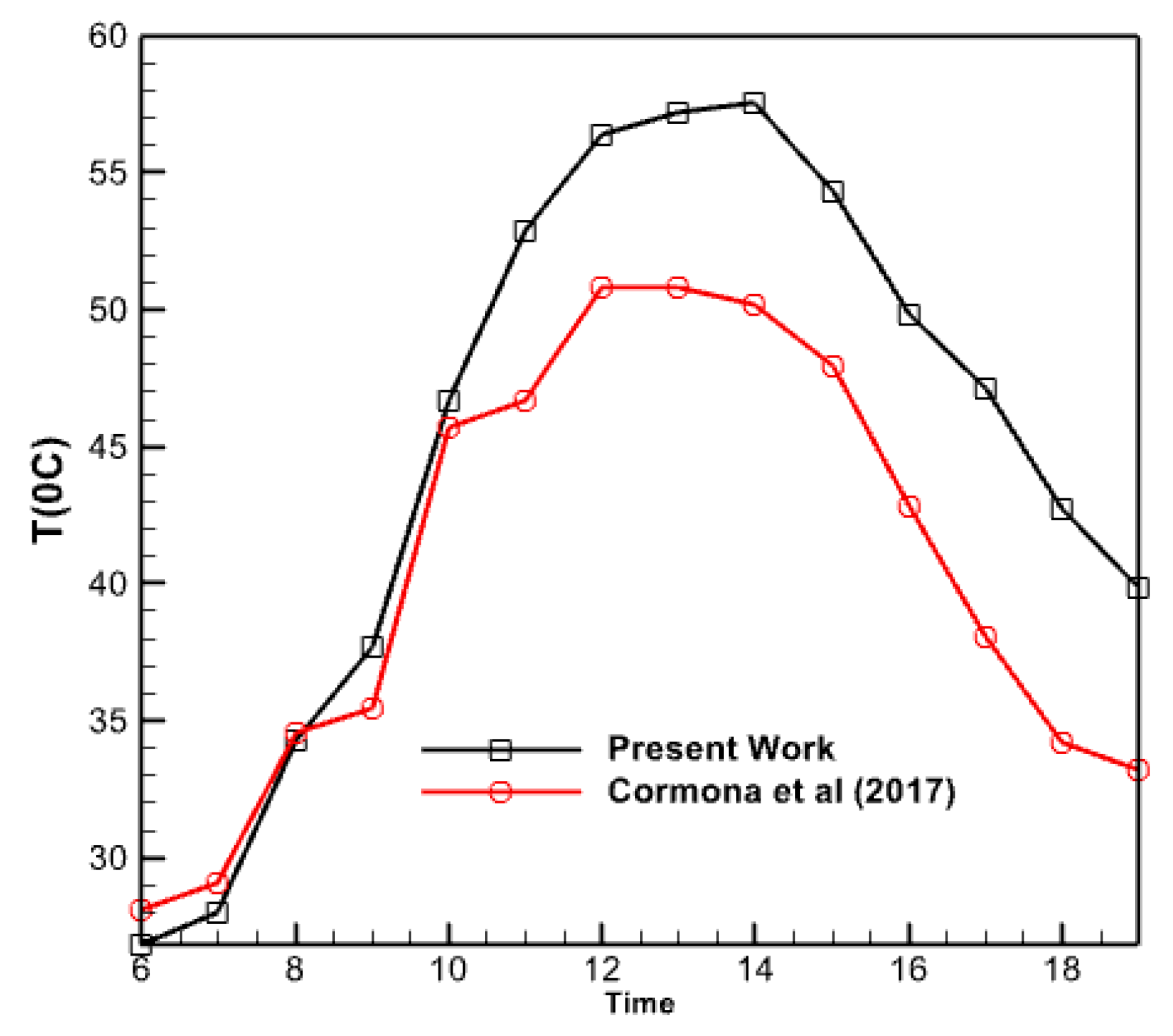
3.1. Verification
3.2. Analysis of the Fluid Inside The Pipes
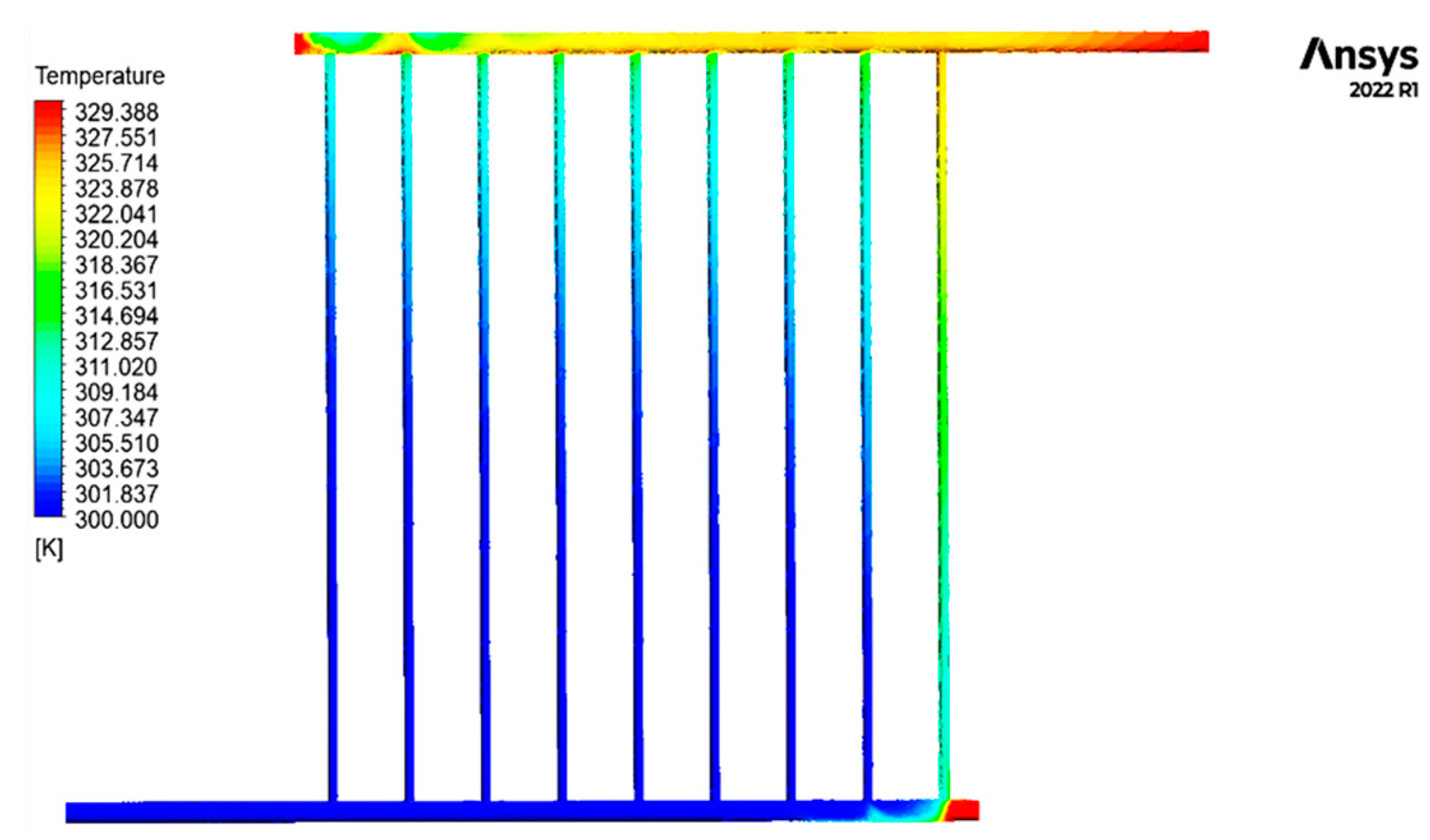
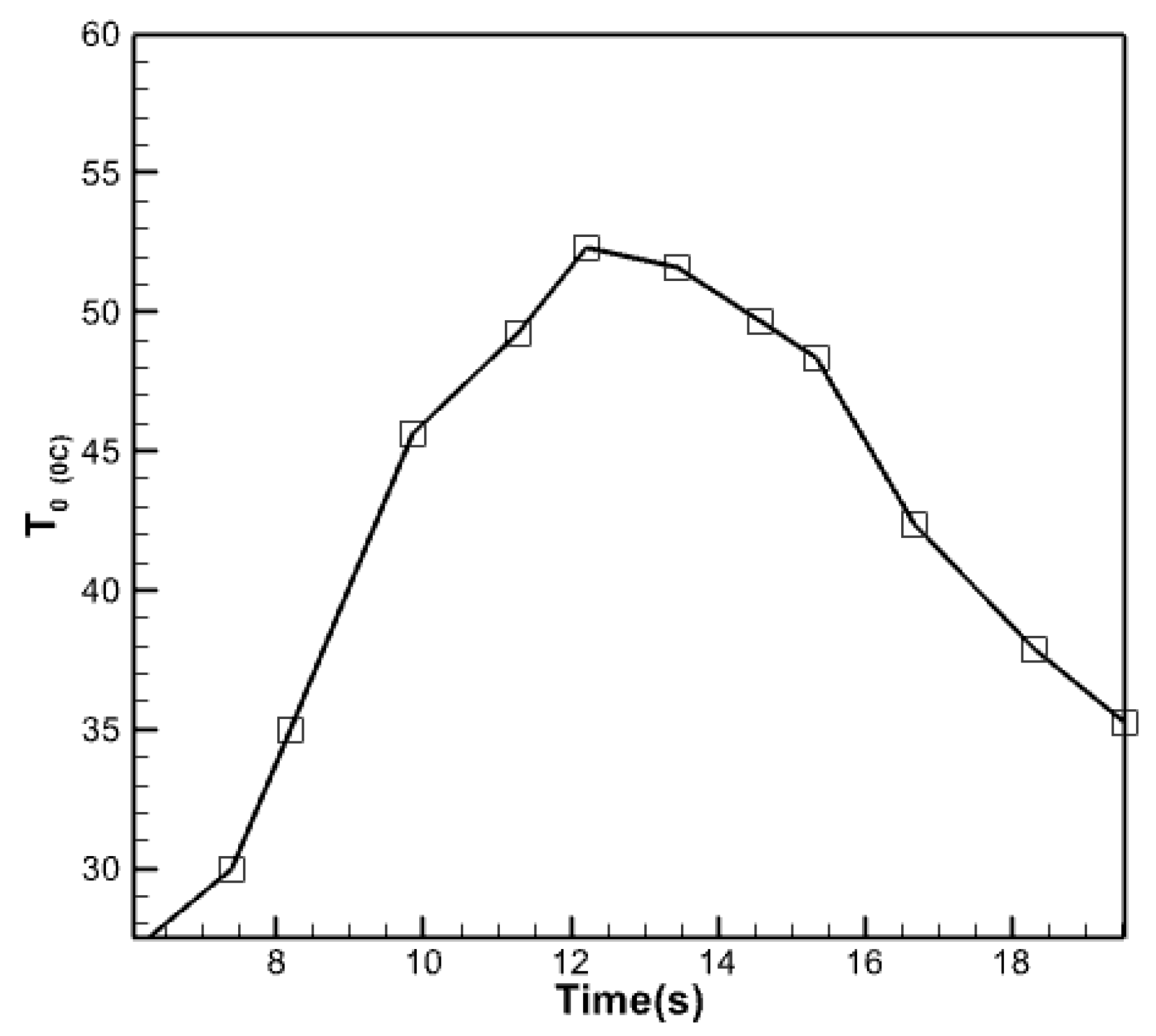
3.2.1. Analysis of Fluid Temperature Inside the Collector
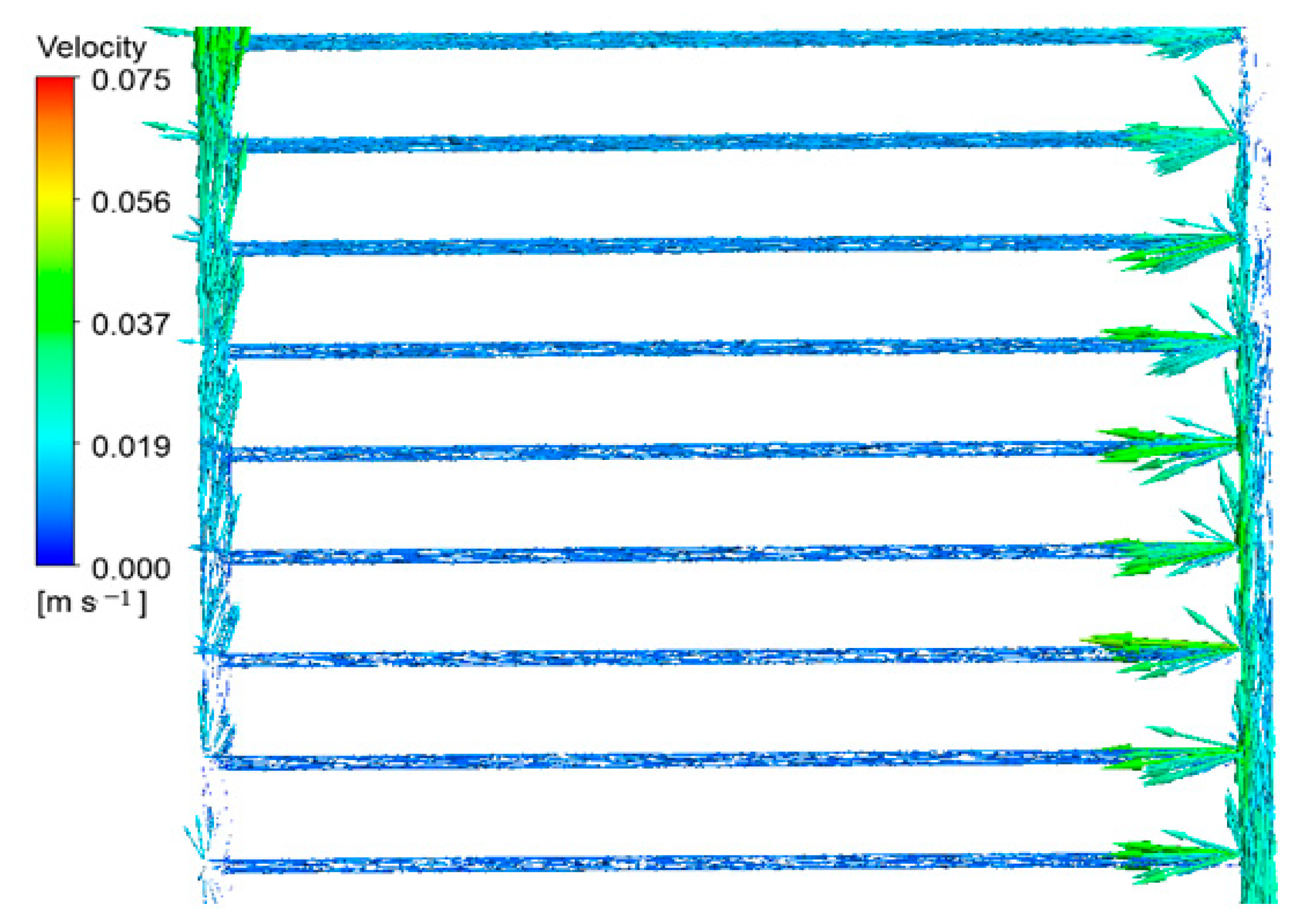
3.2.2. Velocity Distribution Inside the Collector Tubes
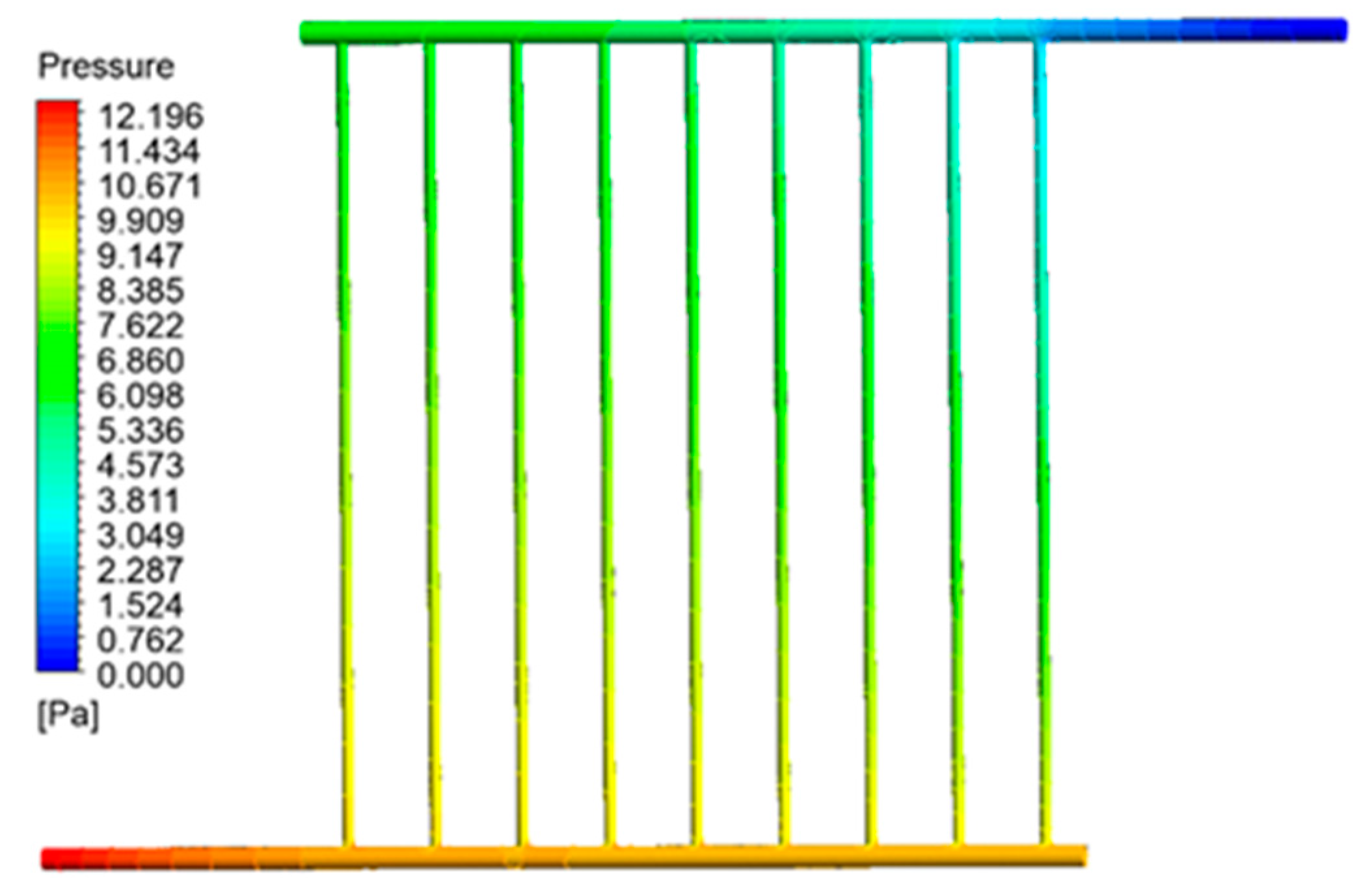
3.2.3. Pressure Distribution in Collector Pipes
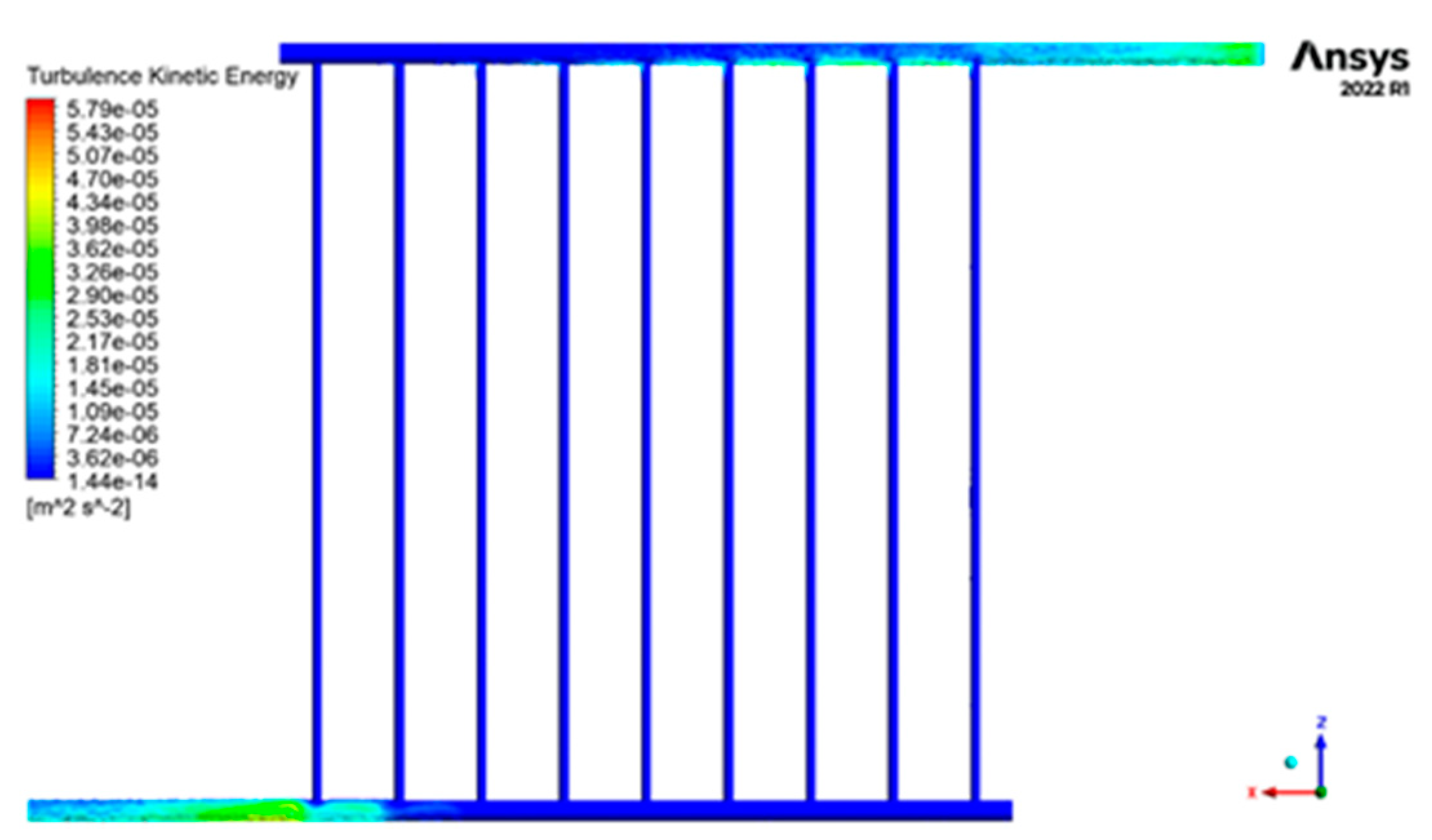
3.2.4. Kinetic Energy of Turbulence
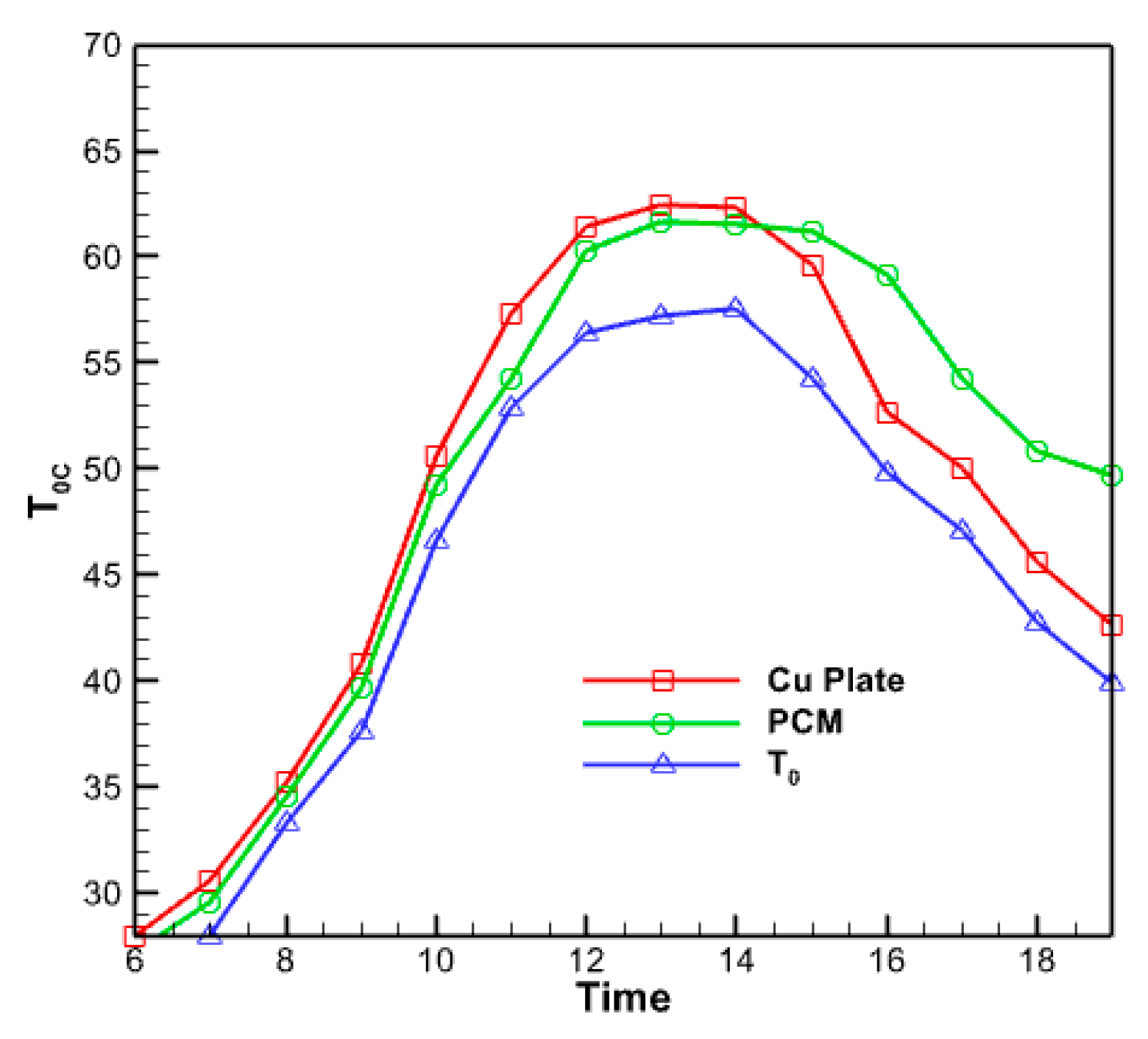
3.2.5. Analysis of the Temperature of the Absorbent Copper Plate
3.2.6. Analysis of the Compartment Containing the Nano Encapsulated Phase Change Agent
3.2.7. Comparison of Obtained Results with Experimental Results
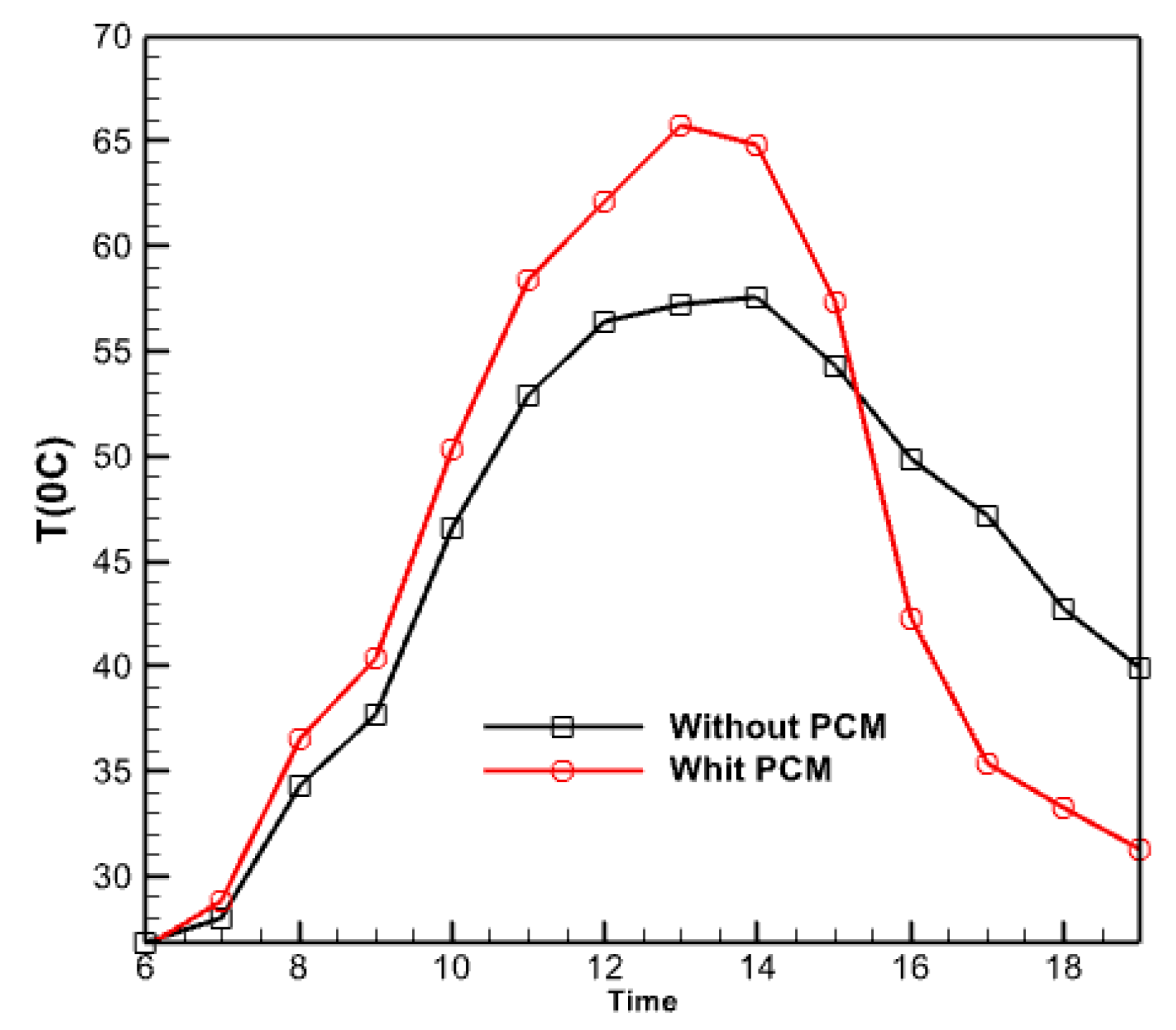
3.2.8. The Effect of Using a Phase Changer
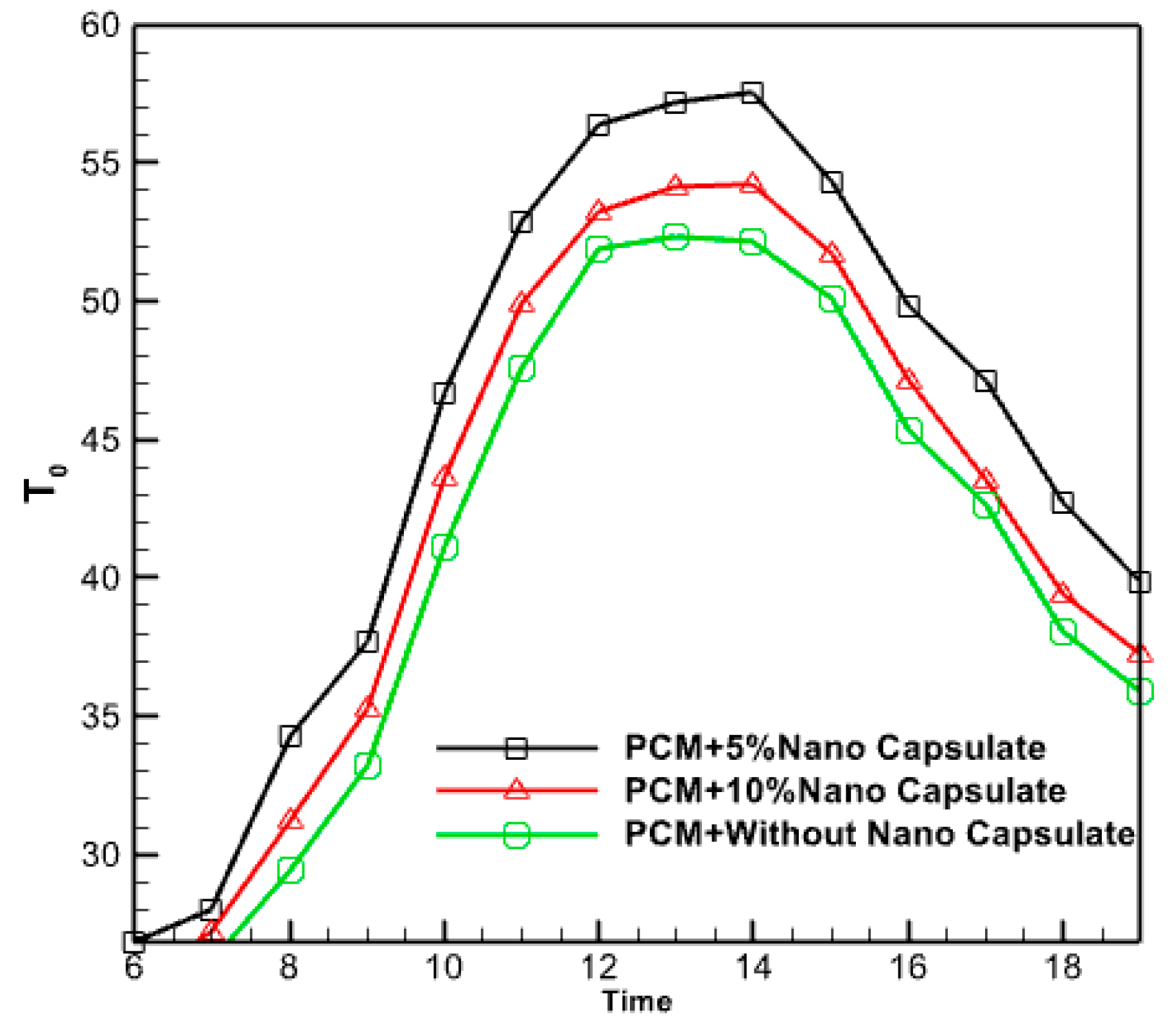
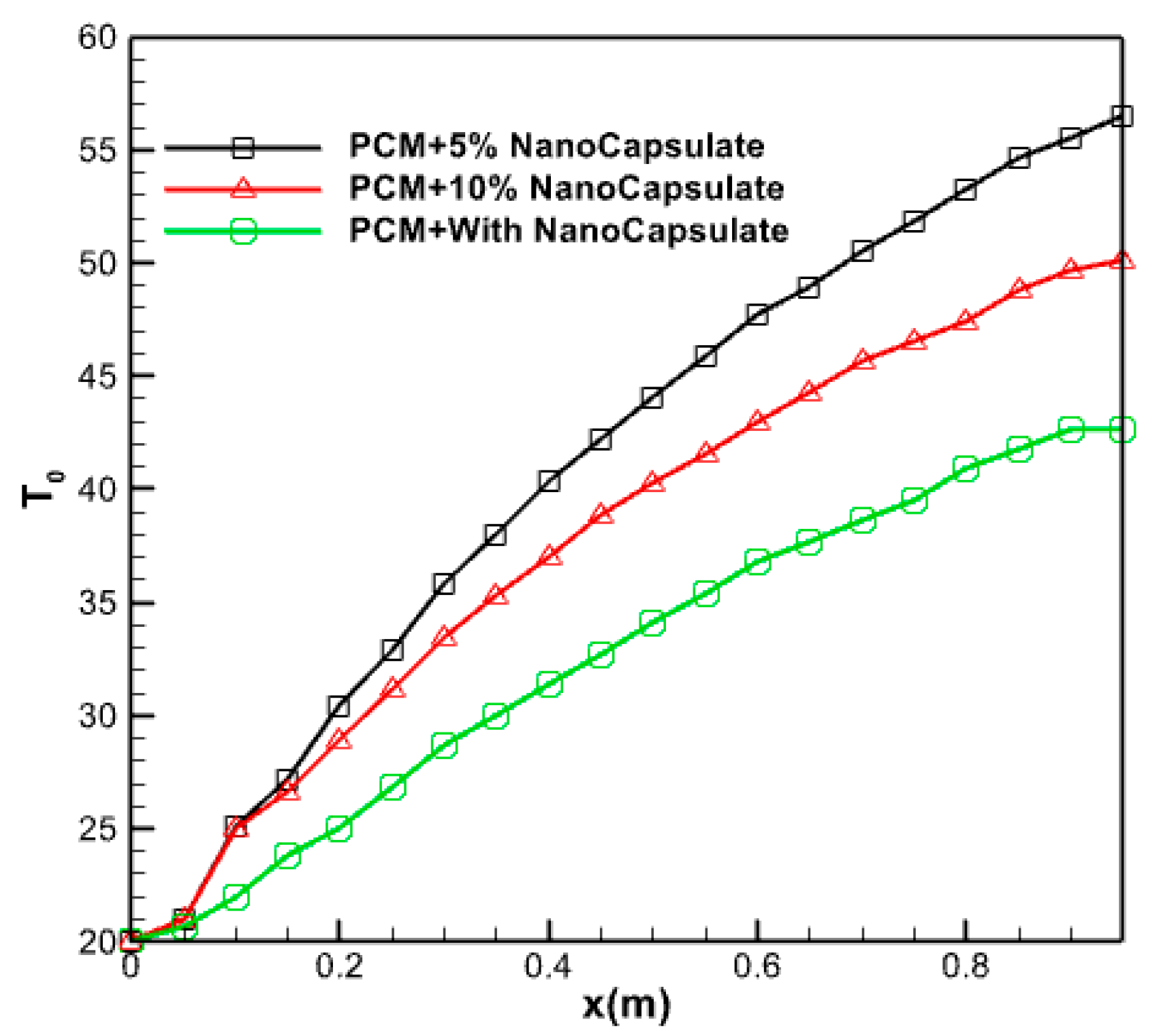
3.2.9. Effect of Nano Capsule Volume Fraction
3.2.10. Effect of Nano Capsule Volume Fraction
3.2.11. In Thermal Efficiency of the Collector with the Addition of Nano Capsule Phase Change Materials
4. Conclusions
- The fluid becomes stagnant and reaches its highest temperature in this section of the collecting tubes at the end of the pipe due to the obstruction and absence of movement.
- The utilization of nano encapsulated phase change material, which releases and transfers stored energy to the fluid inside the collector tubes, reduces the outlet fluid’s temperature with a smaller gradient. This phenomenon can be attributed to the decreased slope observed in the terminal sections of the temperature change diagram of the fluid exiting the collector.
- The peak fluid velocity is observed at the collection’s entry and exit points. Nevertheless, the velocity of the fluid is at its minimum at the termination of the intake pipe and the commencement of the outflow pipe.
- The density and magnitude of the velocity vectors progressively increase in the outflow pipe as the fluid particles reach the end of the conduit.
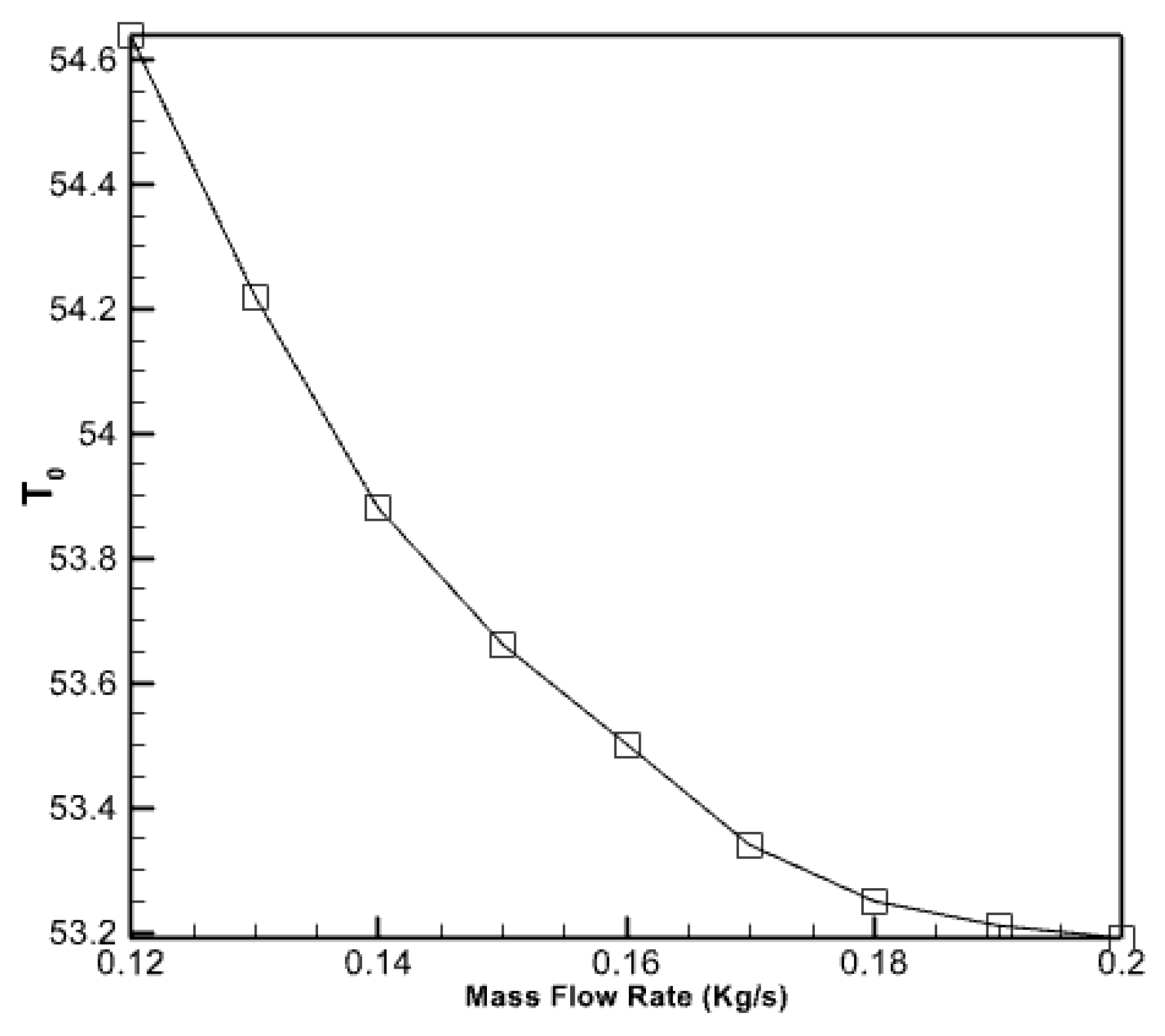
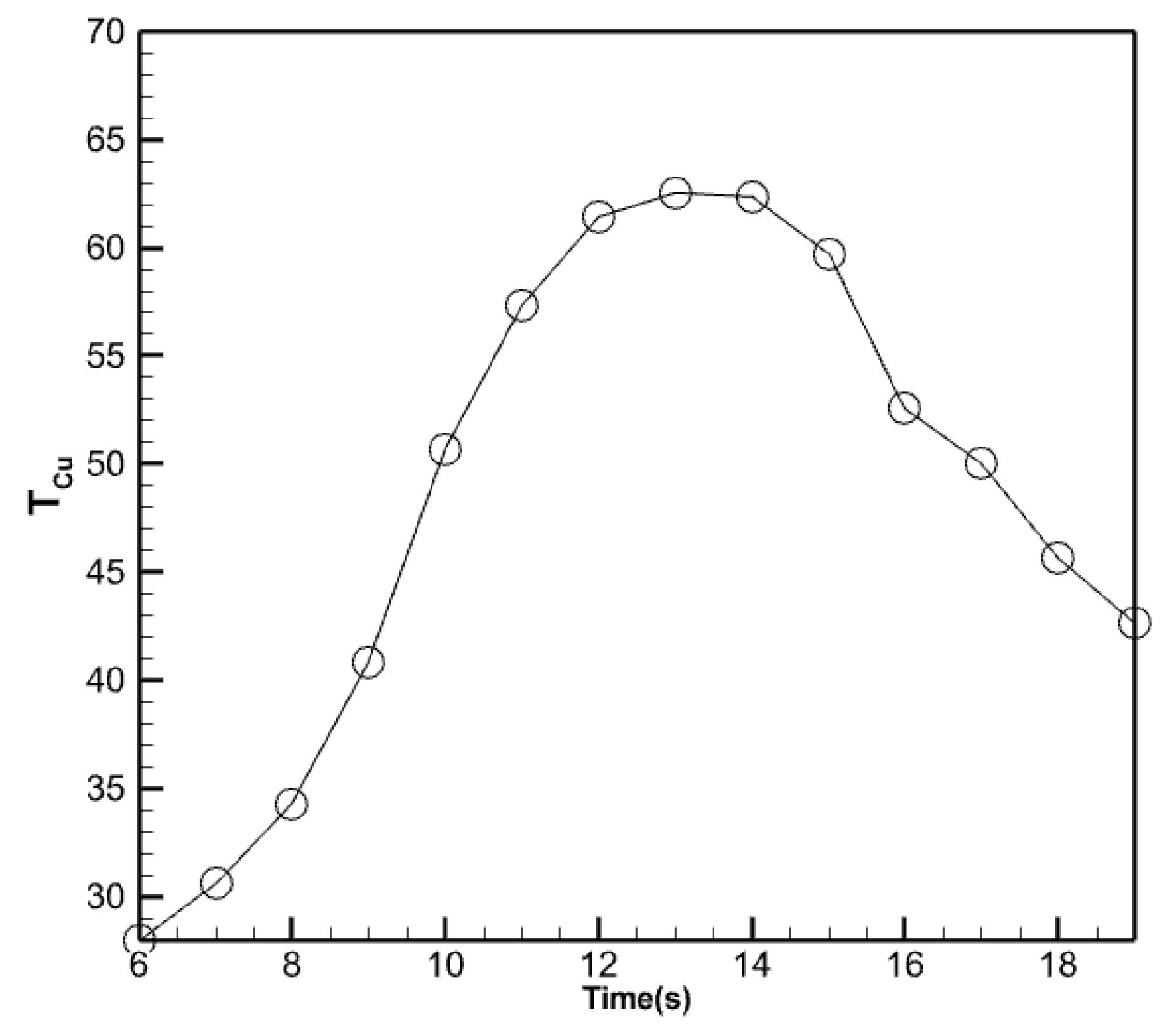
- The outflow fluid reaches its peak temperature between 12:00 and 14:00 and remains relatively stable.
- At 6 PM, there is a 25% disparity between the graphs computed in this study and the graph given by Carmona et al. in 2017. There is a 10% disparity between the two plots during the daytime. Their values might differ by a maximum of 15%.
- The study determined that the ideal proportion of nanoparticles was 5%. Augmenting the volume fraction of nanoparticles within the PCM does not invariably enhance performance and elevate the temperature of the fluid emerging from it.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Jin, X.; Zhang, Y.; Du, K. Recent development on heat transfer and various applications of phase-change materials. J. Clean. Prod. 2020, 287, 124432. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Babayan, M.; Chamkha, A. Investigation of using multi-layer PCMs in the tubular heat exchanger with periodic heat transfer boundary condition. Int. J. Heat Mass Transf. 2020, 147, 118970. [Google Scholar] [CrossRef]
- Jahangiri, A.; Ahmadi, O. Numerical investigation of enhancement in melting process of PCM by using internal fins. J. Therm. Anal. Calorim. 2019, 137, 2073–2080. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Solidification of NEPCM under the effect of magnetic field in a porous thermal energy storage enclosure using CuO nanoparticles. J. Mol. Liq. 2018, 263, 303–315. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Doostanidezfuli, A.; Zargartalebi, H.; Chamkha, A.J. MHD phase change heat transfer in an inclined enclosure: Effect of a magnetic field and cavity inclination. Numer. Heat Transf. Part A Appl. 2017, 71, 91–109. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Doostani, A.; Chamkha, A.J.; Ismael, M.A. Melting of nanoparticles-enhanced phase change materials in an enclosure: Effect of hybrid nanoparticles. Int. J. Mech. Sci. 2017, 134, 85–97. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.X.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.; Zhu, H.J. Preparation and evaluation of a novel solar storage material: Microencapsulated paraffin. Int. J. Sol. Energy 2000, 20, 227–238. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Ruprecht, J.; Liew, W.Y.H.; Jiang, Z.-T. A Binary Salt Mixture LiCl–LiOH for Thermal Energy Storage. Materials 2023, 16, 1434. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for building cooling applications: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Alisetti, E.L.; Roy, S.K. Forced convection heat transfer to phase change material slurries in circular ducts. J. Thermophys. Heat Transf. 2000, 14, 115–118. [Google Scholar] [CrossRef]
- Roy, S.K.; Avanic, B.L. Laminar forced convection heat transfer with phase change material suspensions. Int. Commun. Heat Mass Transf. 2001, 28, 895–904. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Kohl, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Zhang, G.; Li, X. Experimental research on the effective heating strategies for a phase change material-based power battery module. Int. J. Heat Mass Transf. 2019, 128, 392–400. [Google Scholar] [CrossRef]
- Kürklü, A.; Özmerzi, A.; Bilgin, S. Thermal performance of a water-phase change material solar collector. Renew. Energy 2002, 26, 391–399. [Google Scholar] [CrossRef]
- Papadimitratos, A.; Sobhansarbandi, S.; Pozdin, V.; Zakhidov, A.; Hassanipour, F. Evacuated tube solar collectors integrated with phase change materials. Sol. Energy 2016, 129, 10–19. [Google Scholar] [CrossRef]
- Allouhi, A.; Ait Msaad, A.; Benzakour Amine, M.; Saidur, R.; Mahdaoui, M.; Kousksou, T.; Pandey, A.K.; Jamil, A.; Moujibi, N.; Benbassou, A. Optimization of melting and solidification processes of PCM: Application to integrated collector storage solar water heaters (ICSSWH). Sol. Energy 2018, 171, 562–570. [Google Scholar] [CrossRef]
- Badiei, Z.; Eslami, M.; Jafarpur, K. Performance improvements in solar flat plate collectors by integrating with phase change materials and fins: A CFD modeling. Energy 2020, 192, 116719. [Google Scholar] [CrossRef]
- Syahruddin, A.S.; Jalaluddin, J.; Hayat, A. Performance Analysis Of Solar Water Heating System With Plate Collector Integrated Pcm Storage. EPI Int. J. Eng. 2021, 3, 143–149. [Google Scholar] [CrossRef]
- Pawar, V.R.; Sobhansarbandi, S. Design optimization and heat transfer enhancement of energy storage-based solar thermal collector. Sustain. Energy Technol. Assess. 2021, 46, 101260. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Lin, S.C. Performance evaluation of a solar water heater integrated with a PCM nanocomposite TES at various inclinations. Sol. Energy 2014, 109, 82–92. [Google Scholar] [CrossRef]
- Li, B.; Zhai, X. Experimental investigation and theoretical analysis on a mid-temperature solar collector/storage system with composite PCM. Appl. Therm. Eng. 2017, 124, 34–43. [Google Scholar] [CrossRef]
- Li, B.; Zhai, X.; Cheng, X. Experimental and numerical investigation of a solar collector/storage system with composite phase change materials. Sol. Energy 2018, 164, 65–76. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; El Qarnia, H. Performance evaluation of a solar thermal energy storage system using nanoparticle-enhanced phase change material. Int. J. Hydrogen Energy 2019, 44, 2013–2028. [Google Scholar] [CrossRef]
- Kumar, P.M.; Mylsamy, K. A comprehensive study on thermal storage characteristics of nano-CeO2 embedded phase change material and its influence on the performance of evacuated tube solar water heater. Renew. Energy 2020, 162, 662–676. [Google Scholar] [CrossRef]
- Iachachene, F.; Haddad, Z.; Arıcı, M.; Abu-Nada, E. The effect of nano encapsulated phase change materials and nanoparticles on turbulent heat transport: A conical diffuser scenario. J. Energy Storage 2022, 52, 104703. [Google Scholar] [CrossRef]
- Carmona, M.; Palacio Vega, M.; Martinez, A. Experimental analysis of a flat plate solar collector with integrated latent heat thermal storage. Contemp. Urban Aff. 2017, 1, 7–12. [Google Scholar] [CrossRef]
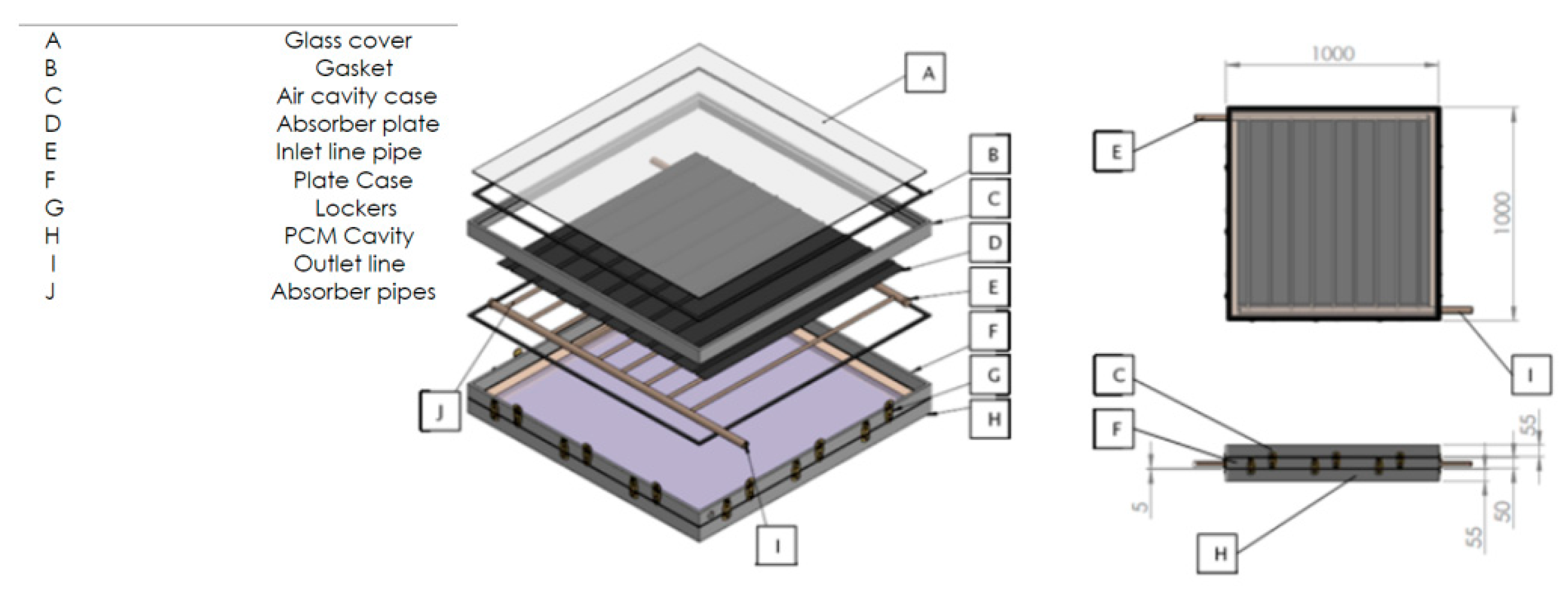
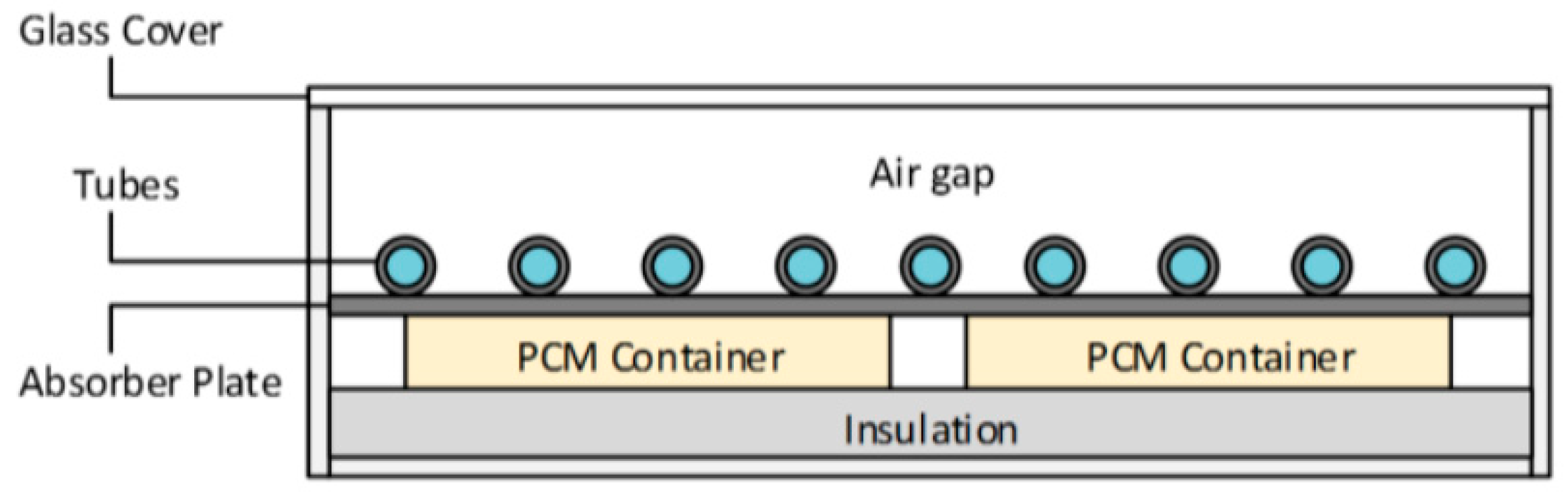
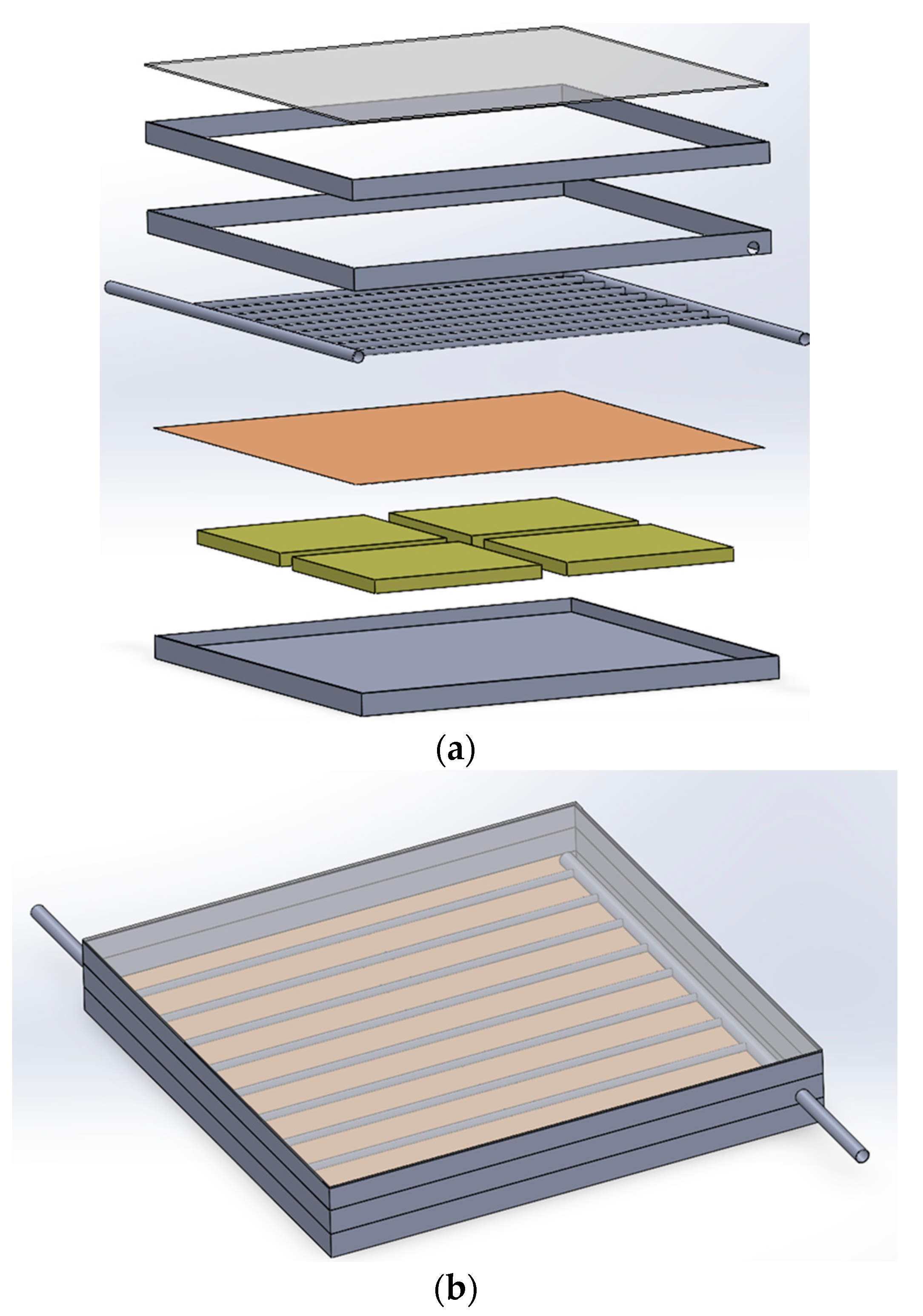



| Description | Specifications |
|---|---|
| cover | 3 mm: Glass |
| Pipe | 80-mm-outside diameter: copper |
| Connecting pipes | 1-mm-thick pipes: Copper |
| outlet pipe | 1-mm-thick pipes: Copper |
| Inlet pipe | 1-mm-thick pipes: Copper |
| Single wall frame | Aluminum with final dimensions of 0.78 by 1.60 m |
| air cavity | 3.5 cm spacing between the glass cover and the absorber |
| Solid Styrofoam board | Density of 40 kg/m3 and a thickness of 50 mm |
| Density | 815 (Kg/m3) |
|---|---|
| hidden heat | 244 (kj/kg) |
| Thermal conductivity | 0.18 (W/m.k) |
| heat capacity | 2000(J/kg) |
| Particle diameter | 100(nm) |
| latent heat of fusion | 190 kg/kj |
| melting point | 53.7 °C |
| ρ (Kg/m3) | Cp (J/KgK) | ||
|---|---|---|---|
| aluminum | 2719 | 871 | 4.202 |
| copper | 8978 | 381 | 6.387 |
| Glass | 2800 | 750 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahi, M.R.; Ostadi, S.; Rahmani, A.; Dibaj, M.; Akrami, M. Studying the Improvement of Solar Collector Mechanism with Phase Change Materials. Energies 2024, 17, 1432. https://doi.org/10.3390/en17061432
Rahi MR, Ostadi S, Rahmani A, Dibaj M, Akrami M. Studying the Improvement of Solar Collector Mechanism with Phase Change Materials. Energies. 2024; 17(6):1432. https://doi.org/10.3390/en17061432
Chicago/Turabian StyleRahi, Maha Rahman, Saba Ostadi, Amin Rahmani, Mahdieh Dibaj, and Mohammad Akrami. 2024. "Studying the Improvement of Solar Collector Mechanism with Phase Change Materials" Energies 17, no. 6: 1432. https://doi.org/10.3390/en17061432





