Ash Formation and Associated Interactions during Co-Combustion of Wheat Straw and Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fuel Samples
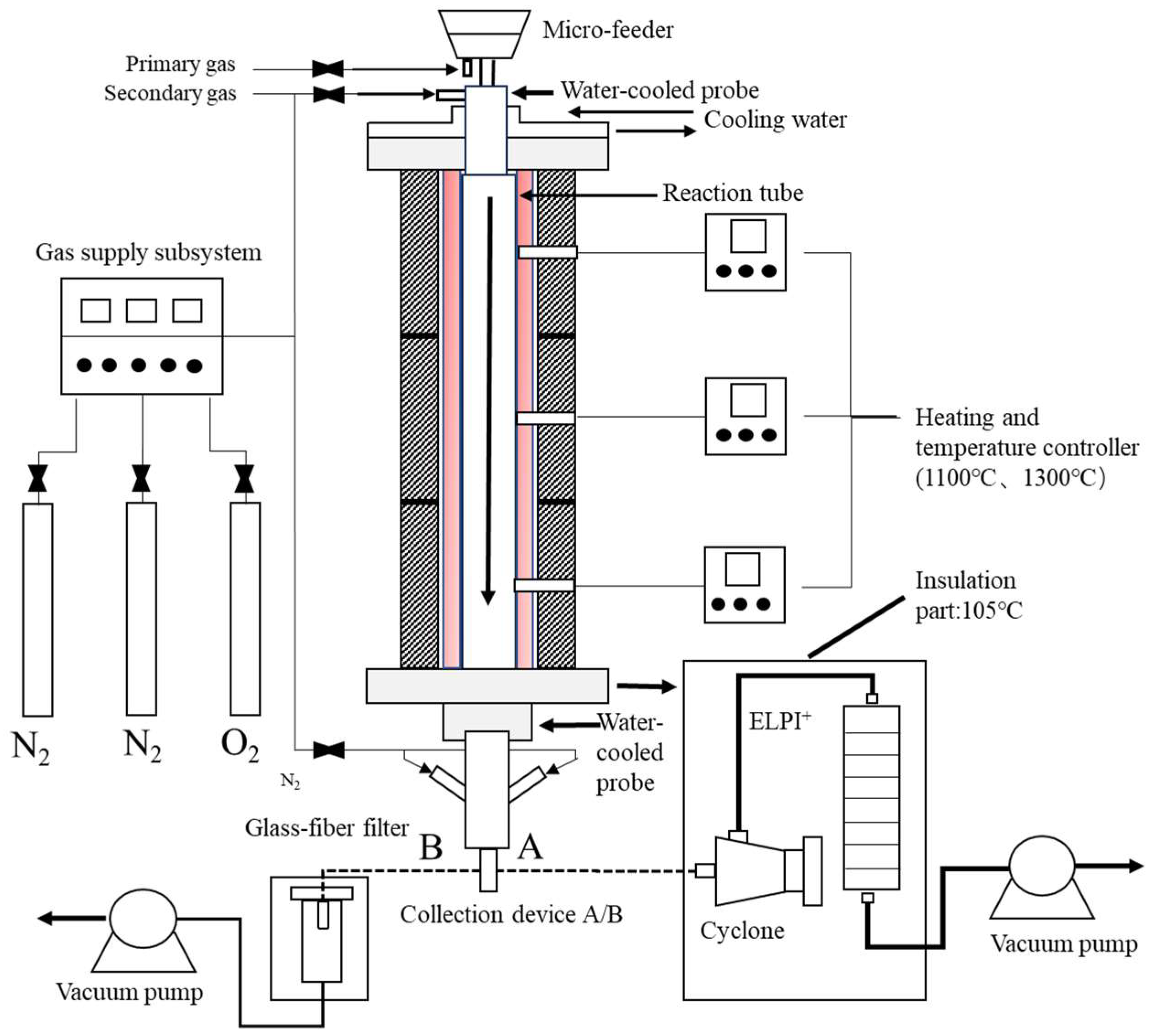
2.2. Combustion Experiments
2.3. Characterization of Ash Samples
3. Results and Discussion
3.1. Characteristics of the Residual Ash Formation
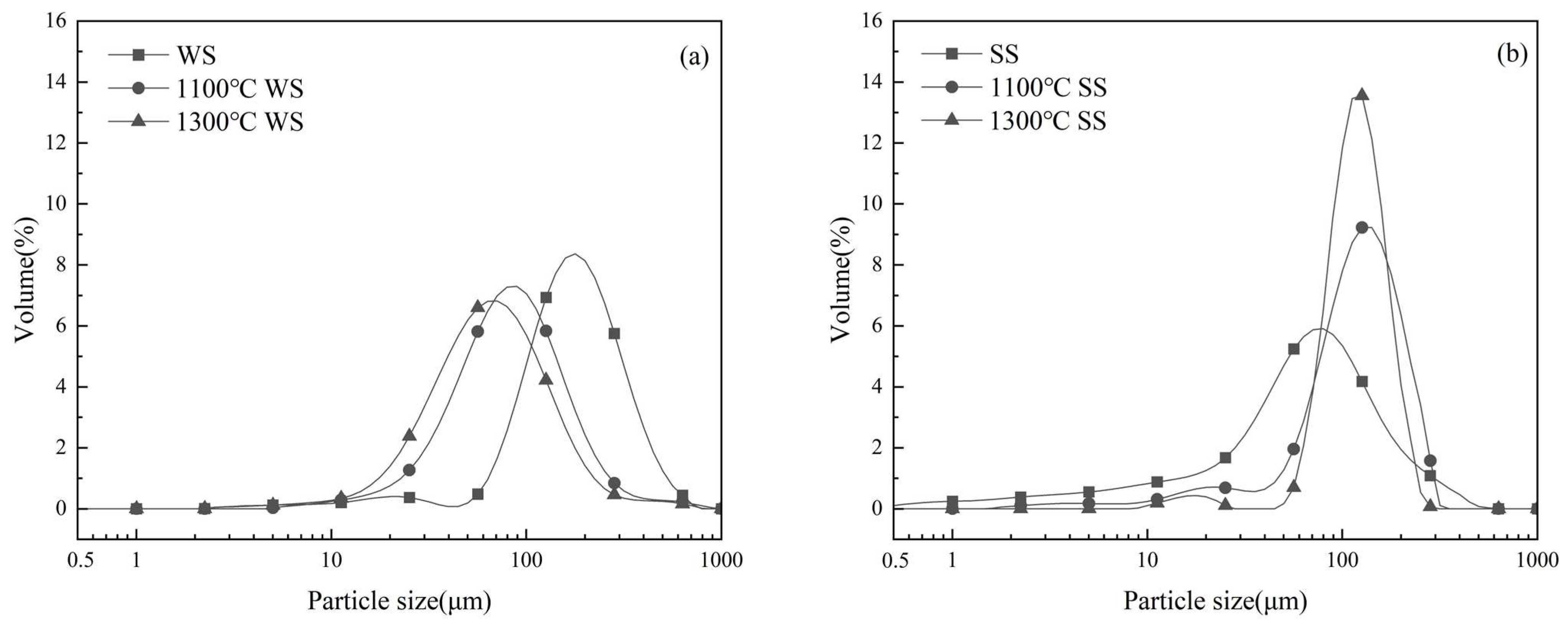
3.1.1. PSDs and Morphology of the Residual Ashes
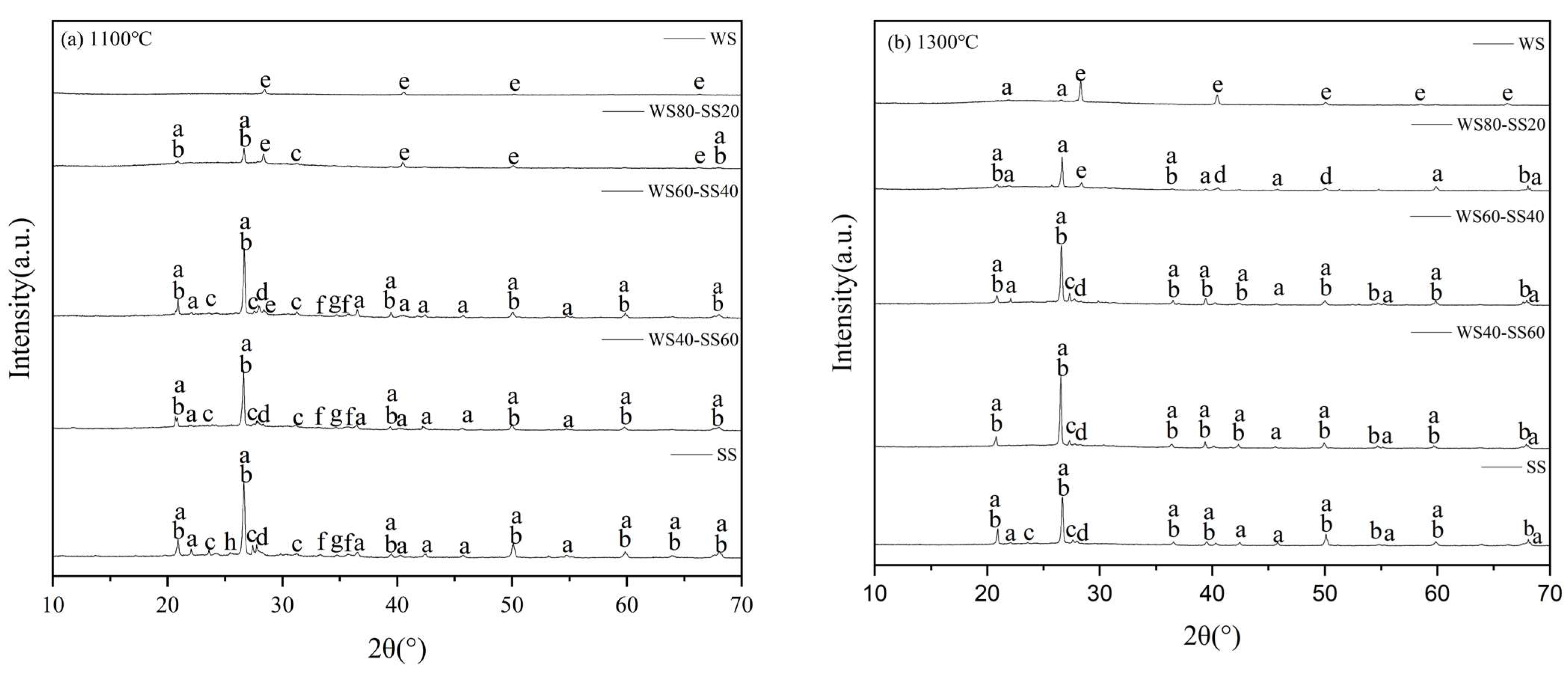
3.1.2. XRD Analysis of the Bulk Ashes
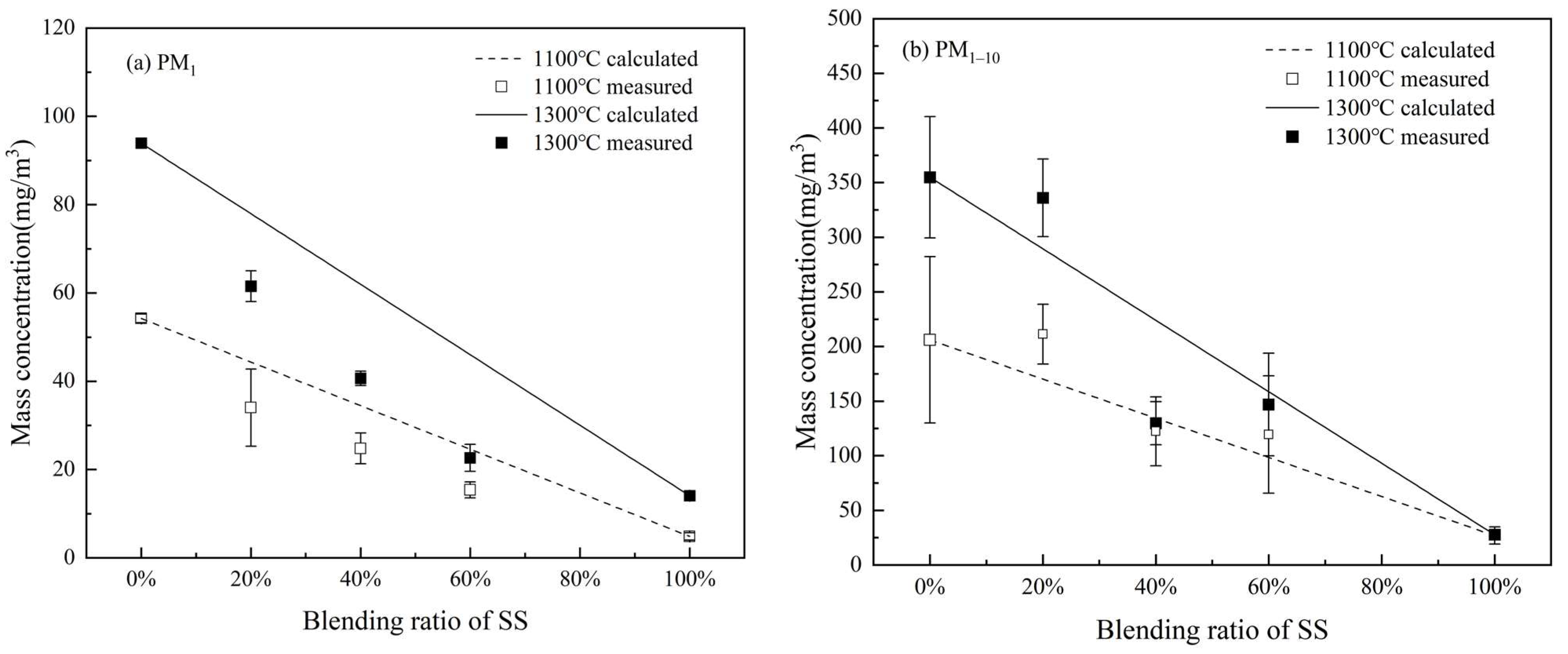
3.2. The Formation of PM10
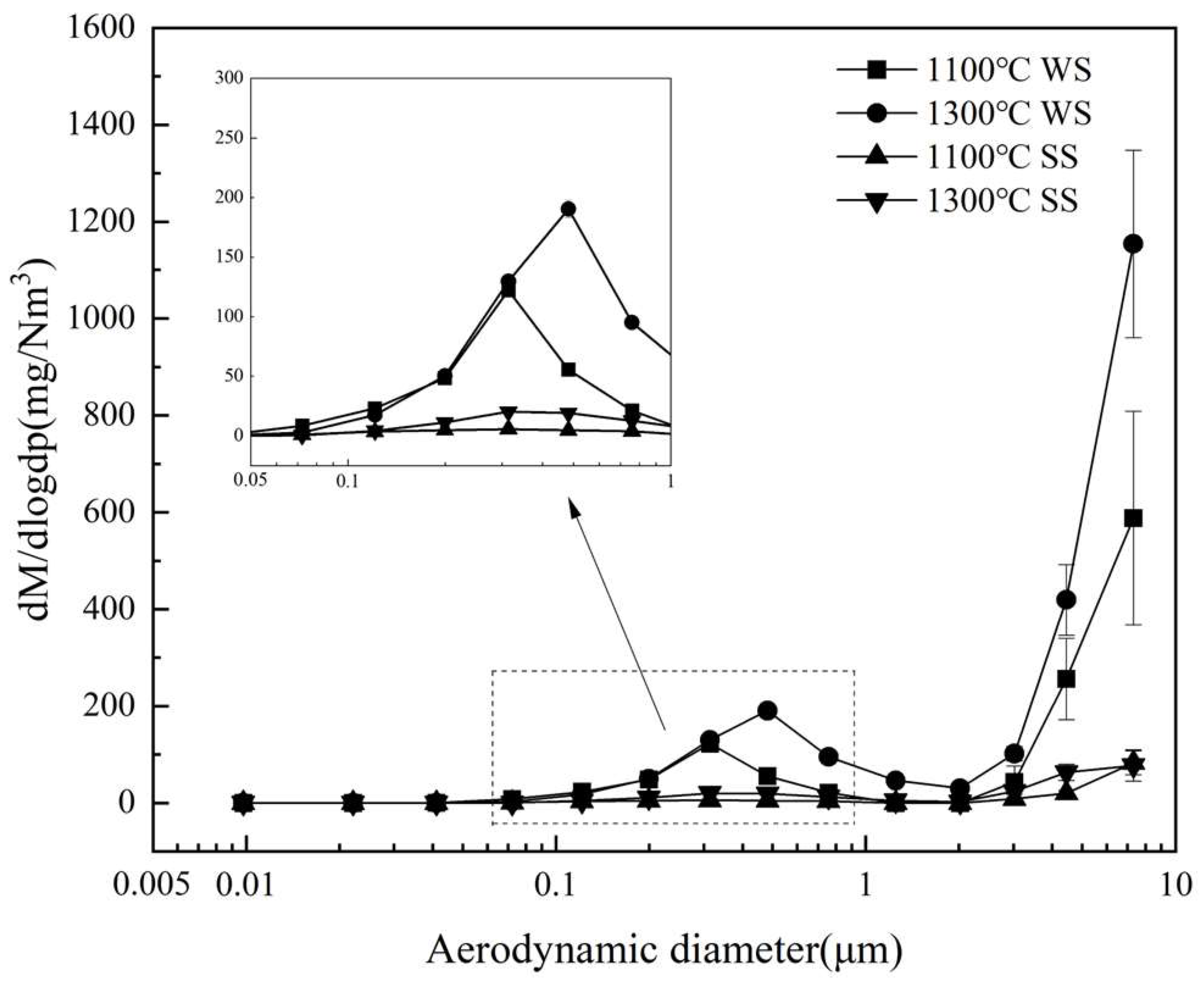
3.2.1. PSD and Production of PM10
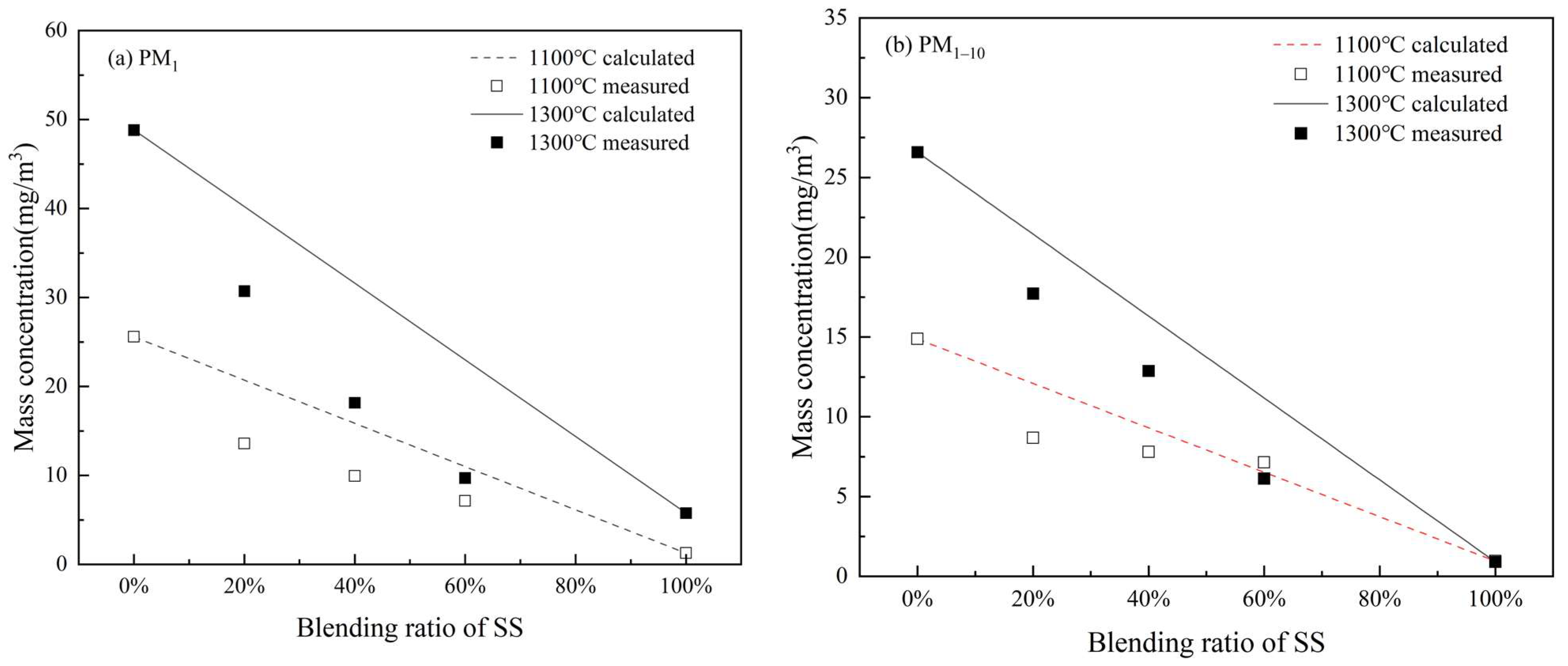
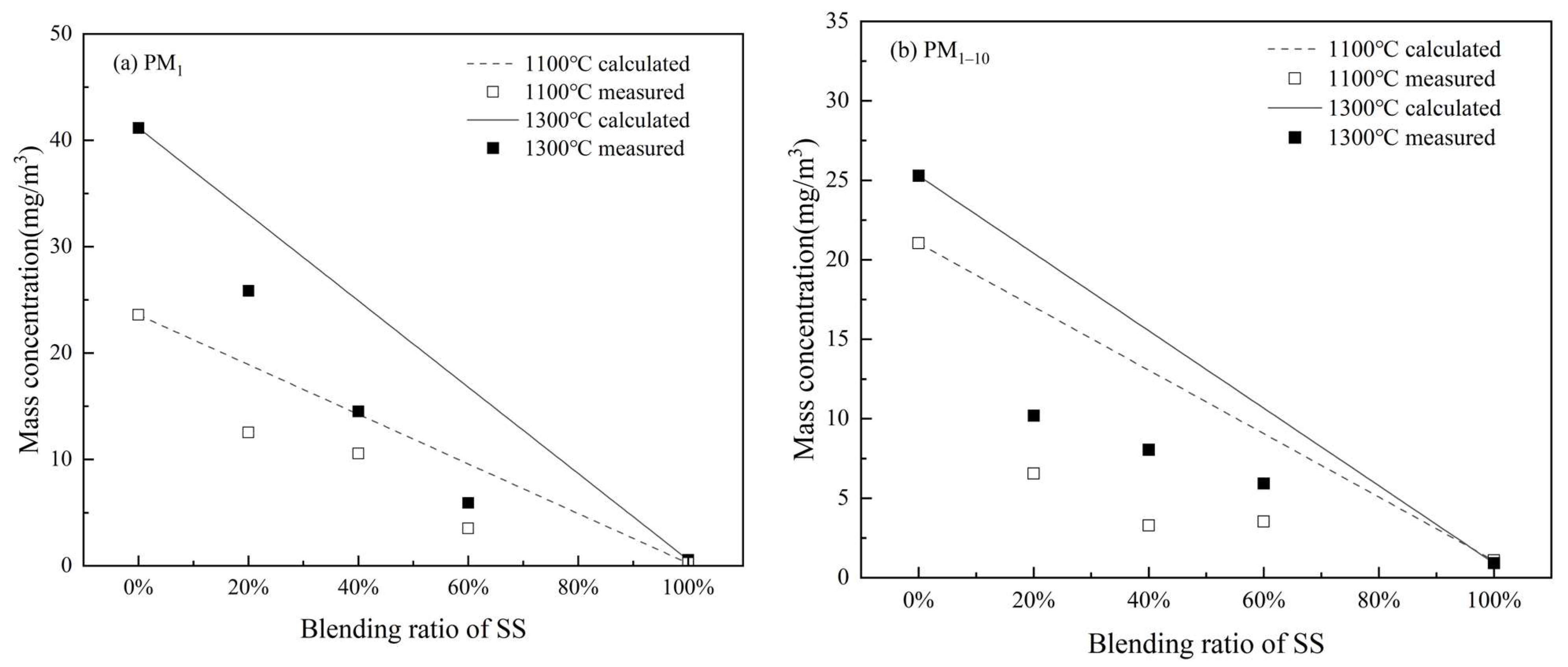
3.2.2. Elemental Composition of PM10
4. Conclusions
- (1)
- Co-combustion influences the PSD of the residual ash particles formed. Co-combustion generally generates larger residual ash particles close to those of SS combustion. The interaction of K capture enhances the melting and therefore increases the production of large and melting ash particles during co-combustion, which may increase the propensity of slag formation in pulverized fuel combustion systems.
- (2)
- Blending SS with WS involves the positive interaction of K captured by aluminosilicates as well as silicate and quartz minerals from SS ash to significantly reduce submicron ash formation. The reduction is 18.6~37.5% and 21.1–50.8% during co-combustion at 1100 °C and 1300 °C, respectively, which increases with the blending ratio of SS from 20% to 60%. It also has the positive interaction of transforming alkali chlorides into alkali sulfates, which reduced 25.9~63.2% and 21.8–64.7% of Cl and 34.4~37.3% and 23.6–57.8% of K in PM1 at 1100 °C and 1300 °C, respectively, to reduce the corrosive potential of the submicron ash particles. Co-combustion of SS with WS can also reduce the condensing of alkali chloride on PM1–10 to lower the potentials of deposition and corrosion of the fine residual ash particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviation | Explanation |
| WS | Narrowly sized wheat straw |
| SS | Narrowly sized sewage sludge |
| DTF | Drop tube furnace |
| ELPI | Electric low-pressure impactor |
| PSD | Particle size distribution |
| SEM | Scanning electron microscopy |
| PM10 | Particulate matter with an aerodynamic diameter of <10 μm |
| PM1–10 | Particulate matter with an aerodynamic diameter of 1–10 μm |
| PM1 | Particulate matter with an aerodynamic diameter of <1 μm |
| XRD | X-ray diffraction |
| EDS | Energy-dispersive X-ray spectroscopy |
References
- Agency, I.E. The Role of Low-Carbon Fuels in the Clean Energy Transitions of the Power Sector; IEA Publications: Paris, France, 2021. [Google Scholar]
- Jones, J.; Darvell, L.; Gudka, B. Bioenergy with Carbon Capture and Storage. International Centre for Sustainable Carbon. 18 May 2023, p. 122. Available online: https://www.sustainable-carbon.org/report/bioenergy-with-carbon-capture-and-storage/ (accessed on 15 January 2024).
- Zhang, X.; Meloni, S. Technology Developments in the Cofiring of Biomass; IEA, Clean Coal Centre: London, UK, 2021. [Google Scholar]
- Mo, W.; Du, K.; Sun, Y.; Guo, M.; Zhou, C.; You, M.; Xu, J.; Jiang, L.; Wang, Y.; Su, S.; et al. Technical-economic-environmental analysis of biomass direct and indirect co-firing in pulverized coal boiler in China. J. Clean. Prod. 2023, 426, 139119. [Google Scholar] [CrossRef]
- European, C.; Joint Research, C.; Camia, A.; Giuntoli, J.; Jonsson, R.; Robert, N.; Cazzaniga, N.; Jasinevičius, G.; Avitabile, V.; Grassi, G.; et al. The Use of Woody Biomass for Energy Production in the EU; Publications Office: Luxembourg, 2021. [Google Scholar]
- Rabaçal, M.; Pereira, S.; Costa, M. Review of Pulverized Combustion of Non-Woody Residues. Energy Fuels 2018, 32, 4069–4095. [Google Scholar] [CrossRef]
- Liu, H.; Qiao, H.; Liu, S.; Wei, G.; Zhao, H.; Li, K.; Weng, F. Energy, environment and economy assessment of sewage sludge incineration technologies in China. Energy 2023, 264, 126294. [Google Scholar] [CrossRef]
- Branco, V.; Costa, M. Effect of particle size on the burnout and emissions of particulate matter from the combustion of pulverized agricultural residues in a drop tube furnace. Energy Convers. Manag. 2017, 149, 774–780. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Bashir, M.S.; Jensen, P.A.; Frandsen, F.; Wedel, S.; Dam-Johansen, K.; Wadenbäck, J.; Pedersen, S.T. Ash transformation and deposit build-up during biomass suspension and grate firing: Full-scale experimental studies. Fuel Process. Technol. 2012, 97, 93–106. [Google Scholar] [CrossRef]
- Tobiasen, L.; Skytte, R.; Pedersen, L.S.; Pedersen, S.T.; Lindberg, M.A. Deposit characteristic after injection of additives to a Danish straw-fired suspension boiler. Fuel Process. Technol. 2007, 88, 1108–1117. [Google Scholar] [CrossRef]
- Wu, H.; Glarborg, P.; Frandsen, F.J.; Dam-Johansen, K.; Jensen, P.A. Dust-Firing of Straw and Additives: Ash Chemistry and Deposition Behavior. Energy Fuels 2011, 25, 2862–2873. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the use of additives to mitigate operational problems associated with the combustion of biomass with high content in ash-forming species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Häggström, G.; Karl Hannl, T.; Holmgren, P.; Broström, M.; Skoglund, N.; Öhman, M. Fate of phosphorus in pulverized fuel co-combustion of sewage sludge and agricultural residues. Fuel 2023, 335, 127059. [Google Scholar] [CrossRef]
- Damoe, A.J.; Jensen, P.A.; Frandsen, F.J.; Wu, H.; Glarborg, P. Fly Ash Formation during Suspension Firing of Biomass: Effects of Residence Time and Fuel Type. Energy Fuels 2017, 31, 555–570. [Google Scholar] [CrossRef]
- Garba, M.U.; Ingham, D.B.; Ma, L.; Porter, R.T.J.; Pourkashnian, M.; Tan, H.; Williams, A. Prediction of Potassium Chloride Sulfation and Its Effect on Deposition in Biomass-Fired Boilers. Energy Fuels 2012, 26, 6501–6508. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wendt, J.O.L. On Ash Deposition Rates from Air and Oxy-Combustion of Pulverized Coal, Petroleum Coke, and Biomass. Energy Fuels 2019, 33, 5849–5858. [Google Scholar] [CrossRef]
- Jiménez, S.; Ballester, J. Influence of operating conditions and the role of sulfur in the formation of aerosols from biomass combustion. Combust. Flame 2005, 140, 346–358. [Google Scholar] [CrossRef]
- Jiménez, S.; Ballester, J. Formation of alkali sulphate aerosols in biomass combustion. Fuel 2007, 86, 486–493. [Google Scholar] [CrossRef]
- Aho, M.; Vainikka, P.; Taipale, R.; Yrjas, P. Effective new chemicals to prevent corrosion due to chlorine in power plant superheaters. Fuel 2008, 87, 647–654. [Google Scholar] [CrossRef]
- Sengeløv, L.W.; Hansen, T.B.; Bartolomé, C.; Wu, H.; Pedersen, K.H.; Frandsen, F.J.; Jensen, A.D.; Glarborg, P. Sulfation of Condensed Potassium Chloride by SO2. Energy Fuels 2013, 27, 3283–3289. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; Li, Z.; Aldén, M.; Jakobsen, J.G.; Hansen, S.; Glarborg, P. Post-flame gas-phase sulfation of potassium chloride. Combust. Flame 2013, 160, 959–969. [Google Scholar] [CrossRef]
- Wang, G.; Poulsen, J.; Poulsen, S.; Jensen, P.A.; Frandsen, F.J. Influence of kaolin and coal fly ash addition on biomass ash deposition in an entrained flow reactor. Fuel 2022, 313, 123041. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.-C.; Li, W.-Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, C. Correlation of Sub-micrometer Ash Formation from Pulverized Biomass Combustion with Ash Composition. Energy Fuels 2019, 33, 5893–5902. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Li, Y.; Cheng, W.; Zhang, W.; Yang, H.; Tan, Z.; Chen, H. Mitigation of particulate matter emissions from co-combustion of rice husk with cotton stalk or cornstalk. Renew. Energy 2022, 190, 893–902. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Cheng, W.; Sang, H.; Yang, H.; Chen, H. Characteristics of Particulate Matter Emitted from Agricultural Biomass Combustion. Energy Fuels 2017, 31, 7493–7501. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Wang, G.; Luo, X.; Ruan, R.; Jin, Q.; Tan, H. Influence of coal co-firing on the particulate matter formation during pulverized biomass combustion. J. Energy Inst. 2019, 92, 450–458. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, H.; Xu, K.; Xu, Q.; Li, L. Investigation on the fusion characterization and melting kinetics of ashes from co-firing of anthracite and pine sawdust. Renew. Energy 2020, 145, 835–846. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H. Effect of Sampling Temperature on the Properties of Inorganic Particulate Matter Collected from Biomass Combustion in a Drop-Tube Furnace. Energy Fuels 2010, 24, 4571–4580. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Beckmann, G.; Wadenbäck, J.; Holm, J.K.; Velaga, S.P.; Weber, R. One way of representing the size and shape of biomass particles in combustion modeling. Fuel 2017, 206, 675–683. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, C. Modelling particle size distribution of residual fly ash from pulverized biomass combustion. J. Biobased Mater. Bioenergy 2021, 15, 75–82. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Zhu, H.; Liu, Z.; Lu, X.; Zhang, D. Investigation into the fusibility of biomass ashes and their mineral phase transformations at elevated temperatures by using the HT-XRD technique. Biomass Bioenergy 2023, 173, 106812. [Google Scholar] [CrossRef]
- Hong-wei, S.; Wei, L.; Rui-yang, L. Study on Problems of Alkali Metal during Straw Combustion. Energy Conserv. Technol. 2009, 27, 24–31. [Google Scholar]
- Gale, T.K.; Wendt, J.O.L. Mechanisms and Models Describing Sodium and Lead Scavenging by a Kaolinite Aerosol at High Temperatures. Aerosol Sci. Technol. 2003, 37, 865–876. [Google Scholar] [CrossRef]
- JimÉNez, S.; Ballester, J. Particulate matter formation and emission in the combustion of different pulverized biomass fuels. Combust. Sci. Technol. 2006, 178, 655–683. [Google Scholar] [CrossRef]
- Gao, X.; Rahim, M.U.; Chen, X.; Wu, H. Inorganic PM10 emission from the combustion of individual mallee components and whole-tree biomass. Proc. Combust. Inst. 2017, 36, 3313–3319. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Adeosun, A.; Liu, B.; Ruan, R.; Li, S.; Tan, H. Particulate matter emission and K/S/Cl transformation during biomass combustion in an entrained flow reactor. J. Energy Inst. 2018, 91, 835–844. [Google Scholar] [CrossRef]
- Liaw, S.B.; Wu, H. High-Phosphorus Fuel Combustion: Effect of Oxyfuel Conditions on PM10 Emission from Homo- and Heterogeneous Phases. Energy Fuels 2017, 31, 2317–2323. [Google Scholar] [CrossRef]





| Samples | Proximate Analysis (wt.%, Air-Dried) | Ultimate Analysis (wt.%, Air-Dried) | Lower Heating Value (MJ/kg, Air-Dried) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile Matter | Ash | Fixed Carbon | C | H | O | N | S | ||
| WS | 5.59 | 64.78 | 11.25 | 18.38 | 37.69 | 5.17 | 39.73 | 0.57 | <0.05 | 17.02 |
| SS | 5.25 | 36.11 | 53.73 | 4.91 | 21.18 | 3.47 | 12.64 | 3.17 | 0.56 | 8.95 |
| Na | Mg | Al | Si | K | Ca | Fe | P | S | Cl | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | 0.72 | 0.82 | 0.23 | 15.69 | 50.78 | 7.04 | 0.71 | 0.88 | 1.27 | 21.58 | 0.28 |
| SS | 1.21 | 2.47 | 12.17 | 31.04 | 5.24 | 14.50 | 15.29 | 11.09 | 3.20 | 1.00 | 2.79 |
| Combustion Temperature | Blending Ratio of SS | (Na + K)/Cl | (Na + K)/2S | (Na + K)/(2S + P) | (Na + K)/(Cl + 2S) | (Na + K)/(Cl + 2S + P) |
|---|---|---|---|---|---|---|
| 1100 °C | 0% | 1.01 | 9.14 | 7.79 | 0.91 | 0.89 |
| 20% | 1.06 | 5.37 | 3.64 | 0.95 | 0.82 | |
| 40% | 1.09 | 5.27 | 4.07 | 0.90 | 0.86 | |
| 60% | 1.17 | 4.13 | 2.96 | 0.91 | 0.84 | |
| 100% | 6.09 | 1.04 | 0.69 | 0.89 | 0.62 | |
| 1300 °C | 0% | 1.12 | 38.44 | 21.1 | 1.09 | 1.06 |
| 20% | 1.18 | 8.59 | 4.26 | 1.04 | 0.93 | |
| 40% | 1.30 | 4.17 | 3.08 | 1.01 | 0.92 | |
| 60% | 1.57 | 3.23 | 1.66 | 1.05 | 0.80 | |
| 100% | 11.39 | 1.30 | 0.96 | 1.17 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Zhou, H.; Sheng, C. Ash Formation and Associated Interactions during Co-Combustion of Wheat Straw and Sewage Sludge. Energies 2024, 17, 1486. https://doi.org/10.3390/en17061486
Shan Y, Zhou H, Sheng C. Ash Formation and Associated Interactions during Co-Combustion of Wheat Straw and Sewage Sludge. Energies. 2024; 17(6):1486. https://doi.org/10.3390/en17061486
Chicago/Turabian StyleShan, Yingnan, Hongfang Zhou, and Changdong Sheng. 2024. "Ash Formation and Associated Interactions during Co-Combustion of Wheat Straw and Sewage Sludge" Energies 17, no. 6: 1486. https://doi.org/10.3390/en17061486





