Low-Cost Feedstock Conversion to Biodiesel via Ultrasound Technology
Abstract
:1. Introduction
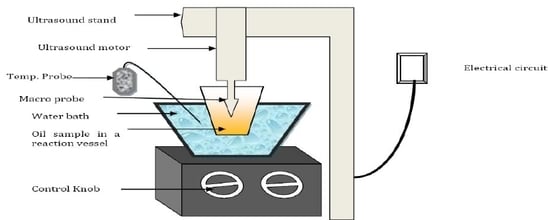
2. Experimental Section
| Property | Waste Oil | Soybean Oil | Sunflower Oil |
|---|---|---|---|
| Fatty Acid Composition (%) | |||
| Palmitic Acid C16:0 | 14 | 11.75 | 6.08 |
| Stearic acid C18:0 | 6.02 | 3.15 | 3.26 |
| Oleic Acid C18:1 | 35.60 | 23.26 | 16.93 |
| Linoleic Acid C18:2 | 44.78 | 55.53 | 73.73 |
| Linolenic Acid C18:3 | 0.6 | 6.31 | 0.00 |
| Mean Molecular weight | 884.16 | ||
| Viscosity (m Pas) at 40 °C | 37 | 29.3 | 29.4 |
| Fatty Acid Value | 1.73 | 0.021 | 0.03 |
3. Results and Discussion
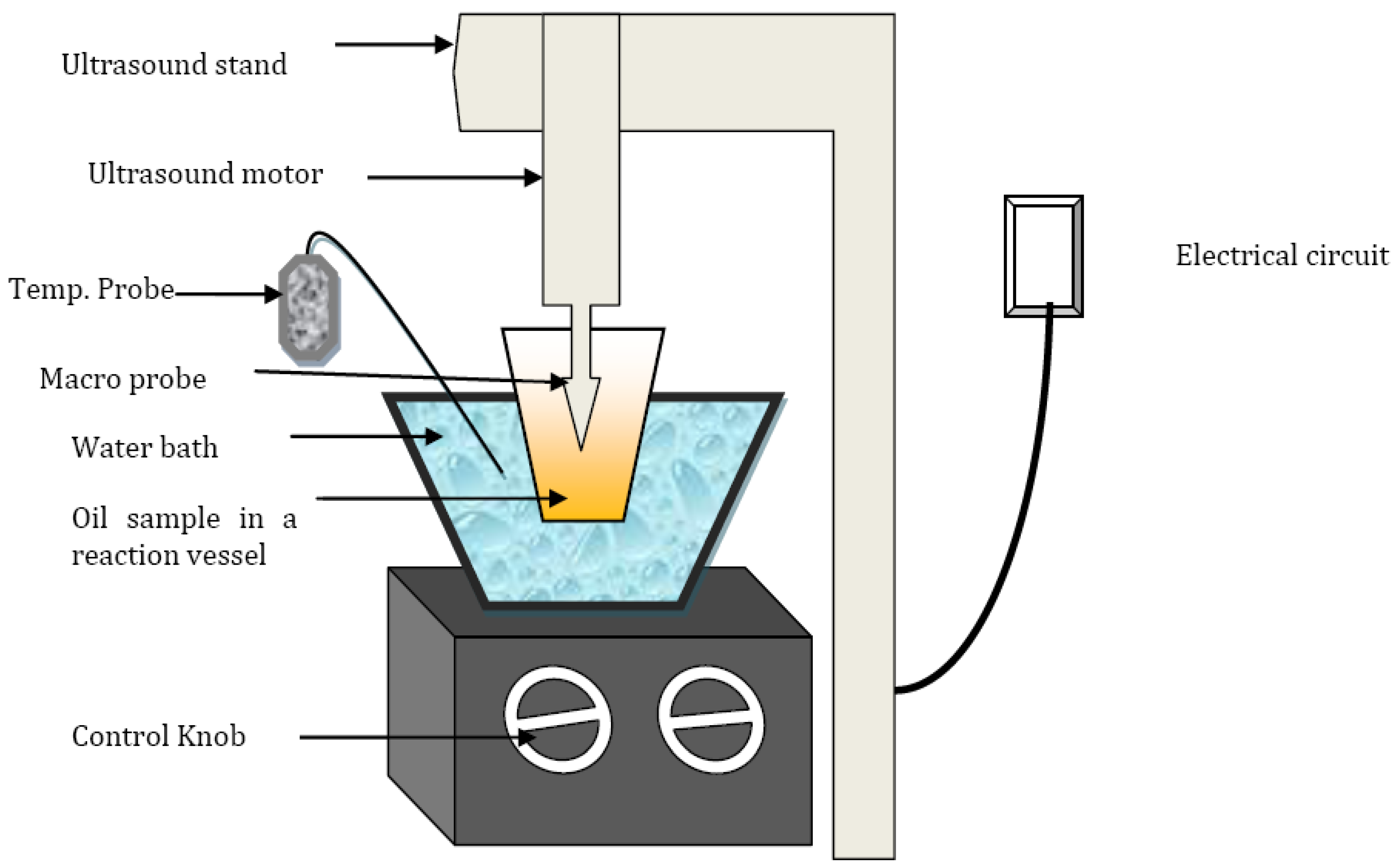
3.1. Effect of Catalyst Concentration
| Catalyst | Mechanical Stirring | Ultrasonication | ||||
|---|---|---|---|---|---|---|
| Concentration | (600 rpm) Yield (%) | (24 kHz) Yield (%) | ||||
| KOH (%wt/wt) | WCO | Sunflower | Soybean | WCO | Sunflower | Soybean |
| 0.5 | 71.3 ± 0.2 | 92.2 ± 0.12 | 90 ± 0.67 | 78.2 ± 0.06 | 95.30 ± 0.8 | 98.23 ± 0.2 |
| 0.75 | 96.8 ± 0.08 | 94.32 ± 0.4 | 97.65 ± 0.04 | |||
| 1.0 | 87.33 ± 0.3 | 93.32 ± 0.2 | 92 ± 1.2 | 95.98 ± 0.7 | 91.78 ± 0.3 | 93.67 ± 0.56 |
| 1.25 | 93.4 ± 0.67 | 90.64 ± 0.5 | 90.03 ± 0.78 | |||
| 1.5 | 91.2 ± 0.1 | 90.12 ± 1.1 | 88.2 ± 0.7 | 94.8 ± 0.03 | 88.78 ± 0.3 | 86.4 ± 0.45 |
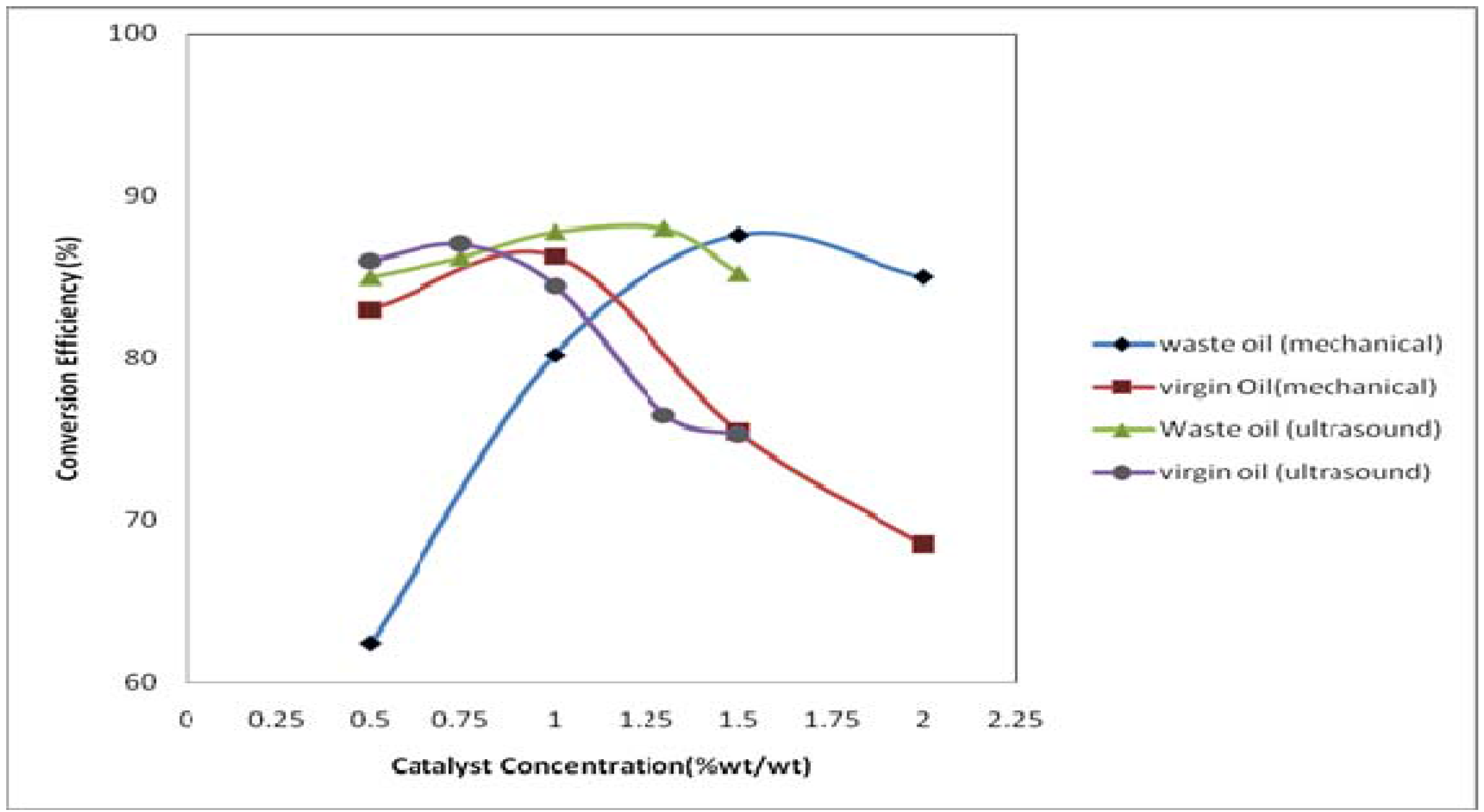
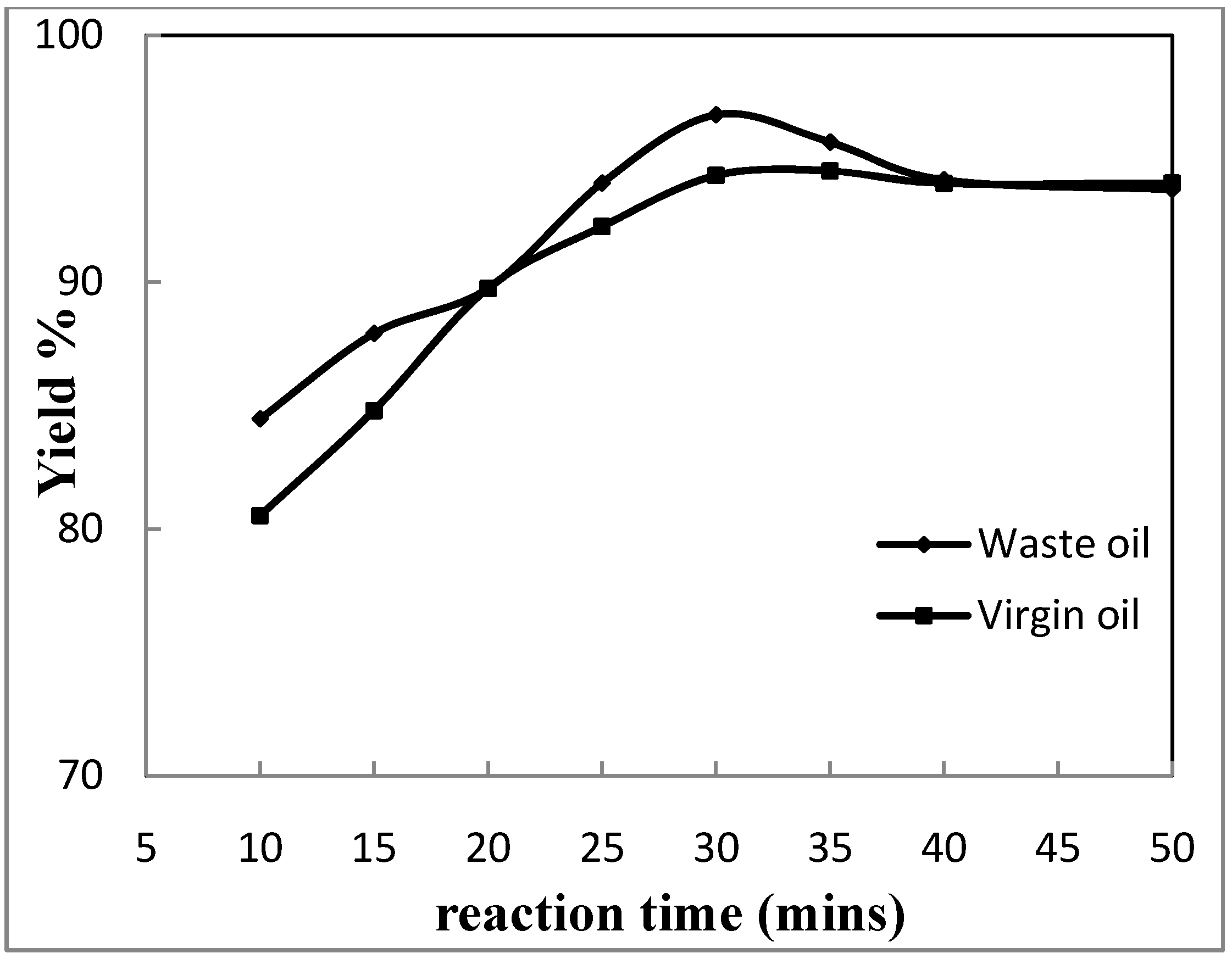
3.2. Effect of Reaction Time
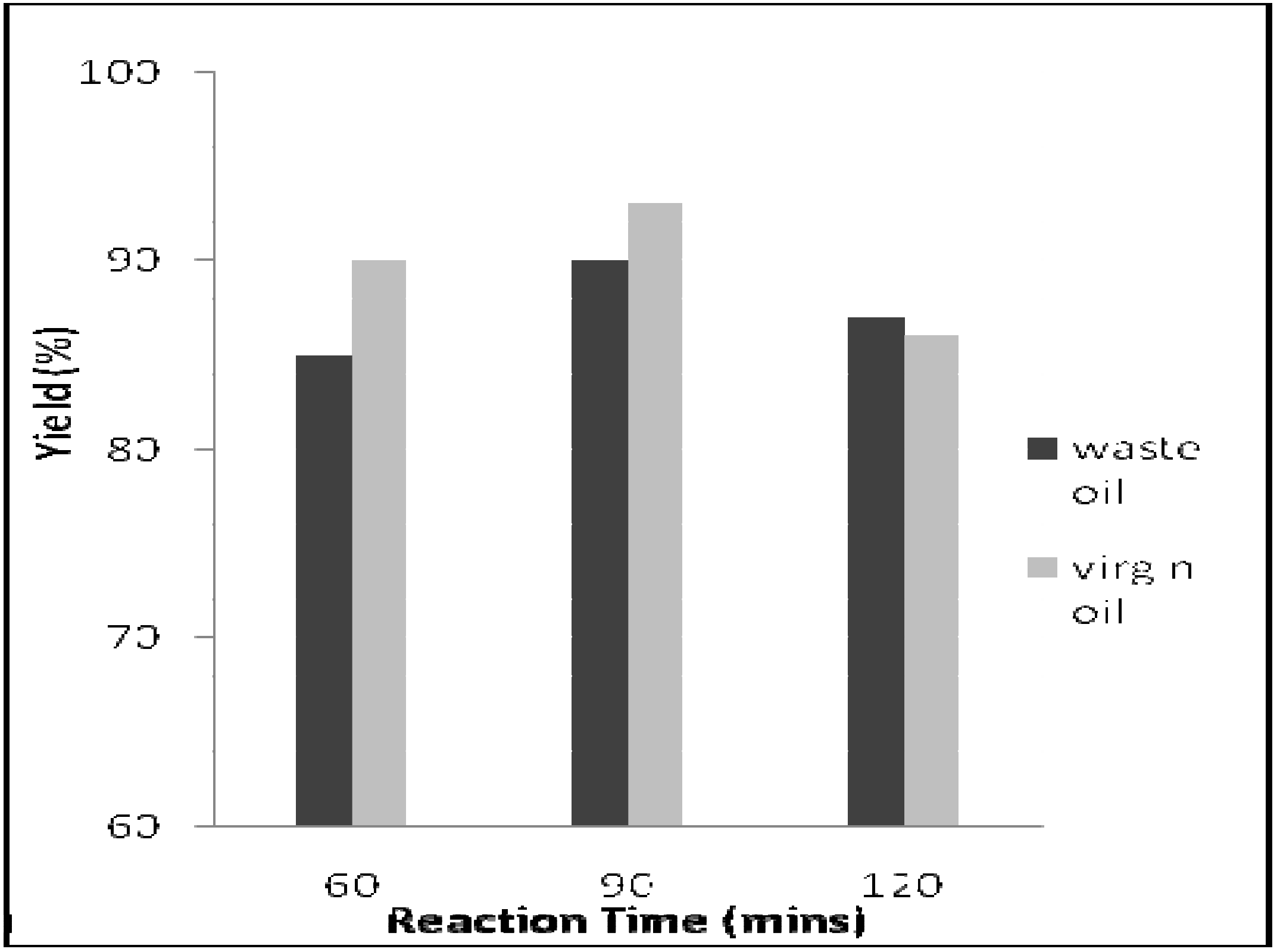
3.3. Effect of Reaction Temperature

| Test parameter | Unit | Result | SANS 1935:2004 | Test method |
|---|---|---|---|---|
| Total ester | Mass% | 96.78 | 96.5 min | ASTM 6584 |
| Total glycerol | Mass% | 0.25 | 0.25 max | ASTM 6584 |
| Free glycerol | Mass% | 0.01 | 0.02 max | AOCS Ca14-56 |
| Monoglyceride | Mass% | 0.65 | 0.8 max | AOCS Ca14-56 |
| Diglyceride | Mass% | 0.17 | 0.2 max | ASTM 6584 |
| Triglyceride | Mass% | 0.18 | 0.2 max | ASTM 6584 |
| Methanol | Mass% | 0.14 | 0.2 max | EN 14110 |
| Viscosity (40 °C) | (mm2/s) | 4.08 | 1.9–6.0 | |
| Flash point | °C | 150 | min 120 | ISO 3679 |
| Density | Kg/m-3 | 891.1 | 860-900 | ISO 3675 |
4. Conclusions
Acknowledgement
References
- The Royal Society. Sustainable Biofuels: Prospects and Challenges; The Royal Society: London, UK, 2008. [Google Scholar]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Energy Environ. Sci. 2008, 1, 542–564.
- Srivastava, A.R.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Vicente, G.; Martınez, M.; Aracil, J. A comparative study of vegetable oils for biodiesel production in spain. Energy Fuels 2006, 20, 394–398. [Google Scholar] [CrossRef]
- Yusuf, C. Biodiesel from microalgae. Biotechnol. Adv. 2007, 3, 294–306. [Google Scholar]
- Canakci, M.; Gerpen, J.V. A pilot plant to produce biodiesel from high free fatty acid feedstocks. Trans. ASAE 2003, 46, 945–954. [Google Scholar]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Batch (one- and two-stage) production of biodiesel fuel from rapeseed oil. Biotechn. Appl. Bioc. 1996, 131, 668–679. [Google Scholar] [CrossRef]
- Bournay, L.; Cassanave, D.; Delfort, B.; Hillion, G.; Chadorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Mittelbach, M. Lipase catalyzed alcoholysis of sunflower oil. J. Am. Oil Chem. Soc. 1990, 67, 168–170. [Google Scholar] [CrossRef]
- Mittelbach, M.; Gangl, S. Long storage stability of biodiesel made from rapeseed and used frying oil. J. Am. Oil Chem. Soc. 2001, 78, 573–577. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Bandow, H.; Maeda, Y. Ultrasonically driven continuous process for vegetable oil transesterification. Ultrason. Sonochem. 2007, 14, 413–417. [Google Scholar] [CrossRef]
- Veronica, C.; Felipa, M.B.; Juan, M.; Campelo, D.L.; Jose, M.M.; Antonio, A.R.; Jose, M.H.; Rafael, L.; Girolamo, G.; Aastacia, M. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1,3-regiospecific alcoholysis of sunflower oil. Process Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables affecting the yield of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Conversion of vegetable oil to Biodiesel using ultrasonic irradiation. Chem. Lett. 2003, 32, 716–717. [Google Scholar] [CrossRef]
- Taleyarkhan, R.P.; Cho, J.S.; West, C.D.; Nigmatulin, R.I.; Block, R.C. Additional evidence of nuclear emissions during acoustic cavitation. Physical Rev. 2004, 69, 361–369. [Google Scholar]
- Encinar, J.M.; González, J.F.; Rodríguez, J.J.; Tejedor, A. Biodiesel fuels from vegetable oils: Transesterification of Cynara cardunculus L. Oils with Ethanol. Energy Fuels 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Encinar, J.M.; Juan, F.; Gonzalez, J.F.; Rodriguez, J.R. Biodiesel from used frying oil: Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renewable Energy 2008, 1–3. [Google Scholar] [CrossRef]
- Mason, T.; Lorimer, J. The Uses of Power Ultrasound in Chemistry and Processing, 2nd ed.; Wiley-VCH: Weinhem, Germany, 2002. [Google Scholar]
- Colucci, J.A.; Borrero, E.E.; Alape, F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. J. Am. Oil Chem. Soc. 2005, 82, 525–530. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Tegou, E.; Avlonitis, D.; Gergis, V. Biodiesel production: Reaction and process parameters of alkali-catalyzed transesterifica- tion of waste frying oils. Energy Fuels 2007, 21, 3023–3027. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.P.; Pomonis, P.J.; Kontominas, M.G. Transesterification of soybean frying oil to biodiesel using heterogeneous catalysts. Fuel Process. Technol. 2009, 90, 671–676. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Bandow, H. Biodiesel production from virgin and waste oils using ultrasonic reactor in pilot scale. Proc. Symp. Ultrason. Electron. 2008, 29, 395–396. [Google Scholar]
- Thanh, L.T.; Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Maeda, Y.; Bandow, H. Ultrasound-assisted production of biodiesel fuel from vegetable oils in a small scale circulation process. Bioresour. Technol. 2010, 101, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.A.; Attia, N.K.; Sibak, H.A.; Sheltawy, S.T.E.; Eldiwani, G.I. Production optimization and quality assessment of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Tech. 2008, 5, 75–82. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel Production from High FFA Rubber Seed Oil. Fuel Process. Technol. 2005, 84, 335–340. [Google Scholar]
- Zhou, C.H.; Beltramini, J.N.; Fana, Y.X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Knothe, G. Analytical Methods used in the production and fuel quality assessment of biodiesel. Trans. ASAE 2001, 44, 193–200. [Google Scholar] [CrossRef]
- Lifka, J.; Ondruschka, B. Influence of mass transfer on the production of biodiesel. Chem. Eng. Technol. 2004, 27, 1156–1159. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Pomonis, P.J.; Avlonitis, D.; Gergis, V. Conventional and in situ transesterification of sunflower seed oil for the production of biodiesel. Fuel Process. Technol. 2008, 89, 503–509. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Maeda, Y.; Bandow, H. A two-step continuous ultrasound assisted production of biodiesel fuel from waste cooking oils: A practical and economical approach to produce high quality biodiesel fuel. Bioresour. Technol. 2010, 101, 5394–5401. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.P.; Rodrigues, S.; Fernandes, F.A.N. Optimisation of the production of biodiesel from soybean by ultrasound assisted methanolysis. Fuel Process. Technol. 2009, 90, 312–316. [Google Scholar] [CrossRef]
- Finigan, I.; Cape-town, South Africa. Private communication, 2008.
- Ramachandran, K.B.; Al-Zuhair, S.; Fong, C.S.; Gak, C.W. Kinetic study on hydrolysis of oils by lipase with ultrasonic emulsification. Biochem. Eng. J. 2006, 32, 19–24. [Google Scholar] [CrossRef]
- Lucena, I.L.; Silva, G.F.; Fernandes, F.A.N. Biodiesel Production by Esterification of Oleic Acid with Methanol Using a Water Adsorption Apparatus. Ind. Eng. Chem. Res. 2008, 47, 6885–6889. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Y.; Li, Y.; Yu, Z.X. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, 411–414. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Babajide, O.; Petrik, L.; Amigun, B.; Ameer, F. Low-Cost Feedstock Conversion to Biodiesel via Ultrasound Technology. Energies 2010, 3, 1691-1703. https://doi.org/10.3390/en3101691
Babajide O, Petrik L, Amigun B, Ameer F. Low-Cost Feedstock Conversion to Biodiesel via Ultrasound Technology. Energies. 2010; 3(10):1691-1703. https://doi.org/10.3390/en3101691
Chicago/Turabian StyleBabajide, Omotola, Leslie Petrik, Bamikole Amigun, and Farouk Ameer. 2010. "Low-Cost Feedstock Conversion to Biodiesel via Ultrasound Technology" Energies 3, no. 10: 1691-1703. https://doi.org/10.3390/en3101691