Fabrication and Characterization of Fullerene-Based Bulk Heterojunction Solar Cells with Porphyrin, CuInS2, Diamond and Exciton-Diffusion Blocking Layer
Abstract
:1. Introduction
2. Experimental Procedures
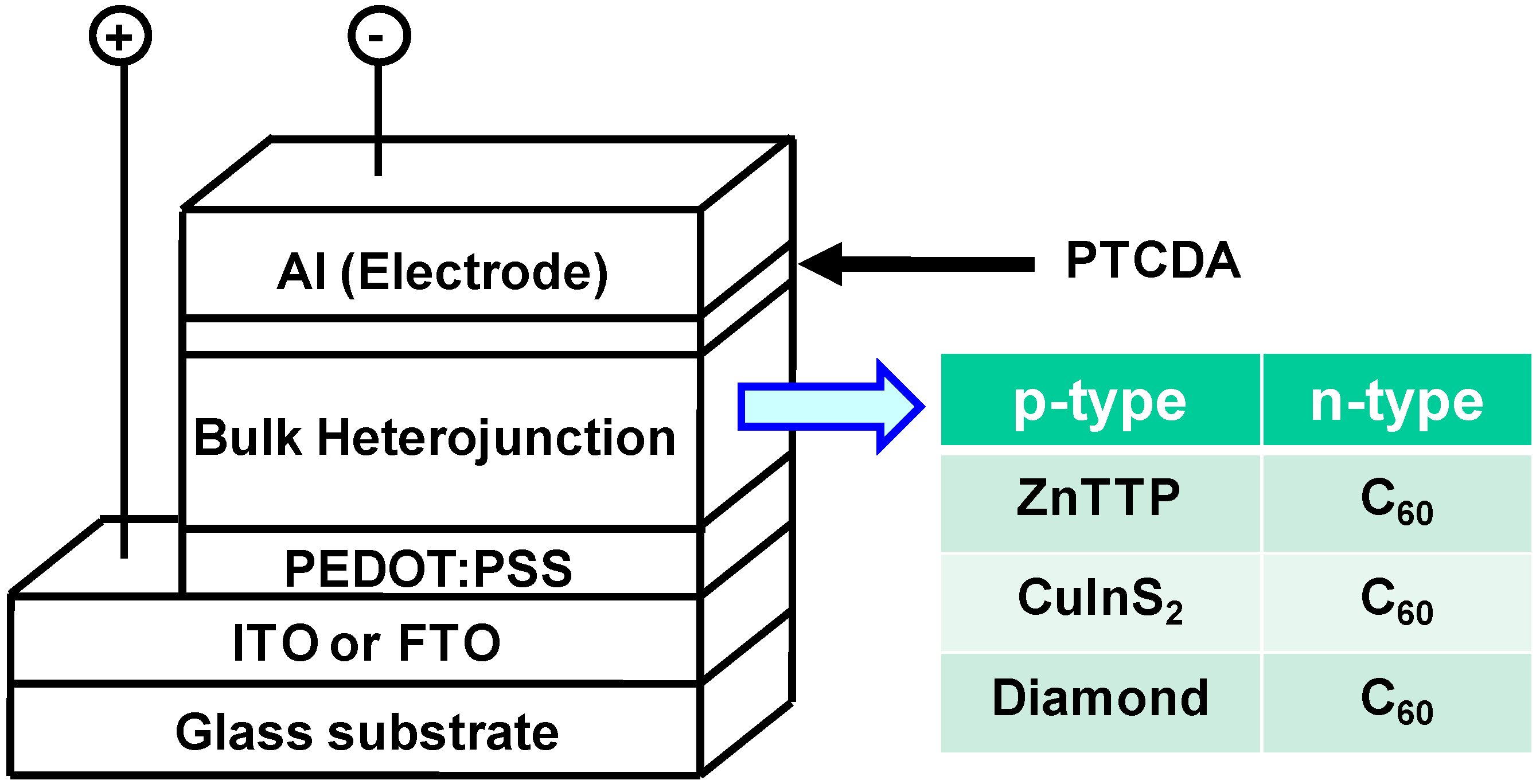
3. Results and Discussion
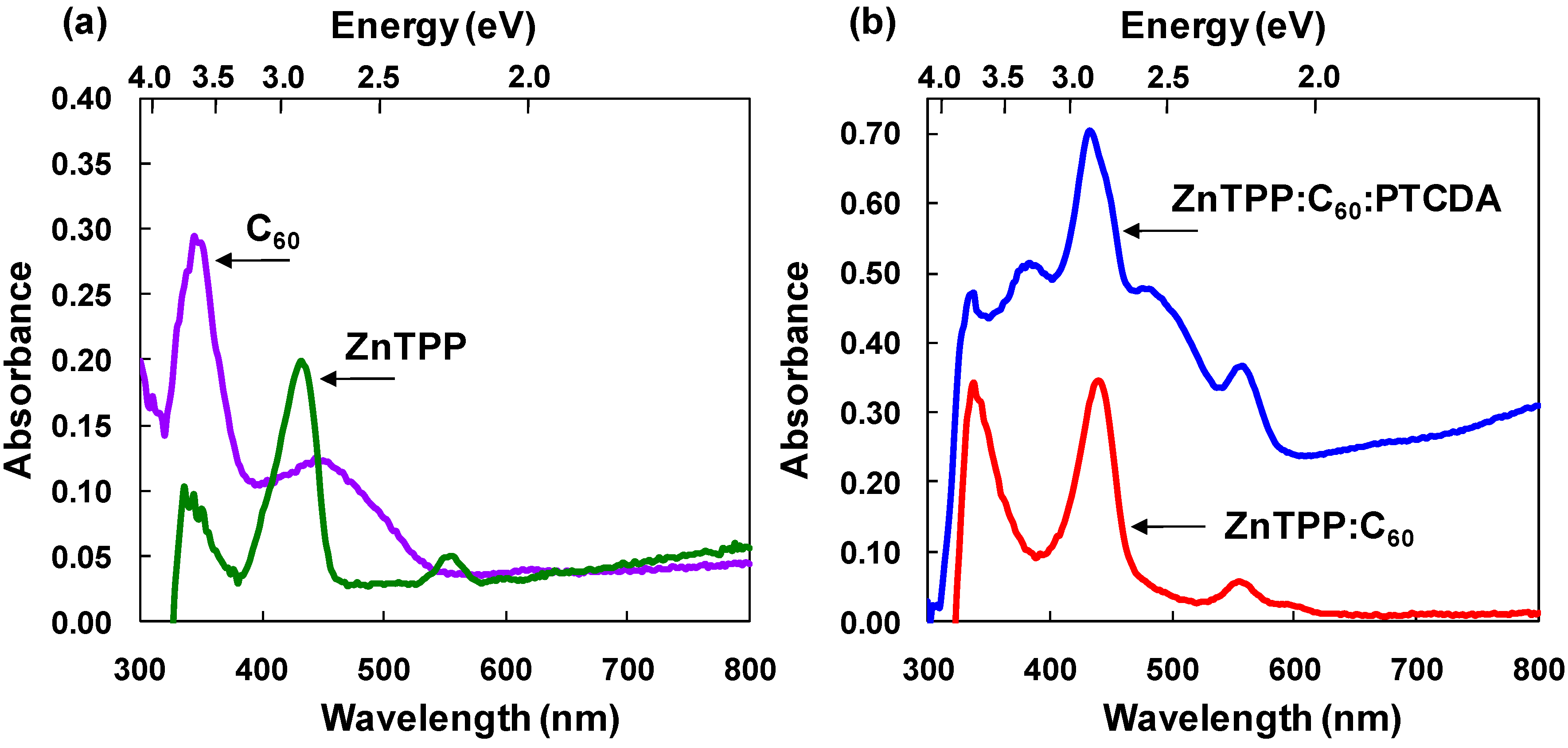
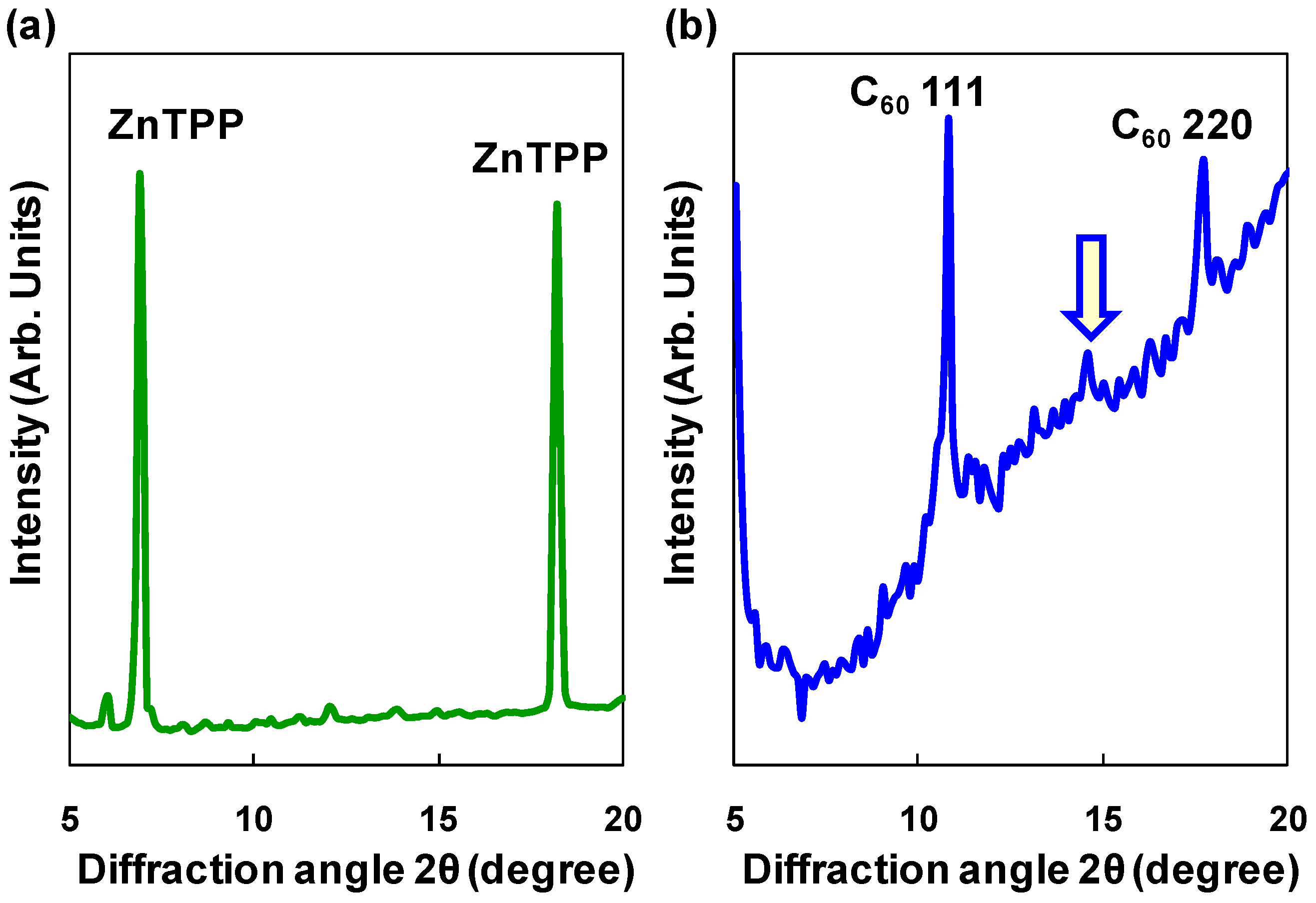
3.1. ZnTPP:C60 bulk heterojunction solar cells
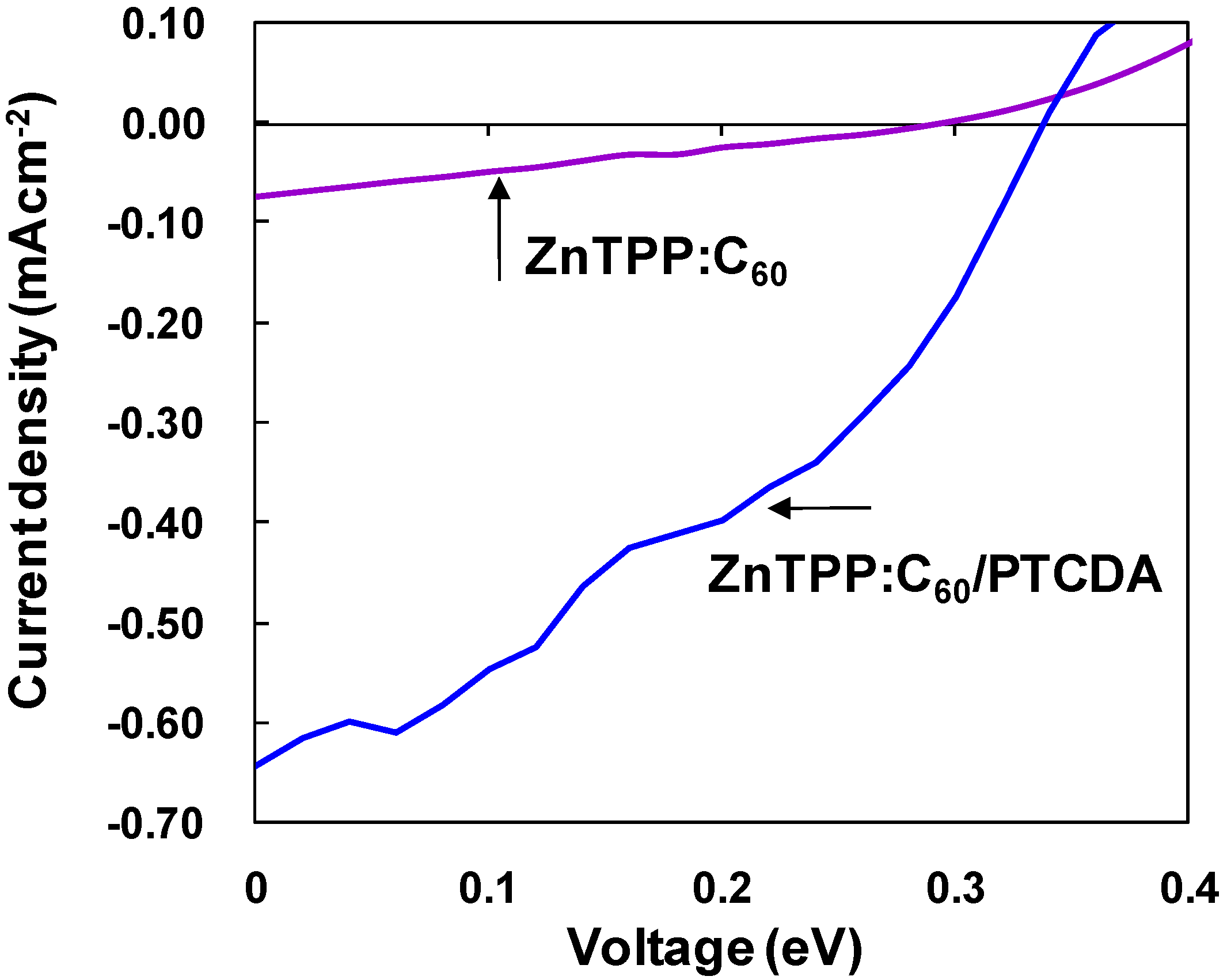
| Sample | VOC (V) | JSC (mAcm−2) | FF | η (%) |
|---|---|---|---|---|
| ZnTTP:C60/PTCDA | 0.33 | 0.62 | 0.38 | 7.8 × 10−2 |
| ZnTTP:C60 | 0.30 | 0.074 | 0.26 | 5.8 × 10−3 |
| CuInS2:C60 | 0.18 | 0.016 | 0.28 | 8.0 × 10−4 |
| Diamond:C60 | 0.023 | 0.0053 | 0.35 | 4.3 × 10−5 |

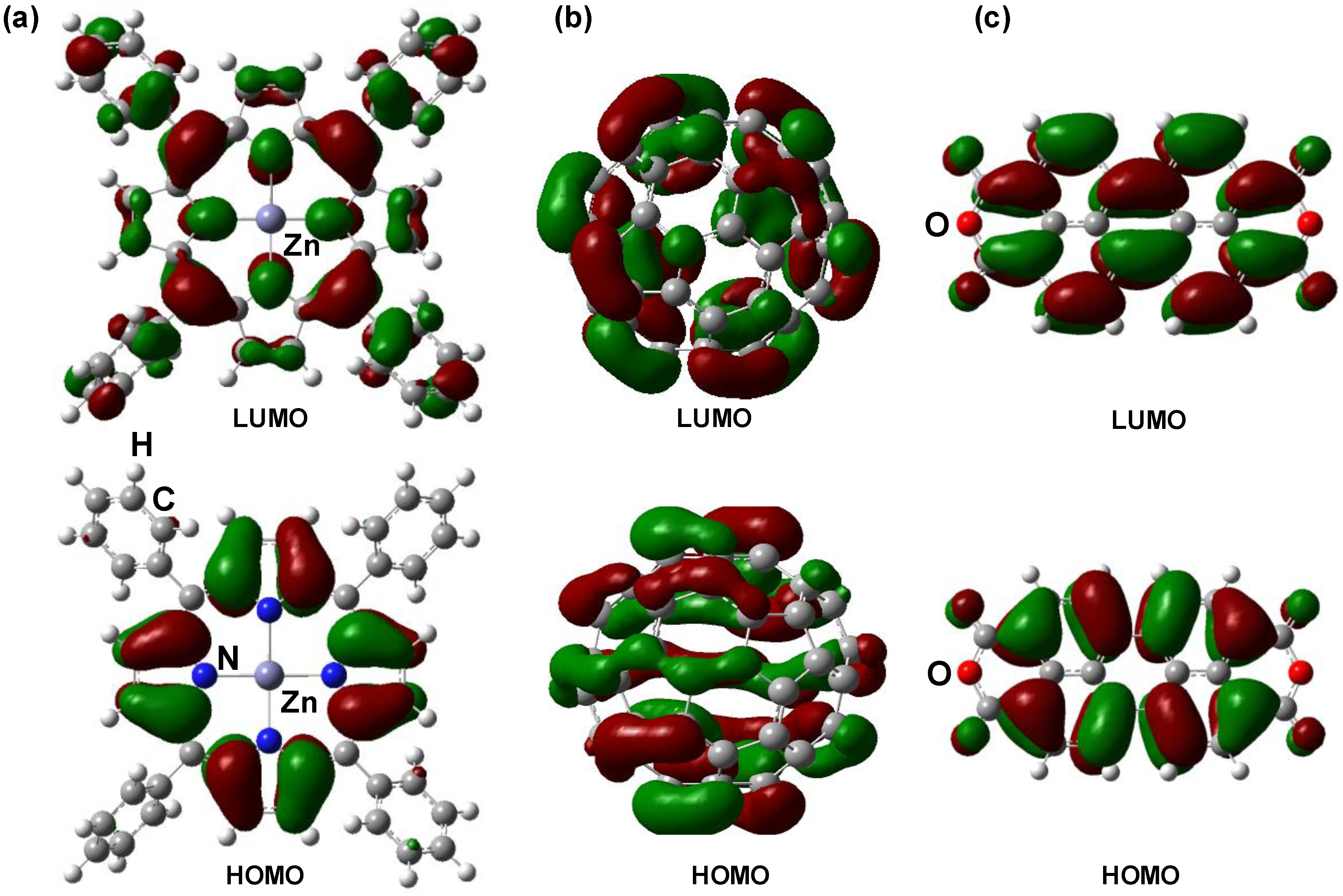
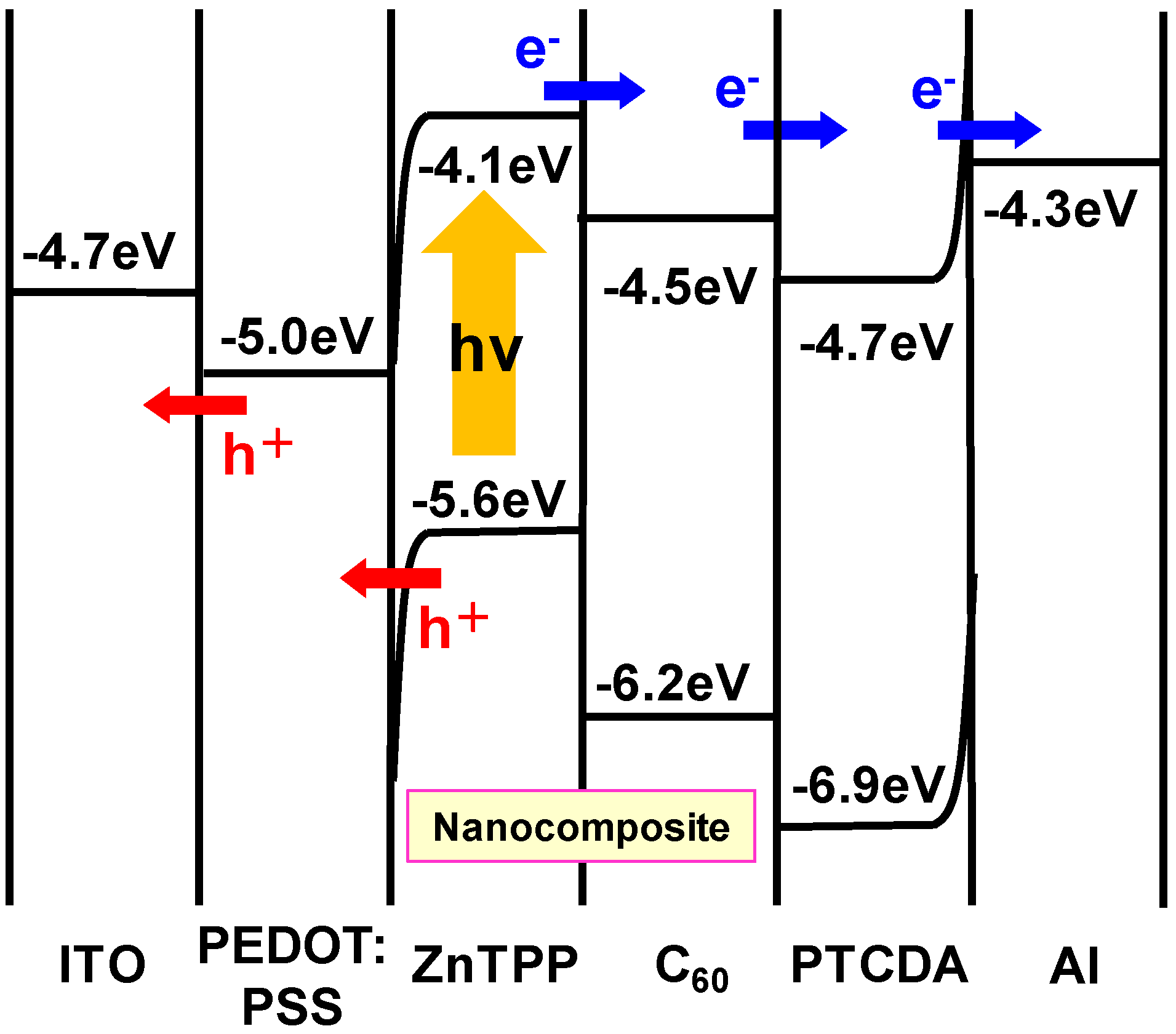
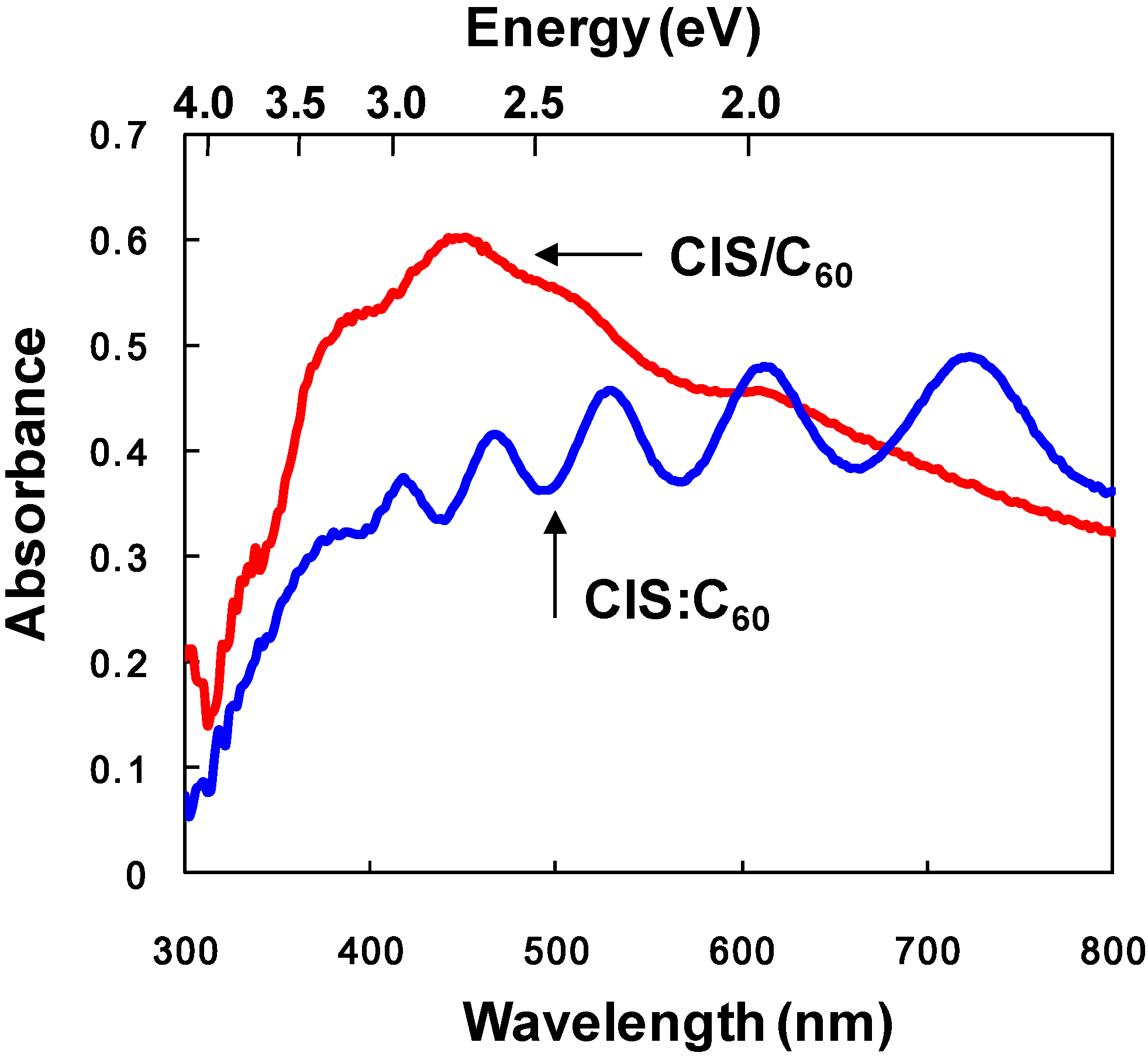
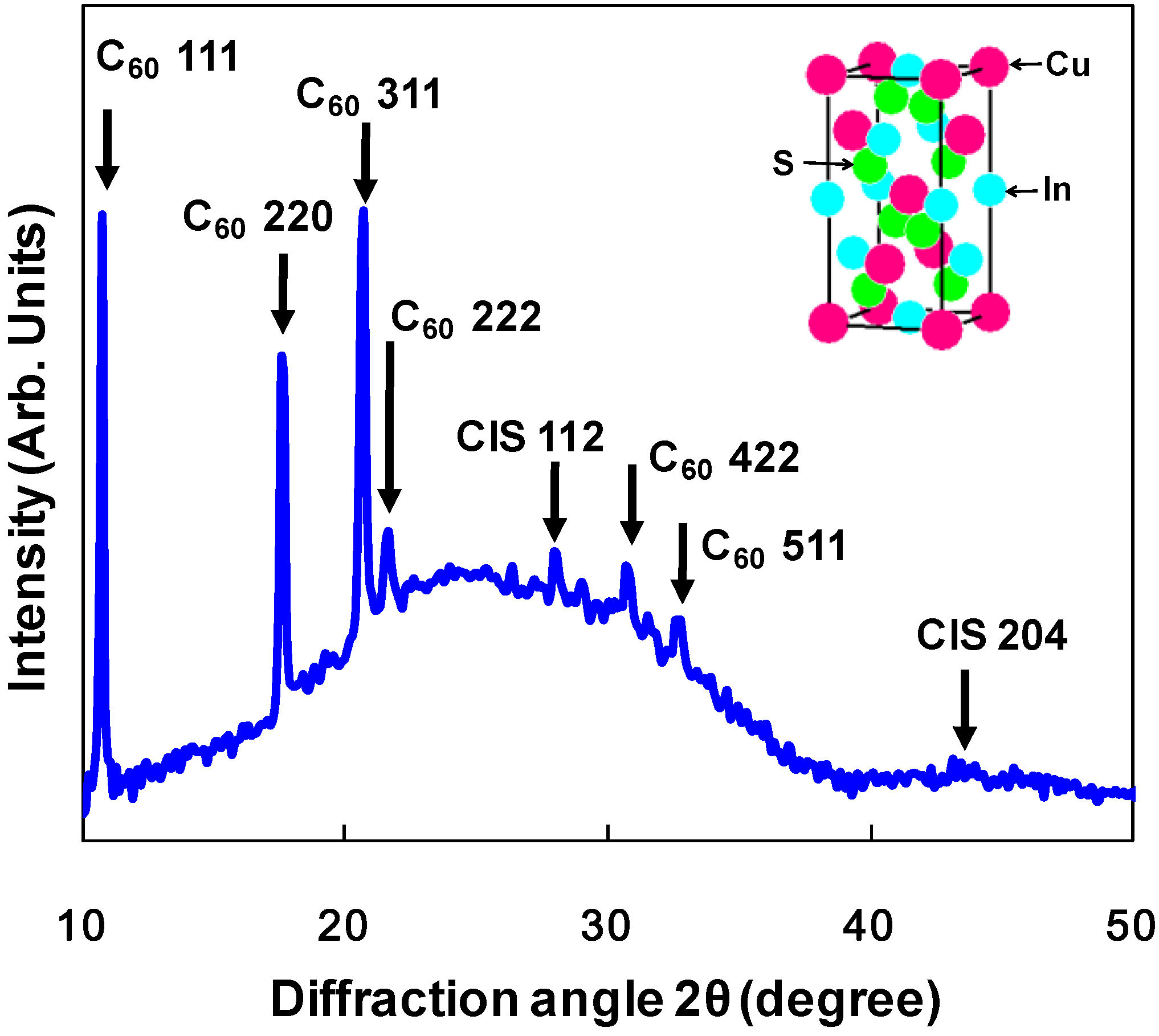
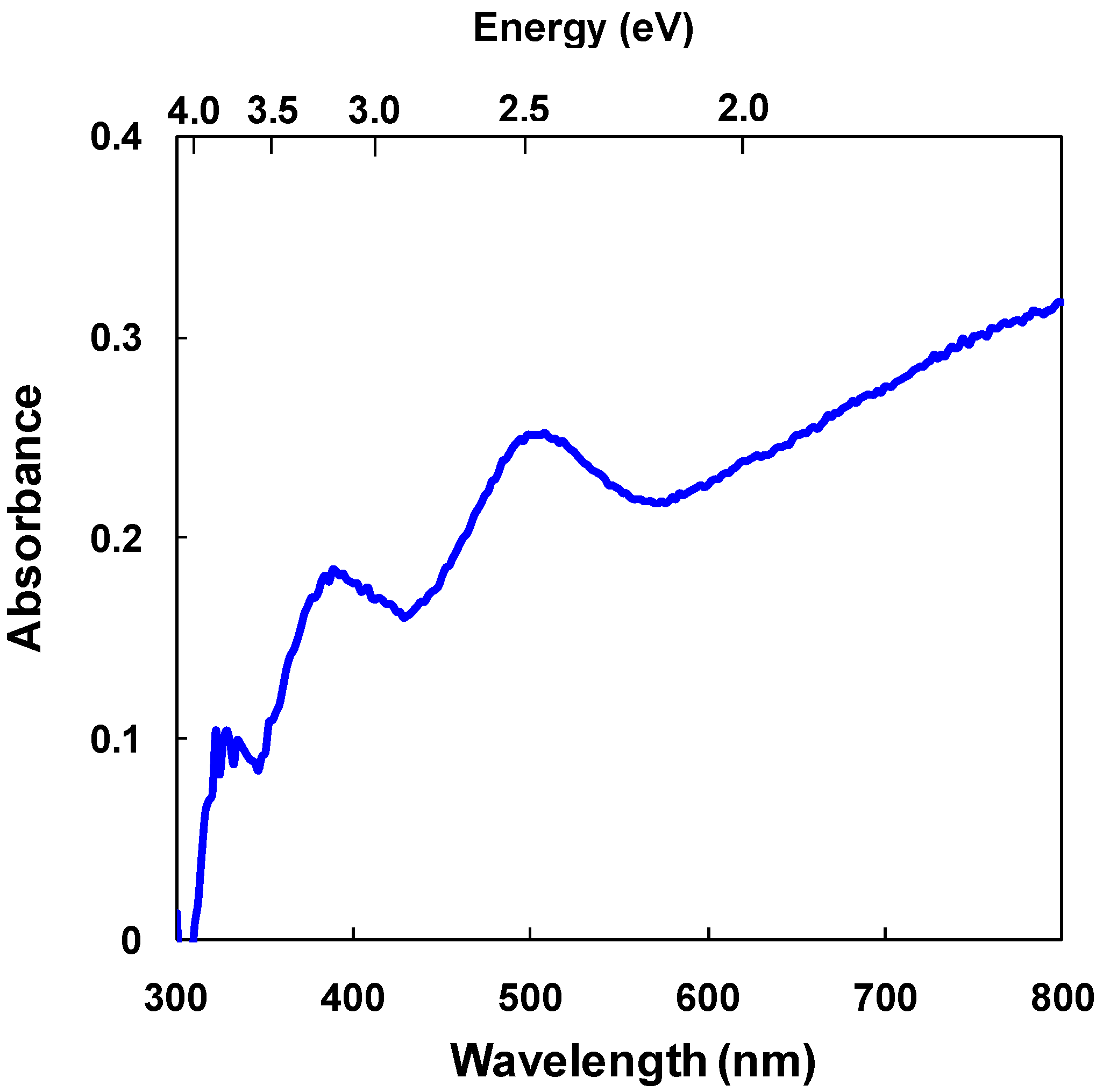
3.2. CuInS2:C60 bulk heterojunction solar cells
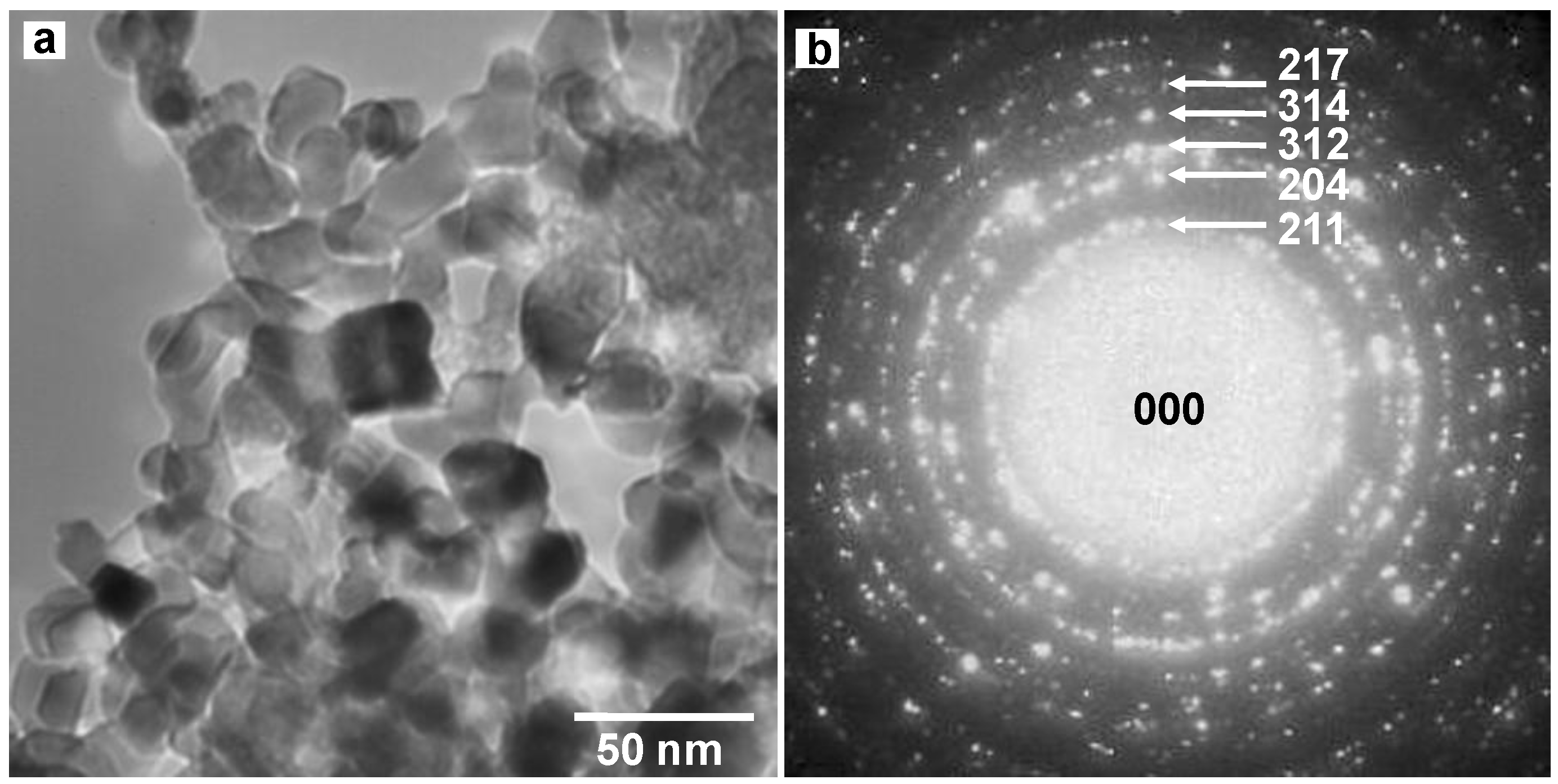
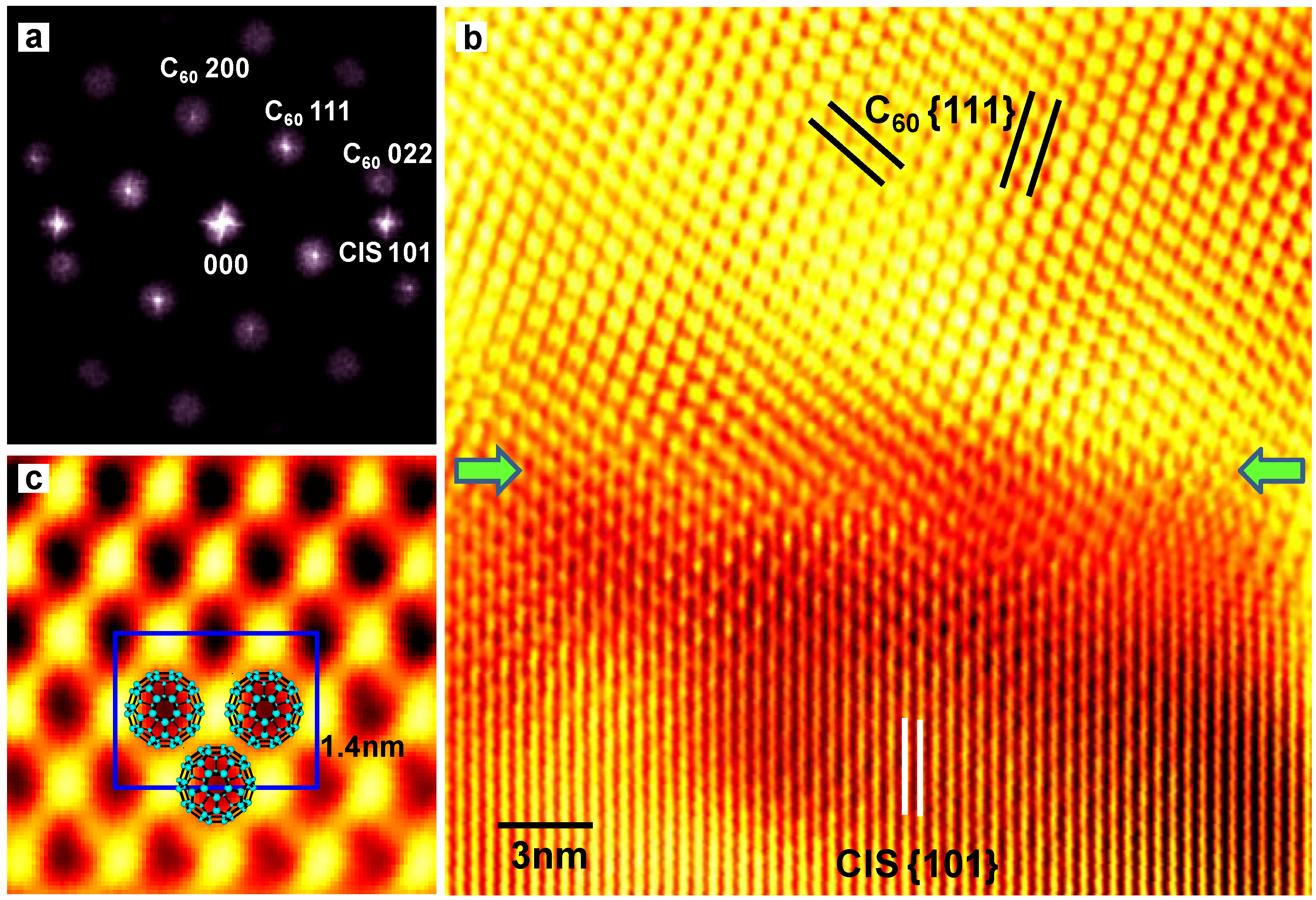
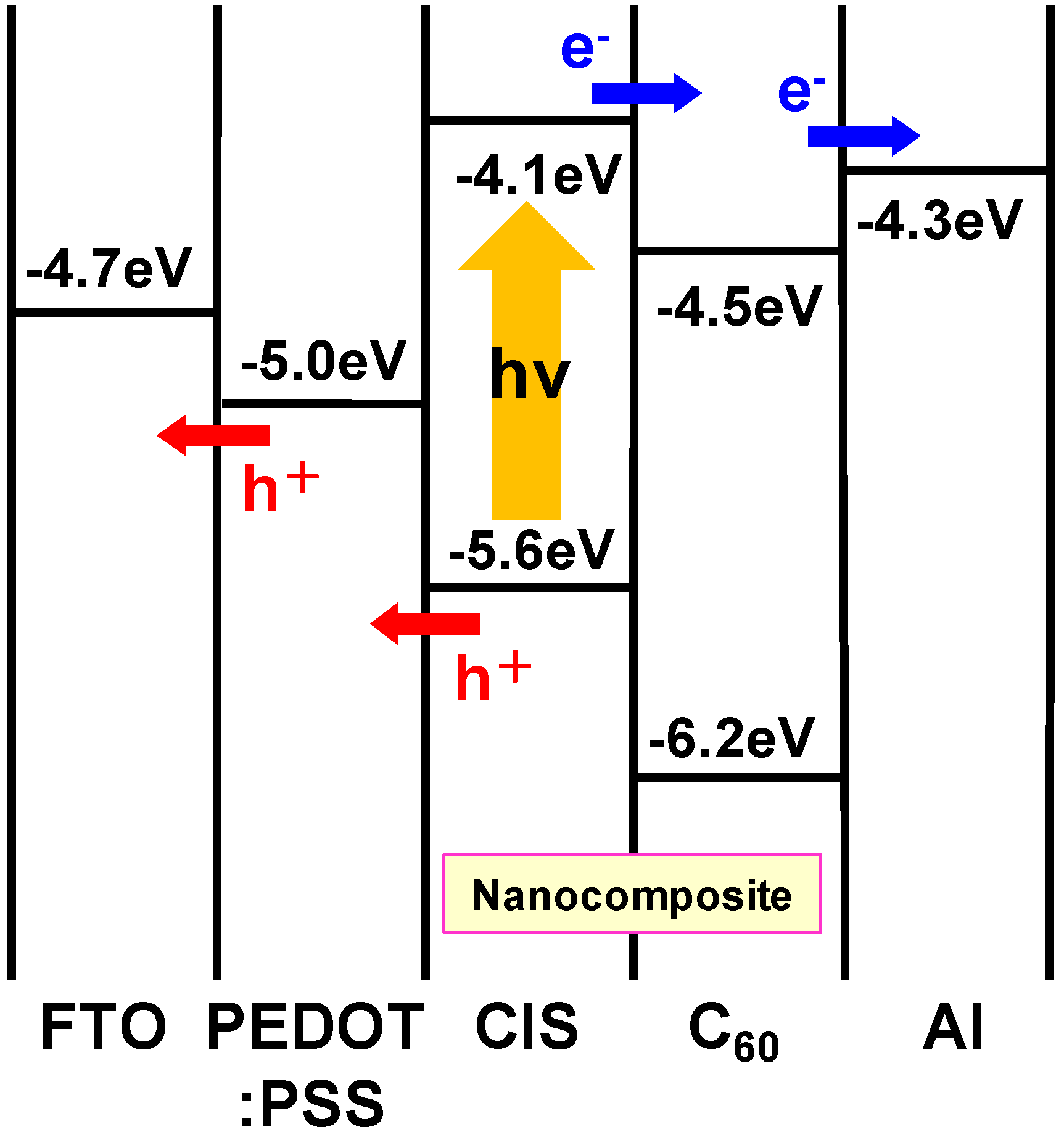
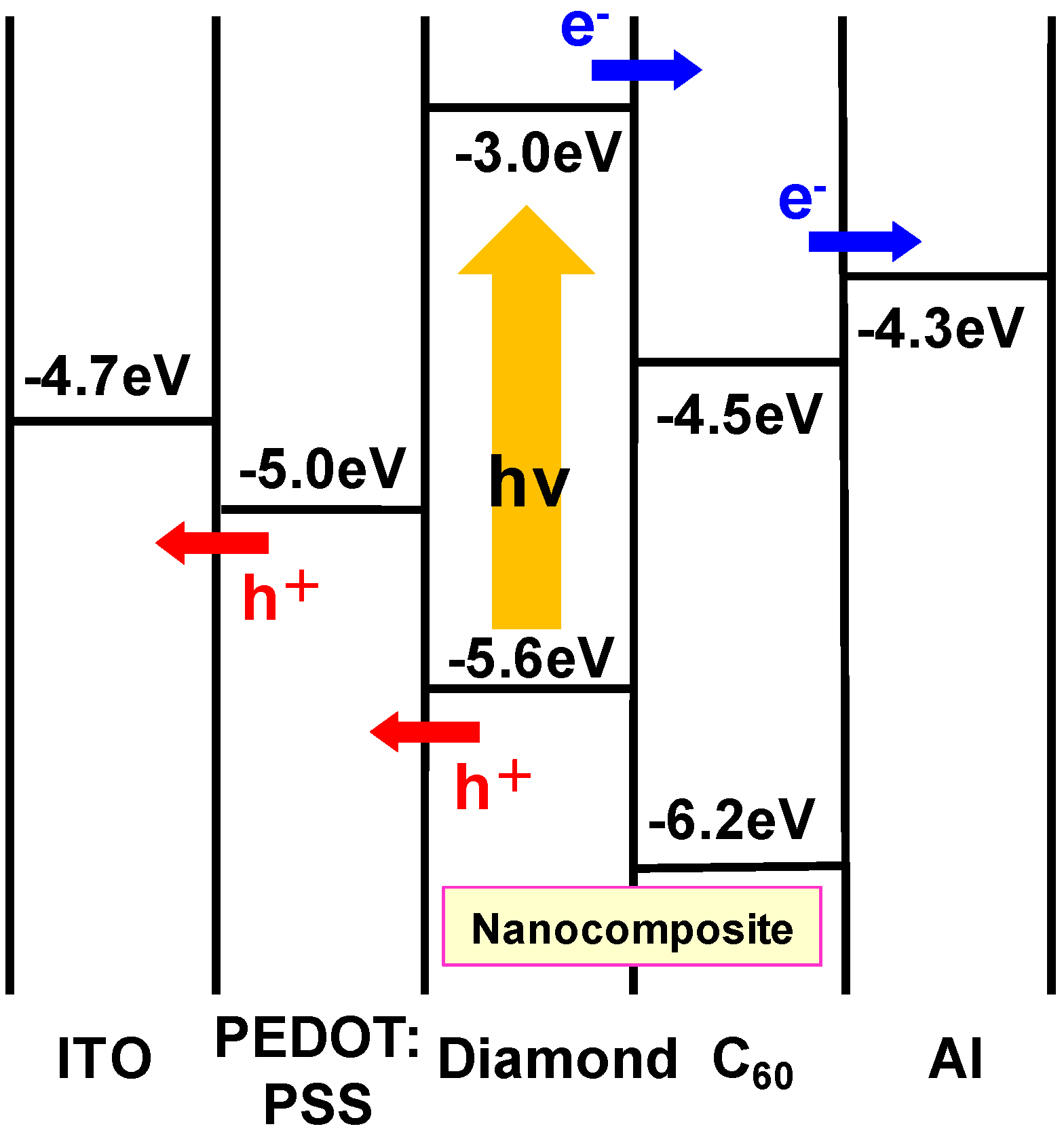
3.3. Diamond:C60 bulk heterojunction solar cells
4. Conclusions
Acknowledgements
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Oku, T.; Narita, I.; Nishiwaki, A.; Koi, N.; Suganuma, K.; Hatakeyama, R.; Hirata, T.; Tokoro, H.; Fujii, S. Formation, atomic structures and properties of carbon nanocage materials. Topics Appl. Phys. 2006, 100, 187–216. [Google Scholar]
- Oku, T.; Hirano, T.; Kuno, M.; Kusunose, T.; Niihara, K.; Suganuma, K. Synthesis, atomic structures and properties of carbon and boron nitride fullerene materials. Mater. Sci. Eng. B 2000, 74, 206–217. [Google Scholar] [CrossRef]
- Oku, T.; Kuno, M.; Kitahara, H.; Narita, I. Formation, atomic structures and properties of boron nitride and carbon nanocage fullerene materials. Int. J. Inorg. Mater. 2001, 3, 597–612. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to Buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamada, I.; Takagi, S.; Takasu, A.; Soga, T.; Jimbo, T. Influence of structure and C60 composition on properties of blends and bilayers of organic donor-acceptor polymer/C60 photovoltaic devices. Jpn. J. Appl. Phys. 2005, 44, 1296–1300. [Google Scholar] [CrossRef]
- Oku, T.; Nagaoka, S.; Suzukia, A.; Kikuchi, K.; Hayashi, Y.; Inukai, H.; Sakuragi, H.; Soga, T. Formation and characterization of polymer/fullerene bulk heterojunction solar cells. J. Phys. Chem. Solids 2008, 69, 1276–1279. [Google Scholar] [CrossRef]
- Oku, T.; Noma, T.; Suzuki, A.; Kikuchi, K.; Kikuchi, S. Fabrication and characterization of fullerene/porphyrin bulk heterojunction solar cells. J. Phys. Chem. Solids 2009, (in press). [Google Scholar]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Granström, M.; Petritsch, K.; Arias, A.C.; Lux, A.; Andersson, M.R.; Friend, R.H. Laminated fabrication of polymeric photovoltaic diodes. Nature 1998, 395, 257–260. [Google Scholar] [CrossRef]
- Oku, T.; Nagaoka, S.; Suzuki, A.; Kikuchi, K.; Hayashi, Y.; Sakuragi, H.; Soga, T. Formation and characterization of MEH-PPV/PCBM-based bulk heterojunction solar cells. J. Ceramic Processing Res. 2008, 9, 549–552. [Google Scholar]
- Yu, G.; Heeger, A.J. Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 1995, 78, 4510–4515. [Google Scholar] [CrossRef]
- Padinger, F.; Rittberger, R.S.; Sariciftci, N.S. Effects of postproduction treatment on plastic solar cells. Adv. Funct. Mater. 2003, 13, 85–88. [Google Scholar] [CrossRef]
- Oku, T.; Kakuta, N.; Kawashima, A.; Nomura, K.; Motoyoshi, R.; Suzuki, A.; Kikuchi, K.; Kinoshita, G. Formation and characterization of bulk hetero-junction solar cells using C60 and perylene. Mater. Trans. 2008, 49, 2457–2460. [Google Scholar] [CrossRef]
- Takahashi, K.; Kuraya, N.; Yamaguchi, T.; Komura, T.; Murata, K. Three-layer organic solar cell with high-power conversion efficiency of 3.5%. Solar Energy Mater. Solar Cells 2000, 61, 403–416. [Google Scholar] [CrossRef]
- Hasobe, T.; Imahori, H.; Kamat, P.V.; Ahn, T.K.; Kim, S.K.; Kim, D.; Fujimoto, A.; Hirakawa, T.; Fukuzumi, S. Photovoltaic cells using composite nanoclusters of porphyrins and fullerenes with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, L.; Zhou, X.; Li, L.; Li, Q. Bilayer- and bulk-heterojunction solar cells using liquid crystalline porphyrins as donors by solution processing. Appl. Phys. Lett. 2007, 91, 253505-1-3. [Google Scholar] [CrossRef]
- Hasobe, T.; Sandanayaka, A.S.; Wada, T.; Araki, Y. Fullerene-encapsulated porphyrin hexagonal nanorods. An anisotropic donor-acceptor composite for efficient photoinduced electron transfer and light energy conversion. Chem. Commun. 2008, 3372–3374. [Google Scholar] [CrossRef]
- Ishii, T.; Aizawa, N.; Kanehama, R.; Yamashita, M.; Sugiura, K.; Miyasaka, H. Cocrystallites consisting of metal macrocycles with fullerenes. Coordination Chem. Rev. 2002, 226, 113–124. [Google Scholar] [CrossRef]
- Konarev, D.V.; Kovalevsky, A.Y.; Li, X.; Neretin, I.S.; Litvinov, A.L.; Drichko, N.V.; Slovokhotov, Y.L.; Coppens, P.; Lyubovskaya, R.N. Design of molecular and ionic complexes of fullerene C60 with metal (II) octaethylporphyrins, MIIOEP (M = Zn, Co, Fe, and Mn) containing coordination M-N(ligand) and M-C(C60-) bonds. Inorg. Chem. 2002, 41, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Belcher, W.J.; Wagner, K.I.; Dastoor, P.C. The effect of porphyrin inclusion on the spectral response of ternary P3HT:porphyrin:PCBM bulk heterojunction solar cells. Solar Energy Mater. Solar Cells 2007, 91, 447–452. [Google Scholar] [CrossRef]
- Dastoor, P.C.; McNeill, C.R.; Frohne, H.; Foster, C.J.; Dean, B.; Fell, C.J.; Belcher, W.J.; Campbell, W.M.; Officer, D.L.; Blake, I.M.; Thordarson, P.; Crossley, M.J.; Hush, N.S.; Reimers, J.R. Understanding and improving solid-state polymer/C60-fullerene bulk-heterojunction solar cells using ternary porphyrin blends. J. Phys. Chem. C 2007, 111, 15415–15426. [Google Scholar] [CrossRef]
- Qi, Y.; Tang, K.; Zeng, S.; Zhou, W. Template-free one-step fabrication of porous CuInS2 hollow microspheres. Microporous Mesoporous Mat. 2008, 114, 395–400. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Zhao, X.; Song, M.; Gu, X. Preparation and characterization of copper indium disulfide films by facile chemical method. Mater. Sci. Eng. B 2007, 139, 88–94. [Google Scholar] [CrossRef]
- Berredjem, Y.; Karst, N.; Cattin, L.; Lakhdar-Toumi, A.; Godoy, A.; Soto, G.; Diaz, F.; Del Valle, M.A.; Morsli, M.; Drici, A.; Boulmokh, A.; Gheid, A.H.; Khelil, A.; Bernède, J.C. The open circuit voltage of encapsulated plastic photovoltaic cells. Dyes and Pigments 2008, 78, 148–156. [Google Scholar] [CrossRef]
- Arici, E.; Sariciftci, N.S.; Meissner, D. Hybrid solar cells based on nanoparticles of CuInS2 in organic matrices. Adv. Funct. Mater. 2003, 13, 165–171. [Google Scholar] [CrossRef]
- Takeda, A.; Oku, T.; Suzuki, A.; Kikuchi, K.; Kikuchi, S. Fabrication and characterization of inorganic-organic hybrid solar cells based on CuInS2. J. Ceram. Soc. Jpn. 2009, 117, 967–969. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C. Design for donors in bulk-heterojunction solar cells-towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Brédas, J.L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and energy-transfer processes in pi-conjugated oligomers and polymers: a molecular picture. Chem. Rev. 2004, 104, 4971–5004. [Google Scholar] [CrossRef] [PubMed]
- Voz, C.; Puigdollers, J.; Cheylan, S.; Fonrodona, M.; Stella, M.; Andreu, J.; Alcubilla, R. Photodiodes based on fullerene semiconductor. Thin Solid Films 2007, 515, 7675–7678. [Google Scholar] [CrossRef]
- Winkler, M.; Griesche, J.; Konovalov, I.; Penndorf, J.; Wienke, J.; Tober, O. CISCuT-solar cells and modules on the basis of CuInS2 on Cu-tape. Solar Energy 2004, 77, 705–716. [Google Scholar] [CrossRef]
- Korobova, M.V.; Avramenko, N.V.; Bogachev, A.G.; Rozhkova, N.V.; Ōsawa, E. Nanophase of water in nanodiamond gel. J. Phys. Chem. C 2007, 111, 7330–7334. [Google Scholar] [CrossRef]
- Barnard, A.; Sternberg, M. Crystallinity and surface electrostatics of diamond nanocrystals. J. Mater. Chem. 2007, 17, 4811–4819. [Google Scholar] [CrossRef]
- Ōsawa, E.; Ho, D.; Huang, H.; Korobov, M.V.; Rozhkova, N.N. Consequences of strong and diverse electrostatic potential fields on the surface of detonation nanodiamond particles. Diam. Rel. Mater. 2009, 18, 904–909. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; King, D.L.; Hishikawa, Y.; Warta, W. Solar cell efficiency tables. Photovolt. Prog. Res. Appl. 2006, 14, 455–461. [Google Scholar] [CrossRef]
- Oku, T.; Narita, I.; Koi, N.; Nishiwaki, A.; Suganuma, K.; Inoue, M.; Hiraga, K.; Matsuda, T.; Hirabayashi, M.; Tokoro, H.; Fujii, S.; Gonda, M.; Nishijima, M.; Hirai, T.; Belosludov, R.V.; Kawazoe, Y. Boron nitride nanocage clusters, nanotubes, nanohorns, nanoparticles, and nanocapsules. In B-C-N Nanotubes and Related Nanostructures; Yap, Y.K., Ed.; Springer: New York, NY, USA, 2009; pp. 149–194. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oku, T.; Takeda, A.; Nagata, A.; Noma, T.; Suzuki, A.; Kikuchi, K. Fabrication and Characterization of Fullerene-Based Bulk Heterojunction Solar Cells with Porphyrin, CuInS2, Diamond and Exciton-Diffusion Blocking Layer. Energies 2010, 3, 671-685. https://doi.org/10.3390/en3040671
Oku T, Takeda A, Nagata A, Noma T, Suzuki A, Kikuchi K. Fabrication and Characterization of Fullerene-Based Bulk Heterojunction Solar Cells with Porphyrin, CuInS2, Diamond and Exciton-Diffusion Blocking Layer. Energies. 2010; 3(4):671-685. https://doi.org/10.3390/en3040671
Chicago/Turabian StyleOku, Takeo, Akihiro Takeda, Akihiko Nagata, Tatsuya Noma, Atsushi Suzuki, and Kenji Kikuchi. 2010. "Fabrication and Characterization of Fullerene-Based Bulk Heterojunction Solar Cells with Porphyrin, CuInS2, Diamond and Exciton-Diffusion Blocking Layer" Energies 3, no. 4: 671-685. https://doi.org/10.3390/en3040671





