1. Introduction
Bio-oils derived from the pyrolysis of biomass or Fischer–Tropsch processes are considered as one of the major future renewable energy sources [
1,
2,
3,
4]. The study of the thermo-mechanical and physical properties of bio-oil, biodiesel, and other liquid bio-oils is therefore very important for obtaining the fundamental characteristics of bio-oil combustion. Due to their high viscosities and larger water contents, effective atomization of bio-oils has been however difficult and faces challenges in powering direct injection (DI) engines [
5,
6,
7]. In order to increase the distribution of fine droplets over the entire reaction zone, the electrostatic atomization method is of great interest. In addition to its application in fuel atomization for gas turbines and internal combustion engines, electrostatic atomization also could be used in other spray systems, e.g., in pesticide processing, industrial painting, film preparation, and ink-jet painting. The implementation of electrostatic atomization can separate a bio-oil stream into fine droplets with a narrow size distribution. This process does not require a high air pressure like conventional atomization techniques do (e.g., 20 MPa for an indirect injection system and up to about 140 MPa for a DI one). When electrostatic force is utilized to break up a bulk liquid into fine and mono-dispersed droplets, the motion and trajectories of these droplets can be controlled by regulating the applied electrical voltage. Moreover, this process can be optimized with relative ease to achieve the desired heat and mass transfer rates. Since the droplets are highly charged in the electrical field, the Coulombic repulsion prevents their agglomeration and this results in a more uniform space distribution of droplet sizes.
In the past 30 years, fuel electrostatic atomization technologies have been developed for diesel, hydrocarbons, and ethanol [
8,
9,
10,
11,
12,
13]. Several researchers have extensively designed and tested several kinds of atomizers to comprehend the effects of parameters such as the nozzle geometry, electrode style, location relative to the grounded surface, orifice size, fuel viscosity, and other physical properties on the specific charge of the resulting spray [
8,
9,
14,
15,
16,
17]. However, electrostatic atomization of bio-oils, as one of the most important and best alternative bio-energy forms, has not been broadly investigated, and atomization of some vegetable and corn oils has been only studied in the rare literature on the topic [
13,
18]. The breakup mechanism of the electrostatic atomization for diesel, kerosene and hydrocarbons was thoroughly investigated in previous work [
19,
20,
21,
22,
23,
24,
25,
26]. Although the previous excellent work has determined the highest possible specific charge and the theoretical interpretation of the breakup mechanism(s) of hydrocarbons using the Rayleigh limit or other energy conservation methods for diesel and other hydrocarbons have been done, the current literature does not contain fundamental understanding of the electrostatic breakup mechanism of bio-oils in sprays.
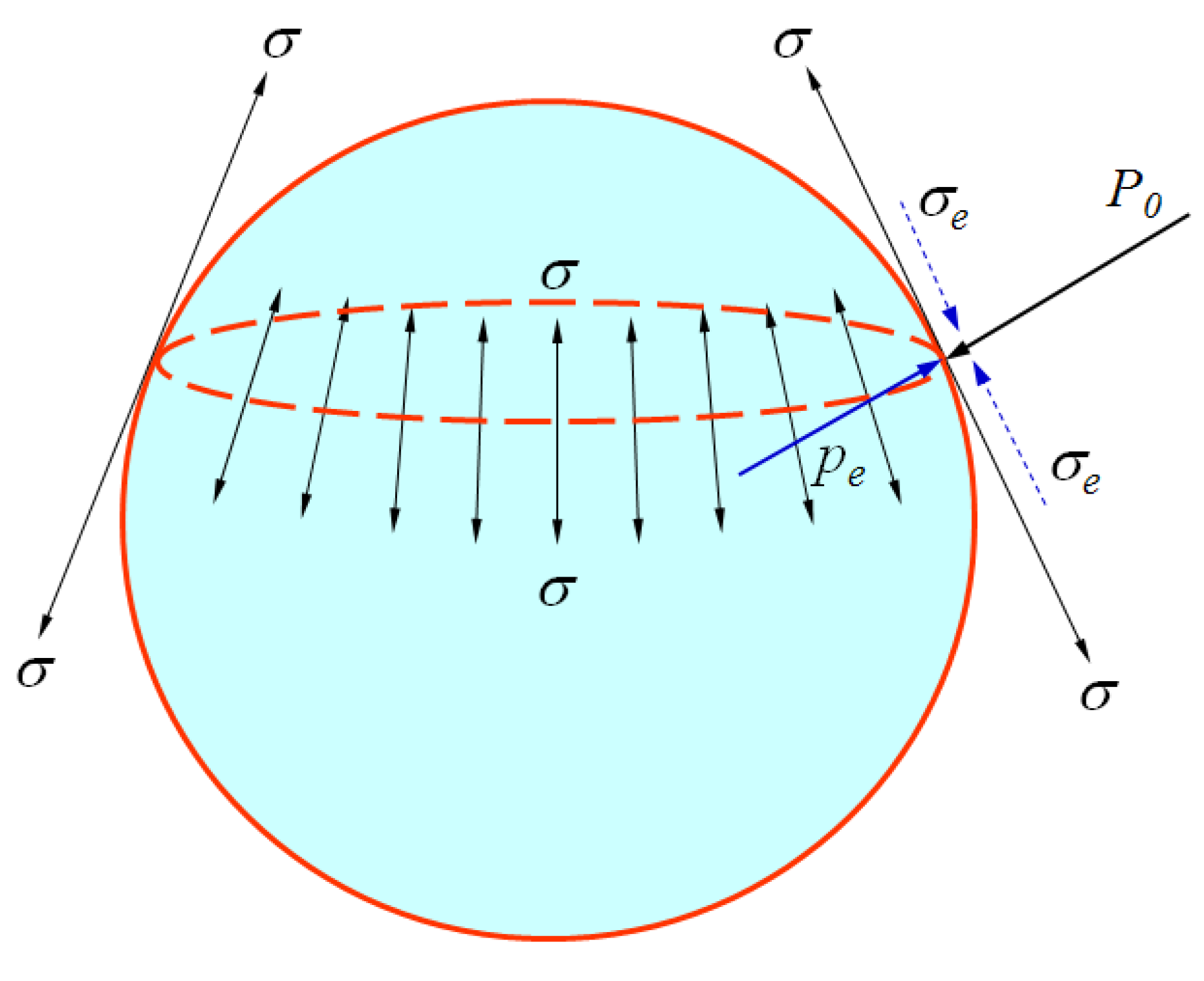
In this work, a theoretical and experimental investigation on the breakup mechanism for biodiesel droplets under different critical instability conditions, which are the bag and strip breakup instability modes, was carried out. This paper aims to interpret the electrostatic breakup mechanism for a single droplet which possesses net charges on the whole surface or partial areas using the Rayleigh instability condition. The parameters controlling the droplet breakup process are identified and correlations among the droplet size distribution, droplets’ specific charges and applied electrostatic voltages are quantified. Experimental results are compared with the theoretical findings.
3. Analysis of the Critical Specific Charges on the Droplet
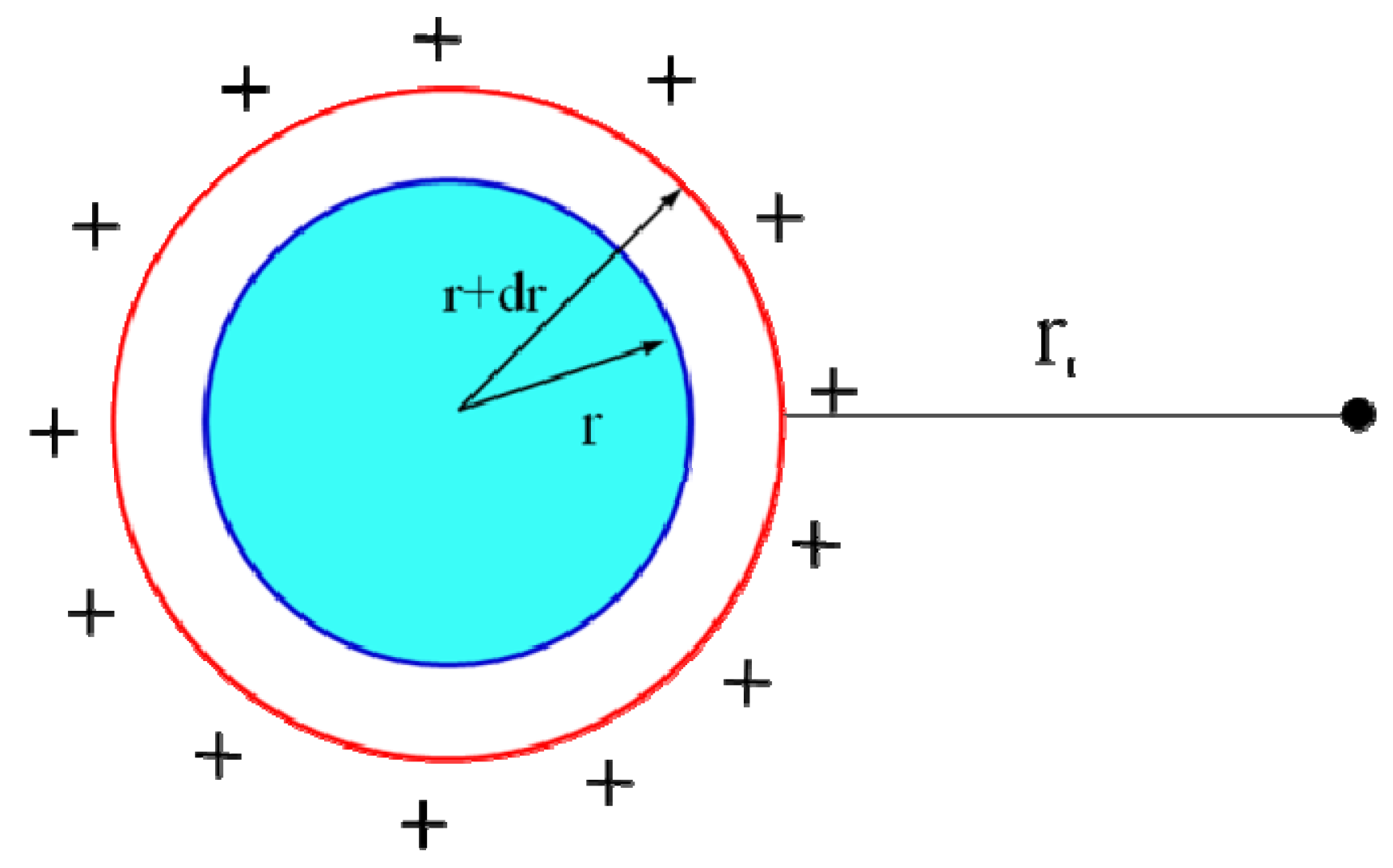
When the specific charge of a droplet exceeds the critical value, atomization will certainly happen. Generally speaking, the specific charges are used to represent the atomization capacity of the droplets. In the model of Equations (14) and (15), which are derived from the Rayleigh instability condition, these factors which can determine the atomization process in an externally applied electrostatic field are identified as the amount of surface charges or the specific charges, original droplet size, bio-oil surface tension, surrounding medium gas density, temperature and droplet velocity. In this paper, for biodiesel, the critical specific charges were obtained from different initial droplet sizes, temperatures, and for standing-still and moving droplets by adopting Equations (14) and (15). The other parameters like some calculated constants and liquid properties for biodiesel during atomization shown in
Table 1 were used [
29]. The gas mass density, viscosity and biodiesel density were calculated by considering their temperature dependences [
30].
Table 1.
Calculated constants and liquid properties for biodiesel.
Table 1.
Calculated constants and liquid properties for biodiesel.
| Density (kg/m3) | Surface tension (N/m) | Critical temperature (K) | Permittivity of air (C2/N∙m2) | Conductivity (s/m) |
|---|
| 880.20 | 0.02635 | 780.00 | 8.85 × 10−12 | 4.06 × 10−10 |
where
p is the international normal atmosphere and set to 101325 Pa,
T the absolute temperature,
Ф the coefficient of temperature, and
T0 = 273.15 K. The specific charge was calculated for different droplet sizes for various temperatures and droplet velocities. The results are shown in
Figure 4 and
Figure 5, using the stability criteria for bag and strip break-up modes, respectively.
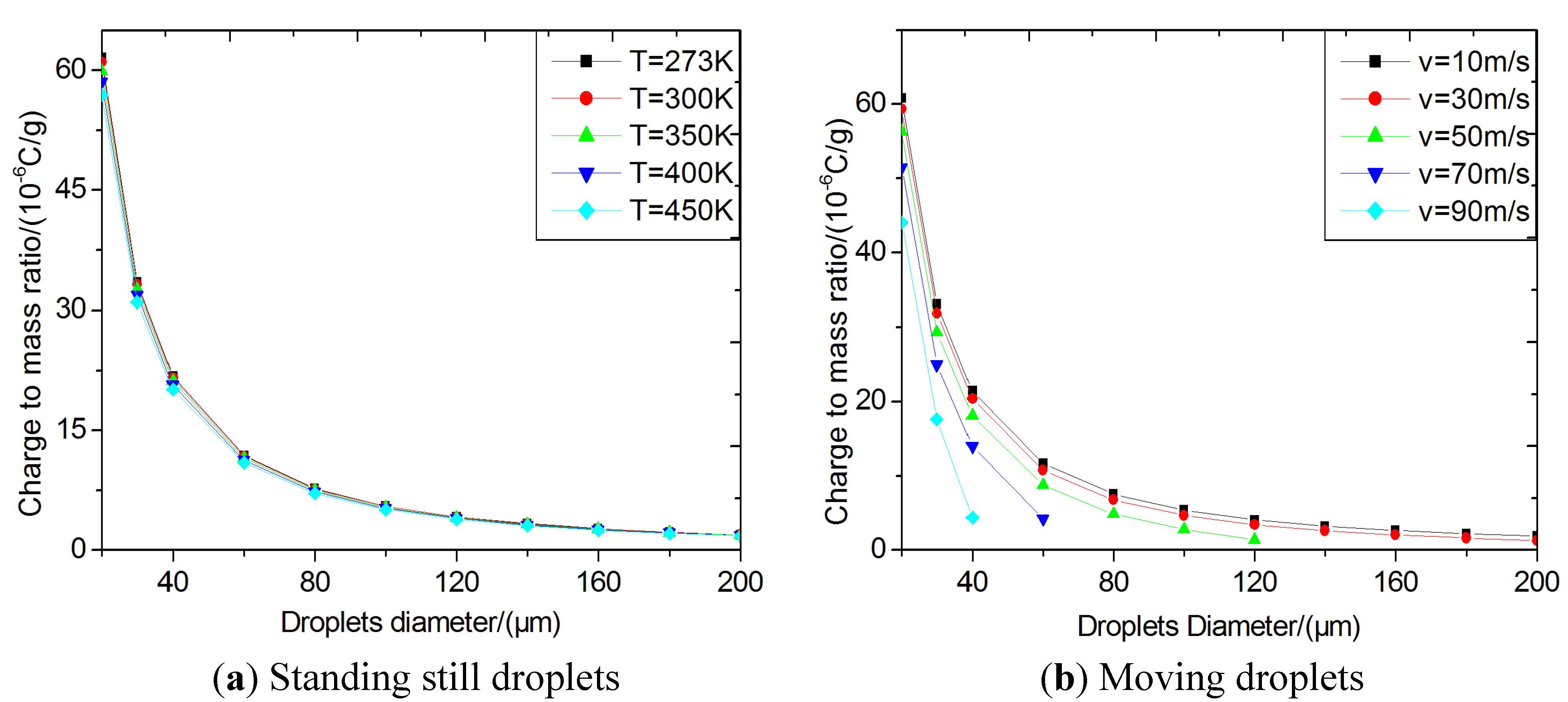
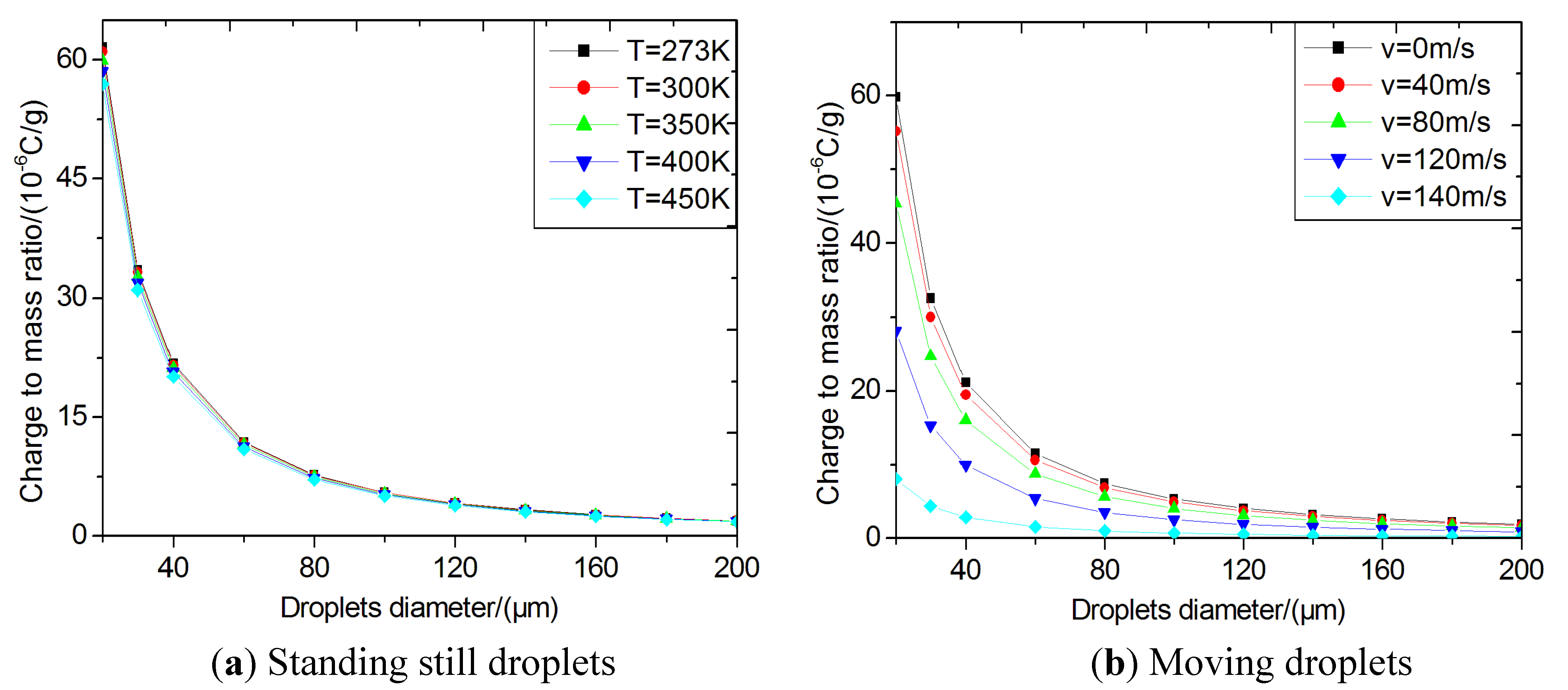
Figure 4(a) shows the specific charges of the standing-still droplets with different sizes and varying temperatures. The critical specific charges of droplets decrease with increasing droplet size and temperature and the temperature dependence becomes insignificant when the droplet size become larger.
Figure 4(b) shows the specific charges of moving droplets at
T = 300 K. The critical specific charges decrease with increasing the droplet velocity for all droplet diameters, indicating that the aerodynamic force is another important factor influencing the breakup process.
Figure 4.
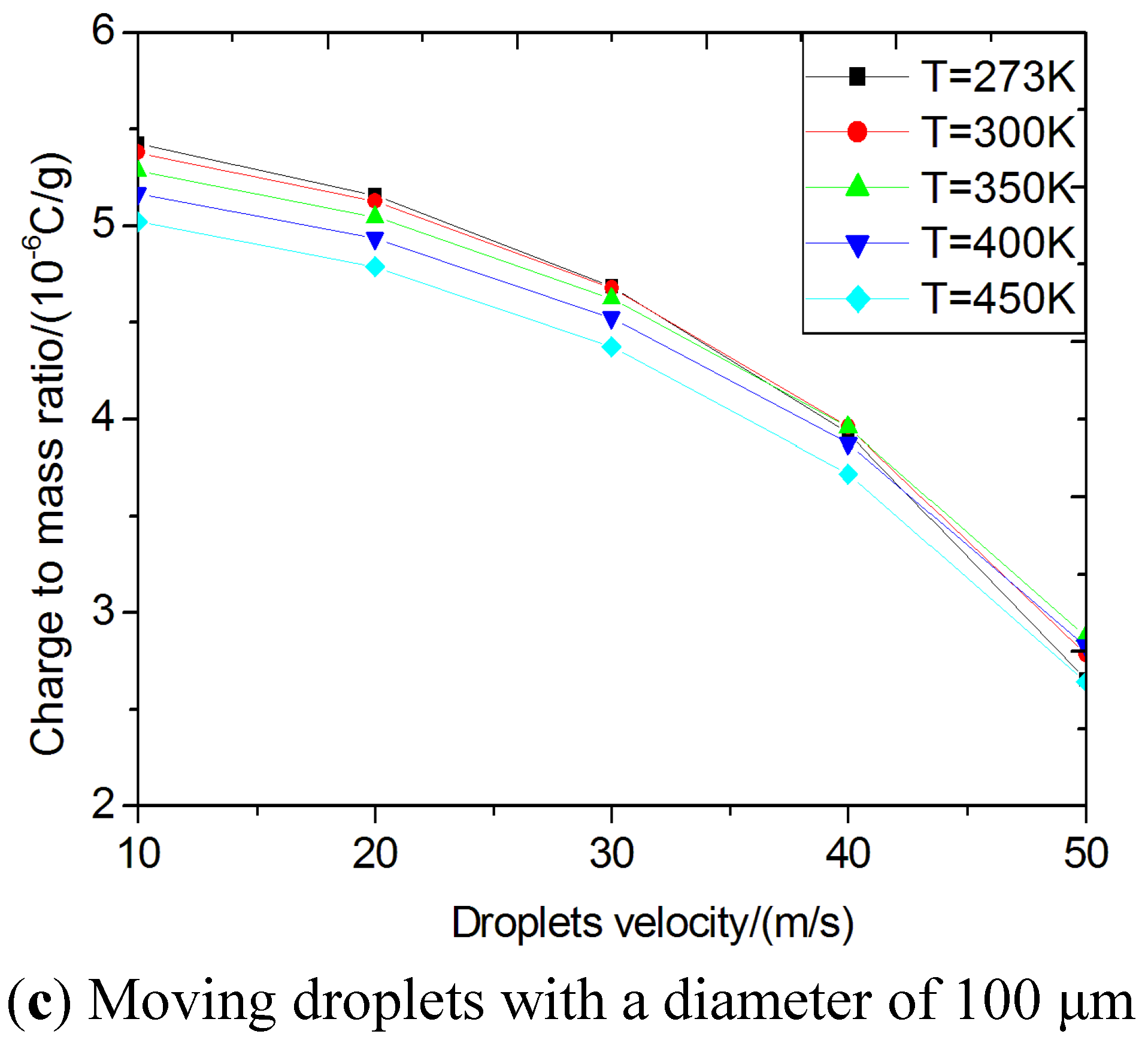
Critical specific charges using the stability criteria for bag break-up mode. (a) standing still droplets; (b) moving droplets; (c) moving droplets with a diameter of 100 μm.
Figure 4.
Critical specific charges using the stability criteria for bag break-up mode. (a) standing still droplets; (b) moving droplets; (c) moving droplets with a diameter of 100 μm.
Figure 4(c) shows the calculated critical specific charges of moving droplets at different temperatures for a droplet of 100 μm in diameter. In general, smaller sized droplets are difficult to break up due to their higher critical specific charges corresponding to the Rayleigh instability condition. In this paper, the Laplace pressure (surface additional pressure) has been considered. Similarly, a lower velocity or temperature will make atomization more difficult. As shown in
Figure 4, the calculated critical specific charges of normal biodiesel droplets in DI engines are on the order of magnitude of 10
−6 C/g and 10
−7 C/g for bigger drops.
Figure 5(a) shows the specific charges of the standing-still droplets with different sizes and for varying temperatures and is the same with
Figure 4(a).
Figure 5.
Critical specific charges using the stability criteria for strip break-up mode. (a) standing still droplets; (b) moving droplets; (c) moving droplets with a diameter of 100 μm.
Figure 5.
Critical specific charges using the stability criteria for strip break-up mode. (a) standing still droplets; (b) moving droplets; (c) moving droplets with a diameter of 100 μm.
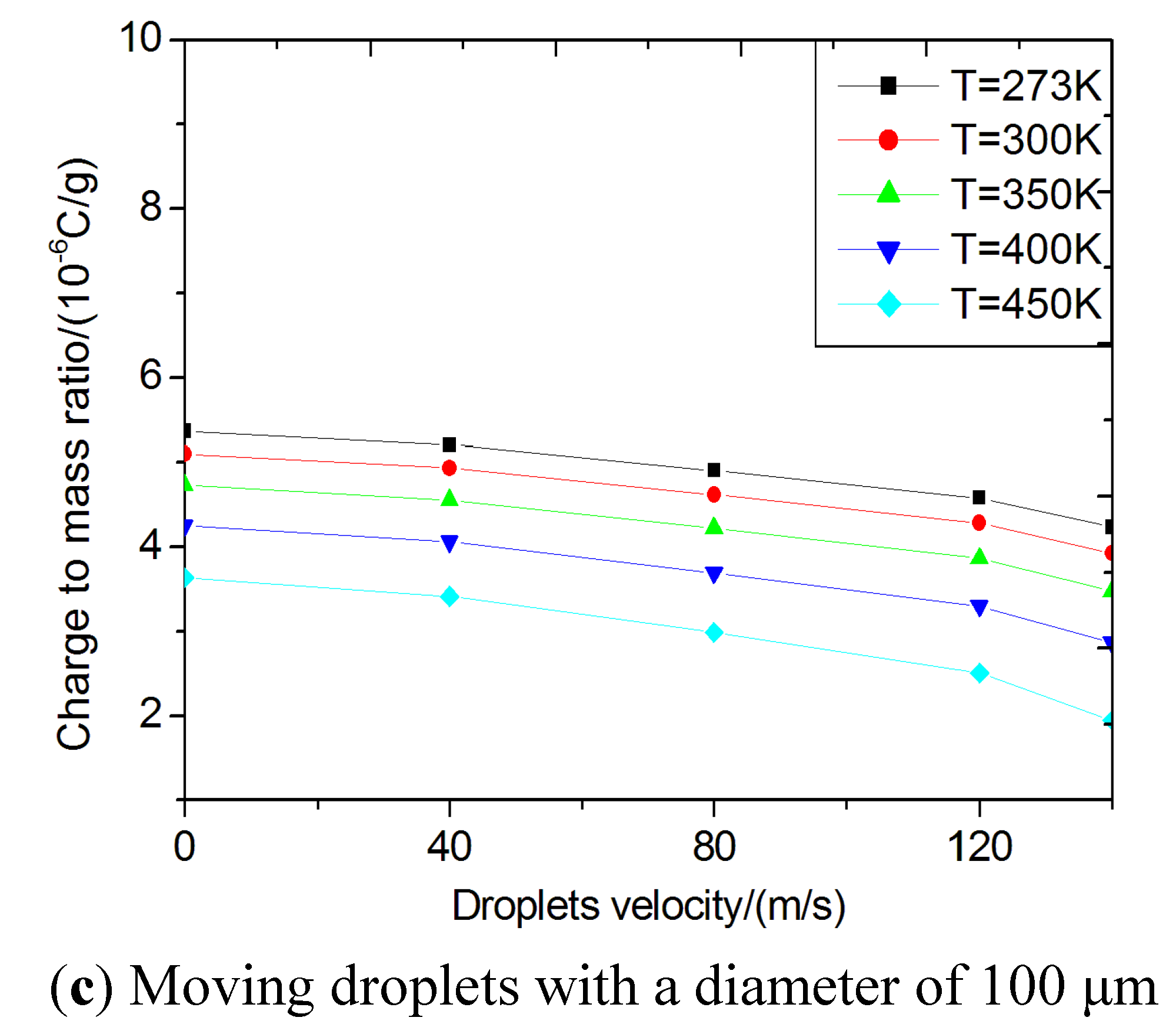
The critical specific charges of droplets usually decrease with increasing the droplet size and temperature. The effect of temperature on the critical specific charge is very small for all the droplets, as shown in
Figure 5(a).
Figure 5(b) shows the specific charges of moving droplets at
T = 300 K. The critical specific charges decrease with increasing the droplet velocity for all droplets, indicating that the aerodynamic force is another factor affecting the breakup process, but in strip breakup mode the effect of aerodynamic force on the droplets’ breakup is smaller than in the bag breakup model.
Figure 5(c) shows the critical specific charges of moving droplets at different temperatures for a droplet of 100 μm in diameter. In strip breakup mode the effect of temperature on the droplet breakup is larger than in the bag breakup model. In general, smaller sized droplets are difficult to break up due to their need for higher critical specific charges corresponding to the Rayleigh instability condition. In the strip breakup mode, the calculated critical specific charges of biodiesel droplets are also on the order of magnitude of 10
−6 C/g for smaller drops and 10
−7 C/g for larger ones. The critical specific charges are on the same order of magnitude as for the bag mode. Comparing the two different breakup modes, we found the bag breakup model just considered the effect of
We number for droplet breakup, while the strip breakup mode took both the
We and
Re numbers into account.
In this paper, the effect of evaporation on droplet size was not taken into account, because in the real combustion process in powering DI energies, the lifetime of unstable drops is usually very short relative to their evaporation and momentum time scales, corresponding to both bag and strip breakup modes. In the breakup theory of the Reitz and Diwakar secondary atomization mode, the lifetimes of unstable drops are:
respectively, where
Bt =
π and
St = 1.0, corresponding to the bag and strip breakup modes, respectively [
28].
Figure 6 shows the lifetime of unstable droplets in internal combustion engines. It is clearly found that the lifetime of unstable droplets is less than 0.5 ms for both droplet breakup modes. For the bag breakup mode, for the droplet diameters which could be formed in powering real DI engines, the lifetime is increasing with varying droplet diameters and the effect of temperature on lifetime is unobvious. For the strip breakup mode, the lifetimes of unstable droplets is less than 0.25 ms, when the droplet velocity is 10.0 m/s; while it is less than 0.025 ms, when the droplet velocity is 100 m/s. It is noted that the breakup timescales are much shorter than their momentum and evaporation timescales for the drops themselves, so the effect of evaporation could be ignored in this paper.
Figure 6.
The lifetimes of unstable drops for two breakup mode: (a) bag breakup mode; (b) strip breakup mode.
Figure 6.
The lifetimes of unstable drops for two breakup mode: (a) bag breakup mode; (b) strip breakup mode.
4. Experimental Investigations of Biodiesel Droplet Breakup
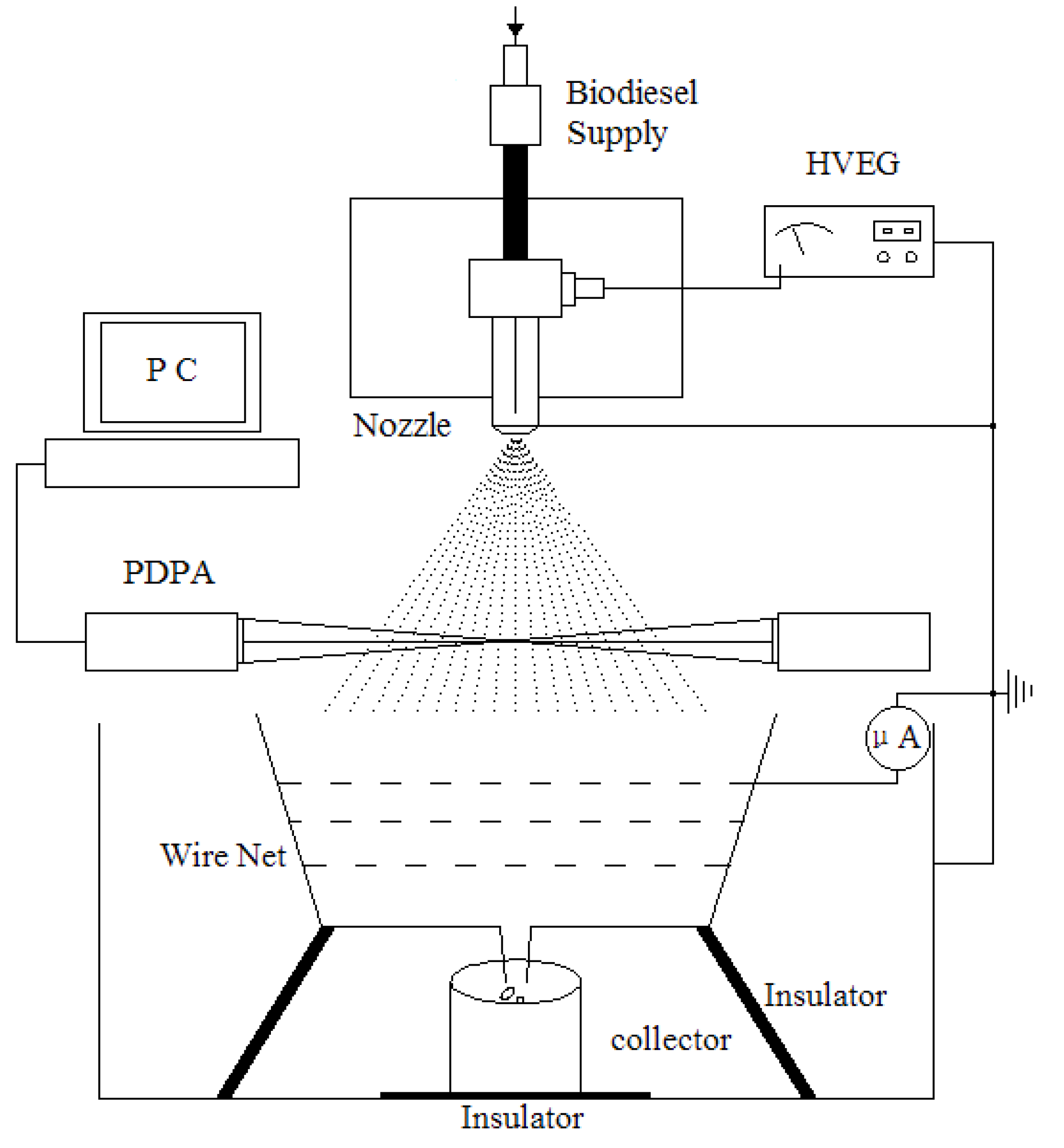
In order to validate the theoretically derived breakup modes shown as Equations (14) and (15), the droplet size and specific charges during the processes of electrostatic atomization were measured. Two data sets were simultaneously characterized using PDPA (Phase Doppler Particle Analyzer) measurements. The design of the atomization nozzle is shown in
Figure 7.
Figure 8 shows the experimental apparatus for PDPA and specific charge (using the Faraday cage principle) measurements. The measurement parameters can be found in
Table 2. In this work, in order to detect the average droplet diameter distribution by varying the electrostatic high voltages, PDPA systems were used to measure the droplet sizes online. The measurement point lies in the center line and the distance between the nozzle and measurement point is set to 180 mm. In this study, we do not obtain the size distribution in space due to the narrow width of the jet spray and not getting enough number drops in the margin of jet spray in this situation. The drop size is just used to interpret the electrostatic breakup in the secondary atomization. Under these experimental conditions, the pressure of 0.25 MPa seems quite low compared to that from modern diesel engine injection systems. But in this paper, the purpose of this experiment is to discuss the effects of the electrostatic force on droplet breakup, so we artificially make the pressure of the air-compressor equal to 0.25 Mpa, which could atomize the liquid. The average specific charge was measured by the Faraday cage principle (as in
Figure 8). This equipment is composed of a conducting material, wire net, collector, insulator and exact ammeter. The equipment cage is linked with the ground by a wire. When the biodiesel drops enter the cage, they can be captured by the wire net, and the charges on these droplets will transfer to the ground to produce a tiny electric current. The specific charge could then be obtained [
31].
Table 2.
Experimental conditions for characterizing the electrostatic atomization of biodiesel.
Table 2.
Experimental conditions for characterizing the electrostatic atomization of biodiesel.
| Room temperature (K) | Relative humidity (%) | Nozzle pressure (MPa) | Needle diameter (mm) | Nozzle aperture (mm) |
|---|
| 298.15 | 72.2 | 0.25 | 2.5 mm | 1.0 mm |
Figure 7.
Schematic of the atomizer nozzle.
Figure 7.
Schematic of the atomizer nozzle.
Figure 8.
Electrodes, PDPA and the Faraday cage.
Figure 8.
Electrodes, PDPA and the Faraday cage.
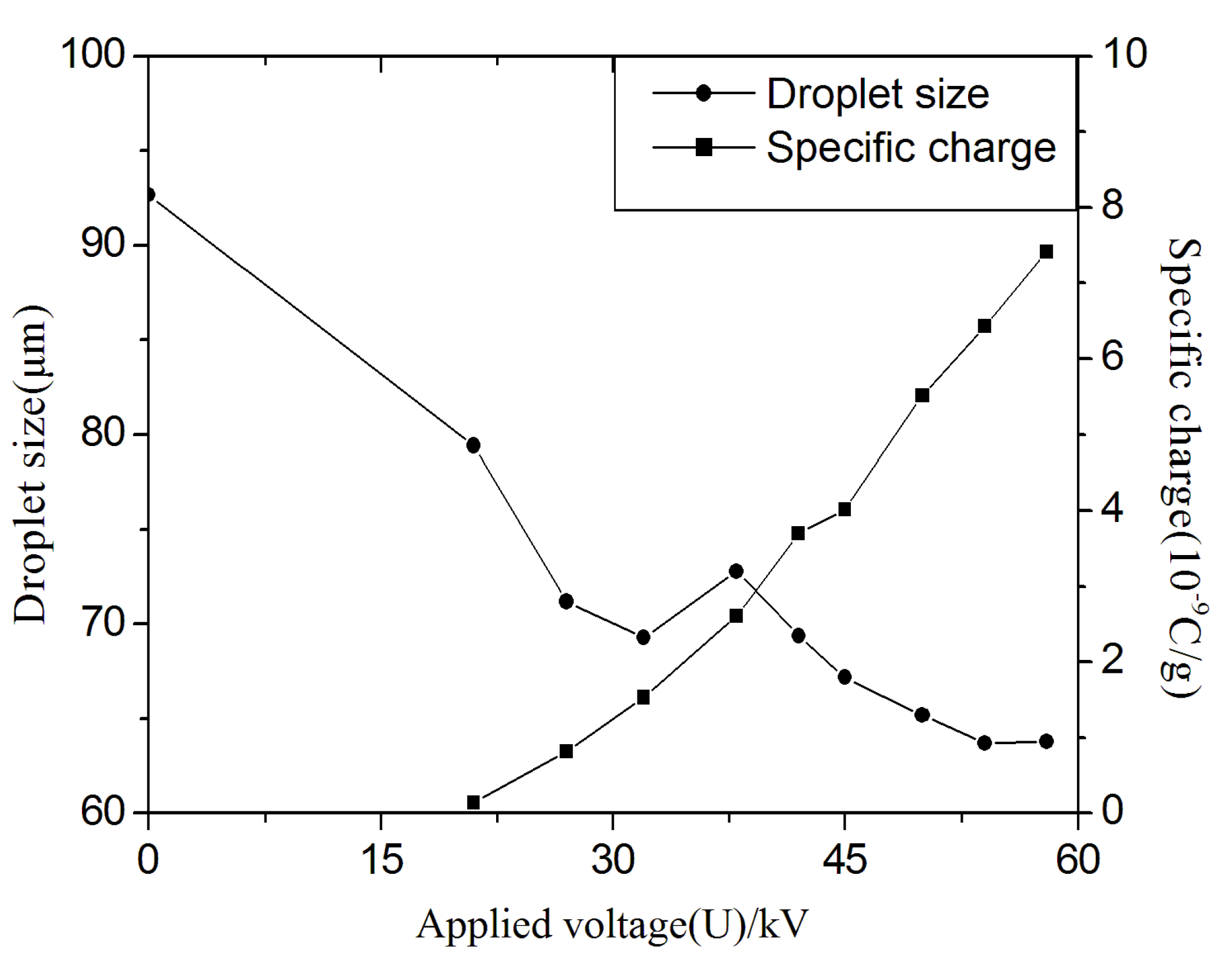
Figure 9 shows the measured correlations among the droplet size, applied voltage and specific charges for biodiesel. As a whole, it was found that by increasing the applied voltage, the droplet size decreases while the droplet specific surface charges increase. Recalling the previously derived breakup mechanism, when the surface tension of the droplet cannot balance the electrostatic stress, the droplet breaks up into much finer droplets. The critical specific surface charges calculated for biodiesel are on the order of magnitude of 10
−7 and 10
−6 C/g for different normal original droplet sizes with bag and strip breakup modes, respectively, while the measurements shown in
Figure 9 are on order of magnitude of 10
−9 C/g and 10
−8 C/g if the voltage is high enough. This would argue against the validity of the Rayleigh instability conditions in describing biodiesel breakup. There exist several explanations for the discrepancy between experimental and theoretical results. The specific charges were measured on droplets after they disintegrated. In electrostatic atomization, droplets formed from secondary breakup will acquire much more surface tension energy and this energy comes from the electrical energy which the original droplet held. The electrical energy will be reduced because of the increasing surface and total energy loss due to discharge towards the surrounding media. In a non-uniform electrical field, the droplets will be polarized and hold negative and positive charges on the opposite poles. Consequently, on the pole area, the surface charge density is higher than on the droplets’ equator area. The surface tension may be less than the electrostatic stress, because the specific charge on this area may higher than the critical charge or measured value. A droplet may be disintegrated into two or more finer drops beginning from one of the pole areas. On the other hand, the shape of the droplet tends to deform into a flat and long ellipsoid due to the polarization force which is generated by a non-superfluous (non-excess) polarization phenomenon. This process is known as the Taylor instability condition. The simplified model derived from the Rayleigh instability condition did not take this Taylor effect into account. Note that the Taylor effect becomes much more significant when the external electrical field becomes stronger and in the theoretical analysis the external field was not considered. When the length of the semi-major axis of the ellipsoid is longer than 1.9 times that of the semi-minor axis, the droplet breakup would happen. Finally, some parameters may be arguable for theoretical calculations and experimental errors exist because the liquid biodiesel has a very low electrical conductivity and the total surface charges collected by the cage could be significantly different from the theoretical values.
Figure 9.
Correlations among the droplets size, applied voltage and specific surface charges.
Figure 9.
Correlations among the droplets size, applied voltage and specific surface charges.
From the experimental results in
Figure 9, it is noted that although the droplet size variation is decreasing overall, the decrease tendency of the droplets’ size is non-monotonic. It is clearly found that the drop size at an applied voltage of 38 kV has a peak value not seen at other applied voltages. This special phenomenon will usually happen during the electrostatic atomization process. In fact, when the applied voltage is lower than 20 kV, the droplets have no net charge and the droplets’ sizes decrease with increasing voltage, and the jet from the nozzle could disintegrate into smaller droplets than in an uncharged situation, due to the existing external electrical field which could increase the disturbance or instability of the jet spray. The electrostatic force increases the disturbance on the jet flow, while on the contrary, uncharged atomization excludes the disturbance caused by electrostatic effects. It is well known that electrostatic atomization will produce much finer drops. When the applied voltage is lower than 32 kV, the very tiny drops are restricted in the main jet flow because the polarization force is not enough to dramatically change the drop motion. When the voltages further increase, many more satellite drops will be produced. The very tiny droplets could be pulled out from the main jet flow area due to the existence of a non-uniform field and flow entrainment, so the number of tiny drops would dramatically decrease in the main jet spray area, and the average size of droplets measured at the same point will increase with an increased applied voltage. When the applied voltage is higher than 38 kV, the droplets will produce secondary atomization due to the Taylor and Rayleigh instability conditions, so in the measurement area, the larger droplets will go on disrupting into smaller drops to maintain the requirements of stable state and the average size of drops will further decrease with increasing applied voltage.
5. Conclusions
These analyses of a few key factors which control the breakup process of biodiesel droplet for the instability criteria for the bag and strip breakup modes were carried out by means of the Rayleigh instability condition. These factors were identified as the original diameter of droplets, applied voltage, specific charge, surface tension force, droplet velocities, and electrical properties of biodiesel such as its dielectric constant and electrical conductivity. The critical specific surface charge was calculated using the Rayleigh instability condition and found being on the order of magnitude of 10−6–10−7 C/g for different original droplet sizes for both the bag and strip breakup modes. In order to validate this data, the droplet size and specific charges were measured simultaneously using a PDPA measurement system and the Faraday cage principle, respectively. The measured specific surface charge was on the order of magnitude of 10−9 C/g which is much smaller than the calculated value. The average droplets diameter is generally decreasing with increasing the voltage, and this decreasing tendency is not monotonic due to produced satellite drops which were dramatically affected by the externally applied non-uniform field and flow entrainment. Some factors such as the energy transformation from the electrical energy to the surface energy, energy loss, Taylor instability breakup mode, non-excess polarization, and system errors were proposed to account for these discrepancies.