Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process
Abstract
:1. Introduction
2. Results and Discussion
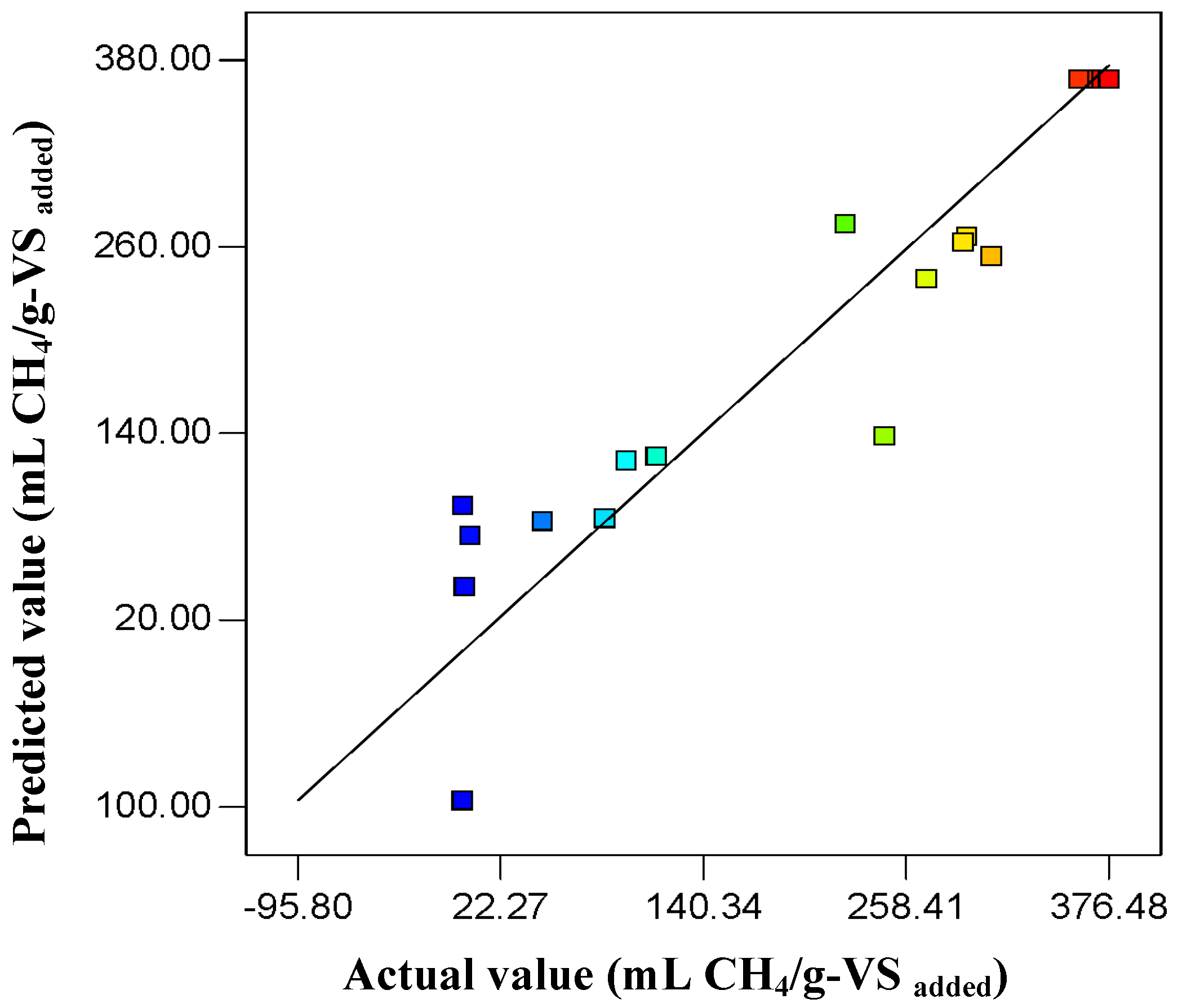
2.1. Statistical Analysis and the Diagnostic Checking of the Fitted Model
| Run | Substrate concentration (mg-COD/L) | NaHCO3 to substrate ratio | Initial pH | MY (mL CH4/g-VS added) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | Actual | Code | Actual | Code | Actual | Observed | Predicted | |||
| 1 | −1.00 | 10,000 | −1.00 | 2.00 | 1.00 | 8.5 | 270 | 270 | ||
| 2 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 364 | 366 | ||
| 3 | −1.00 | 10,000 | 1.00 | 6.00 | −1.00 | 5.5 | 95 | 97 | ||
| 4 | 0.00 | 15,000 | −1.68 | 0.60 | 0.00 | 7.0 | 294 | 290 | ||
| 5 | 0.00 | 15,000 | 0.00 | 4.00 | 1.68 | 9.5 | 49 | 51 | ||
| 6 | 1.00 | 20,000 | 1.00 | 6.00 | −1.00 | 5.5 | 70 | 74 | ||
| 7 | 0.00 | 15,000 | 1.68 | 7.40 | 0.00 | 7.0 | 262 | 263 | ||
| 8 | −1.00 | 10,000 | −1.00 | 2.00 | −1.00 | 5.5 | 83 | 85 | ||
| 9 | −1.00 | 10,000 | 1.00 | 6.00 | 1.00 | 8.5 | 208 | 204 | ||
| 10 | 1.00 | 20,000 | 1.00 | 6.00 | 1.00 | 8.5 | 79 | 84 | ||
| 11 | 1.00 | 20,000 | −1.00 | 2.00 | −1.00 | 5.5 | 89 | 94 | ||
| 12 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 359 | 368 | ||
| 13 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 372 | 368 | ||
| 14 | 1.00 | 20,000 | −1.00 | 2.00 | 1.00 | 8.5 | 113 | 115 | ||
| 15 | −1.68 | 6,591 | 0.00 | 4.00 | 0.00 | 7.0 | 223 | 232 | ||
| 16 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 362 | 368 | ||
| 17 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 376 | 368 | ||
| 18 | 0.00 | 15,000 | 0.00 | 4.00 | 0.00 | 7.0 | 364 | 368 | ||
| 19 | 1.68 | 23,409 | 0.00 | 4.00 | 0.00 | 7.0 | 246 | 251 | ||
| 20 | 0.00 | 15,000 | 0.00 | 4.00 | −1.68 | 4.5 | 0 | 0 | ||
| control | - | 18,500 | - | 2.80 | - | 5.5 | 83 | - | ||
| Source | Sum of Squares | df | Mean Square | Coefficient Estimate | Standard Error | F Value | p-value Prob > F |
|---|---|---|---|---|---|---|---|
| Model | 367670.4 | 9 | 40852.27 | 367.729 | 27.837 | 8.7688 | 0.0011 |
| X1 | 22466.08 | 1 | 22466.08 | −40.559 | 18.469 | 4.8222 | 0.0528 |
| X2 | 17.622 | 1 | 17.622 | −1.135 | 18.469 | 0.0037 | 0.9522 |
| X3 | 22709.11 | 1 | 22709.11 | 40.777 | 18.469 | 4.8744 | 0.0518 |
| X1X2 | 1580.625 | 1 | 1580.625 | −14.056 | 24.131 | 0.3392 | 0.5731 |
| X1X3 | 7501.287 | 1 | 7501.287 | −30.621 | 24.131 | 1.6101 | 0.2332 |
| X2X3 | 253.012 | 1 | 253.012 | −5.623 | 24.131 | 0.0543 | 0.8204 |
| X12 | 46799.54 | 1 | 46799.54 | −56.986 | 17.979 | 10.0453 | 0.01 |
| X22 | 19078.23 | 1 | 19078.23 | −36.384 | 17.979 | 4.095 | 0.0706 |
| X32 | 280988.6 | 1 | 280988.6 | −139.634 | 17.979 | 60.3134 | < 0.0001 |
| R2 = 0.8875; Adequate precision = 9.604; Coefficient of variation (CV) = 32.71% | |||||||
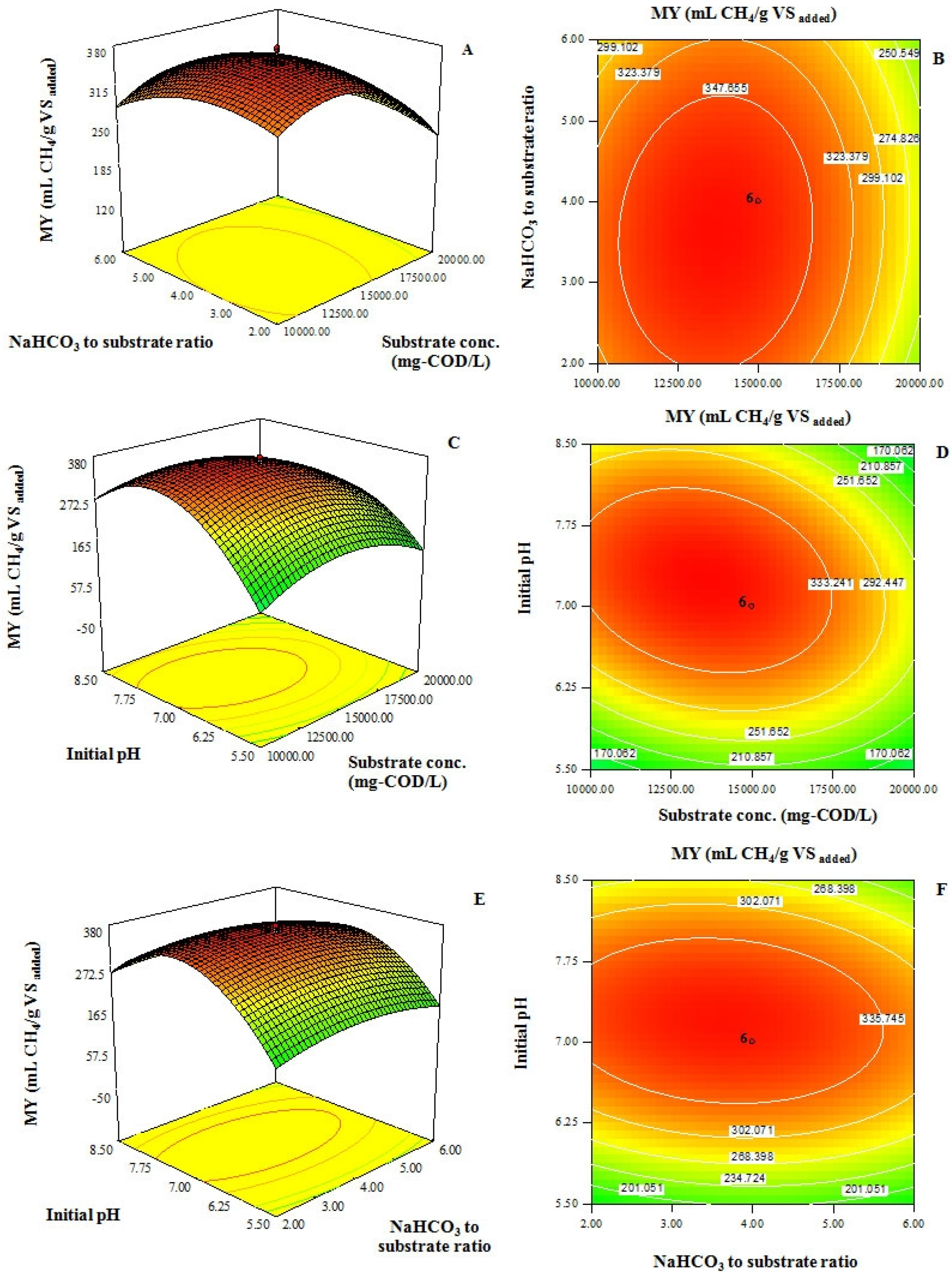
2.2. Effect of Substrate Concentration, Ratio of NaHCO3 to Substrate and Initial pH on MY from Acidic Effluent Coming from Hydrogen Fermentation Process of Sugarcane Juice
2.3. Confirmation Experiments and Sufficiency of the Models
| Run | Variables | MY (mL CH4/g-VSadded) | Bias a (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate concentration (mg-COD/L) | NaHCO3 and substrate ratio | Initial pH | |||||||
| Code | Actual | Code | Actual | Code | Actual | Predicted | Measured | ||
| 21 | 0.00 | 15,000 | 0.00 | 4.00 | 1.00 | 8.5 | 269 | 266 | 0.97 |
| 22 | 0.00 | 15,000 | 1.00 | 6.00 | 0.00 | 7.0 | 331 | 327 | 1.01 |
| 23 | 0.00 | 15,000 | −1.00 | 2.00 | 0.00 | 7.0 | 332 | 331 | 0.33 |
2.4. Energy Analysis
3. Experimental Section
3.1. Methanogenic Anaerobic Seed Sludge
3.2. Substrate
| Chracteristics | Concentration |
|---|---|
| Chemical oxygen demand (mg-COD/L) | 18,500 ± 12 |
| Total organic carbon (TOC, mg/L) | 7,600 ± 10 |
| Total nitrogen (TN, mg/L) | 252 ± 30 |
| Total phosphorus (TP, mg/L) | 73 ± 6 |
| Acetic acid (HAc, mg-COD/L) | 3,390 ± 54 |
| Butyric acid (HBu, mg-COD/L) | 13,000 ± 13 |
| Propionic acid (HPr, mg-COD/L) | 260 ± 20 |
| Chloride (Cl−) (mg/L) | 1,586 ± 56 |
| Volatile solids (VS, mg/L) | 5,870 ± 26 |
3.3. Experimental Design
3.4. Batch Fermentation
| Variables | Range and levels | ||||
|---|---|---|---|---|---|
| −α (−1.682) | Low (−1) | Central (0) | High (1) | +α (1.682) | |
| X1 = Substrate concentration (mg-COD/L) X2 = Ratio of NaHCO3 to substrate concentration X3 = Initial pH of substrate | 6,591 0.64 4.48 | 10,000 2.00 5.50 | 15,000 4.00 7.00 | 20,000 6.00 8.50 | 23,409 7.36 9.52 |
3.5. Analytic Methods
4. Conclusions
Acknowledgments
References
- Wang, X.; Nui, D.J.; Yang, X.S.; Zhao, Y.C. Optimization of methane fermentation from effluent of bio-hydrogen fermentation process using response surface methodology. Bioresour. Technol. 2008, 99, 4292–4299. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.; Maynaed, N.; Cannizzaro, C.; Benemann, J. Two-phase anaerobic digestion for production of hydrogen-methane mixtures. Bioresour. Technol. 2007, 98, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, D.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.H.; Lee, D.J. Production of hydrogen and methane from wastewater sludge using anaerobic fermentation. Int. J. Hydrog. Energy 2007, 32, 677–682. [Google Scholar] [CrossRef]
- Ueno, Y.; Tatara, M.; Fukui, H.; Makiuchi, T.; Goto, M.; Sode, K. Production of hydrogen and methane from organic solid wastes by phase-separation of anaerobic process. Bioresour. Technol. 2007, 98, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Cheng, J.; Zhou, J.; Song, W.; Liu, J.; Cen, K. Production of hydrogen and methane from potatoes by two-phase anaerobic fermentation. Bioresour. Technol. 2008, 99, 5942–5946. [Google Scholar] [CrossRef]
- Zhu, H.; Stadnyk, A.; Beland, M.; Seto, P. Co-production of hydrogen and methane from potato waste using a two-stage anaerobic digestion process. Bioresour. Technol. 2008, 99, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Plangklang, P.; Reungsang, R.; Pattra, S. Enhanced bio-hydrogen production from sugarcane juice by immobilized Clostridium butyricum on sugarcane bagasse. Int. J. Hydrog. Energy 2012, 37, 15525–15532. [Google Scholar] [CrossRef]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimization of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Mosey, F.E.; Fernandes, X.A. Patterns of hydrogen in biogas from the anaerobic digestion of milk-sugars. Water Sci. Technol. 1989, 21, 187–196. [Google Scholar]
- Sandberg, M.; Ahring, B.K. Anaerobic treatment of fish-meal process wastewater in a UASB reactor at high pH. Appl. Microbiol. Biotechnol. 1992, 36, 800–804. [Google Scholar] [CrossRef]
- Guwy, A.J.; Hawkes, F.R.; Wilcoxs, S.J.; Hawker, D.L. Neural network and on-off control of bicarbonate alkalinity in a fluidized-bed anaerobic digester. Water Res. 1997, 31, 2019–2025. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic Biotechnology for Industrial Wastewater, 1st ed.; Archae Press: Nashville, TN, USA, 1996. [Google Scholar]
- Reddy, P.R.M.; Ramesh, B.; Mrudula, S.; Reddy, G.; Seenayya, G. Production of thermostable β-amylase by Clostridium thermosulfurogenes SV2 in solid-state fermentation: optimization of nutrient levels using response surface methodology. Process Biochem. 2003, 39, 267–277. [Google Scholar] [CrossRef]
- Elibol, M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem. 2004, 39, 1057–1062. [Google Scholar] [CrossRef]
- Xiong, C.; Jinhua, W.; Dongsheng, L. Optimization of solid-state medium for the production of inulinase by Kluyveromyces sp. S120 using response surface methodology. Biochem. Eng. J. 2007, 34, 179–184. [Google Scholar]
- Liu, C.F.; Yuan, X.Z.; Zeng, G.M.; Li, W.W.; Li, J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Niladevi, K.N.; Sukumaran, R.K.; Jacob, N.; Anisha, G.S.; Prema, P. Optimization of laccase production from a novel strain Streptomyces psammoticus using response surface methodology. Microbiol. Res. 2009, 164, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Borja, R.; Rincon, B.; Raposo, F.; Alba, J.; Martin, A. A study of anaerobic digestibility of two-phases olive mill solid waste (OMSW) at mesophilic temperature. Process Biochem. 2002, 38, 733–742. [Google Scholar] [CrossRef]
- Murto, M.; Bjornsson, L.; Mattiasson, B. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manag. 2004, 70, 101–107. [Google Scholar] [CrossRef]
- Cho, J.K.; Park, S.C.; Chang, H.N. Biochemical methane potential and solid state anaerobic digestion of Korean food wastes. Bioresour. Technol. 1995, 52, 245–253. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gomez, X.; Escapa, A.; Moran, A. Evaluation and simultaneous optimization of bio-hydrogen production using 32 factorial design and the desirability function. J. Power Source 2007, 169, 131–139. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989.
- Hasyim, R.; Imai, T.; O-Thong, S.; Sulistyowati, L. Biohydrogen production from sago starch in wastewater using an enriched thermophilic mixed culture from hot spring. Int. J. Hydrog. Energy 2011, 36, 14162–14171. [Google Scholar] [CrossRef]
- Owen, W.; Stuckey, C.; Healy, J.; Young, L.; McCarty, P. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1978, 13, 485–493. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reungsang, A.; Pattra, S.; Sittijunda, S. Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process. Energies 2012, 5, 4746-4757. https://doi.org/10.3390/en5114746
Reungsang A, Pattra S, Sittijunda S. Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process. Energies. 2012; 5(11):4746-4757. https://doi.org/10.3390/en5114746
Chicago/Turabian StyleReungsang, Alissara, Sakchai Pattra, and Sureewan Sittijunda. 2012. "Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process" Energies 5, no. 11: 4746-4757. https://doi.org/10.3390/en5114746






