Commercial Biomass Syngas Fermentation
Abstract
:1. Introduction
2. Advantages of Gas Fermentation
| First generation | Second generation | |||
|---|---|---|---|---|
| Corn ethanol (yeast fermentation) | Biochemical (lignocellulosic fermentation) | Thermochemical (Fischer-Tropsch process) | Gas fermentation (hybrid process) | |
| State of the art and political implications | ||||
| Current state of the technology | Commercially established Mature technology producing large quantities of fuel on a commercial scale [5]. The most energy efficient biofuel production technology to date [47]. | Pre-commercial Demonstration and commercial plants in operation [48]. Predicted to partially replace first generation bioethanol technologies within the next decade [26]. | Pre-commercial Technology highly established with coal feedstock. Biomass synthesis gas pilot plants in operation with commercial plants planned [48]. | Pre-commercial Semi-commercial demonstration plants in operation [49]. |
| Energy security | Low Limited feedstock potential [12]. | Variable Uniform feedstock requirement may limit feedstock potential [6,22]. | High Large feedstock potential [22], including non-biomass sources such as industrial waste gas streams. | High Large feedstock potential [22], including non-biomass sources such as industrial waste gas streams [50]. |
| Food security | Poor Feedstock competes directly with food crops [6]. | Variable Feedstock does not compete directly with food crops but could require extensive monocultures, thus competing for agricultural inputs [6]. | High Feedstock does not compete directly with food crops. | High Feedstock does not compete directly with food crops. |
| Overall efficiency | ||||
| Energy capture | High All energy in sugar can be captured through fermentation. Energy-intensive distillation of ethanol from fermentation broth [12]. | Poor Energy and carbon in lignin cannot be captured [41]. Significant process energy loss in converting lignocelluloses into fermentable sugars [51] | High Energy and carbon in both lignin and cellulosic fractions of biomass are converted to syngas by gasification. Gasification energy efficiency is approximately 75%–80% depending on the carbon, moisture and ash content of the biomass feedstock [52]. Overall plant energy efficiency (energy in feedstock converted to final product) of 45% [36]. | High Energy and carbon in both lignin and cellulosic fractions of biomass are converted to syngas by gasification. Gasification energy efficiency is approximately 75%–80% depending on the carbon, moisture and ash content of the biomass feedstock [52]. Overall plant energy efficiency (energy in feedstock converted to final product) of 57% [36]. |
| Upstream process | ||||
| Feedstock | Specific, limited Sugar crops such as wheat, corn, sugar beet, and sugar cane; starch crops such as potato [5]. | Specific, unlimited Lignocellulosic biomass, such as forestry crops, perennial grasses and agricultural residues [5]. Pre-treatment steps are usually biomass type-specific to maximise efficiency and minimise inhibitor production [44] | Flexible, unlimited Lignocellulosic biomass such as forestry crops, perennial grasses and agricultural residues [5]. Gasification process allows a wide range and mixture of feedstock to be used. Pre-treatment steps are biomass and gasifier-specific in order to minimise contaminants and produce required syngas composition [44]. Can also use CO-rich industrial waste gases. | Flexible, unlimited Lignocellulosic biomass such as forestry crops, perennial grasses and agricultural residues, or municipal solid waste (MSW) [5]. Gasification process allows a wide range and mixture of feedstock to be used. Can also use CO-rich industrial waste gases. |
| Gas composition | – | – | Specific Catalyst requires specific syngas composition; for example, cobalt-based FT catalyst has an H2:CO requirement of approximately 2.15 [31]. | Flexible Microbial catalyst can utilise a range of syngas H2:CO composition while retaining product specificity [38]. |
| Reactor | ||||
| Selectivity | High Fermentation organisms produce defined products in a single step [5]. | High Fermentation organisms produce defined products in a single step [5]. | Low Requires large methanol recycle [53]. | High Fermentation organisms produce defined products in a single step. Products as acetate, butanol, or 2,3-butanediol can be produced at predetermined ratios [54]. |
| Tolerance to inhibitors | Medium Refined sugar streams are used. | Low Pre-treatment process releases and creates inhibitors (for example, furfural) to enzymes used in the saccharification process, and bacteria used in the fermentation. | Low Catalysts can be irreversibly poisoned by, for example, sulphur containing compounds [37]. Consequently, the production of purified syngas accounts for 60%–70% of the running costs of a FT plant [31]. | Medium Tolerant to many impurities such as sulphur-containing compounds [39]; consequently, fewer gas clean-up steps are required than thermochemical route. |
| Tolerance to microbial contamination | Medium Refined sugar streams are used. Despite no direct plant material, there is potential for contamination as microorganisms growing on sugars are abundant in nature [55]. Mainly batch or fed-batch processes to minimise contamination. | Poor Great potential for contamination as microorganisms growing on sugars are abundant in nature. Consequently, fermentation strategies require aseptic conditions at each stage. In lignocellulosic fermentation contamination is an issue as inhibitor compounds provide an advantage to contaminating organisms. | – | Good Very few microorganisms are known to be capable of living in the presence of, or utilising CO. As CO and H2 are the only carbon sources this greatly reduces opportunity for microbial contamination. |
| Reactor operation type | Mainly batch or fed batch, and few continuous processes [56]. | Usually batch. | Continuous with low residence times, reactions very fast. | Batch or continuous with low residence times. |
| Reactor temperature | Moderate Optimum temperature of 33 °C–37 °C [57]. | Moderate-Medium Mesophilic bacteria, but also thermophilic bacteria with an optimum around 60 °C. | High 150 °C–300 °C. | Moderate Mainly mesophilic bacteria with an optimum at 37 °C. Few thermophiles with an optimum around 60 °C. |
| Downstream process | ||||
| Cost of product recovery | Low-Moderate Sugar fermentation broths have high solid levels which require separation and treatment. Tolerant to high ethanol levels up to 15%, thus require less energy for distillation [58]. | Moderate Lower solids and ethanol content in fermentation broth than corn ethanol route. Similar separation system as biochemical route can be applied with minor modifications. | Low-Moderate Mixed alcohol separation. Ethanol, methanol, propanol and higher level alcohols. Distillation schemes used to purify approximately 90% ethanol to greater than 99.5% purity. Separation of mixed components increases costs. | Moderate Lower solids and ethanol content in fermentation broth than biochemical route. Similar separation system scheme as biochemical route can be applied with minor modifications. |
3. Biomass Syngas and Gasification
| Partial oxidation: | C + 0.5 O2 ↔ CO | ∆Hvap = −268 MJ/kg mol, ∆G°´= −151 kJ/mol | (1) |
| Complete oxidation: | C + O2 ↔ CO2 | ∆Hvap = −406 MJ/kg mol, ∆G°´= −423 kJ/mol | (2) |
| Water gas reaction: | 2O ↔ CO + H2 | ∆Hvap = +118 MJ/kg mol, ∆G°´= −100 kJ/mol | (3) |
| Water gas-shift reaction: | CO + H2O ↔ CO2 + H2 | ∆Hvap = −42 MJ/kg mol, ∆G°´= −20 kJ/mol | (4) |
| Methane formation: | CO + 3H2 ↔ CH4 + H2O | ∆Hvap = −88 MJ/kg mol, ∆G°´= −151 kJ/mol | (5) |
4. Biochemistry of Gas Fermentation: the Reductive Acetyl-CoA, Wood-Ljungdahl, ACS/CODH Pathway
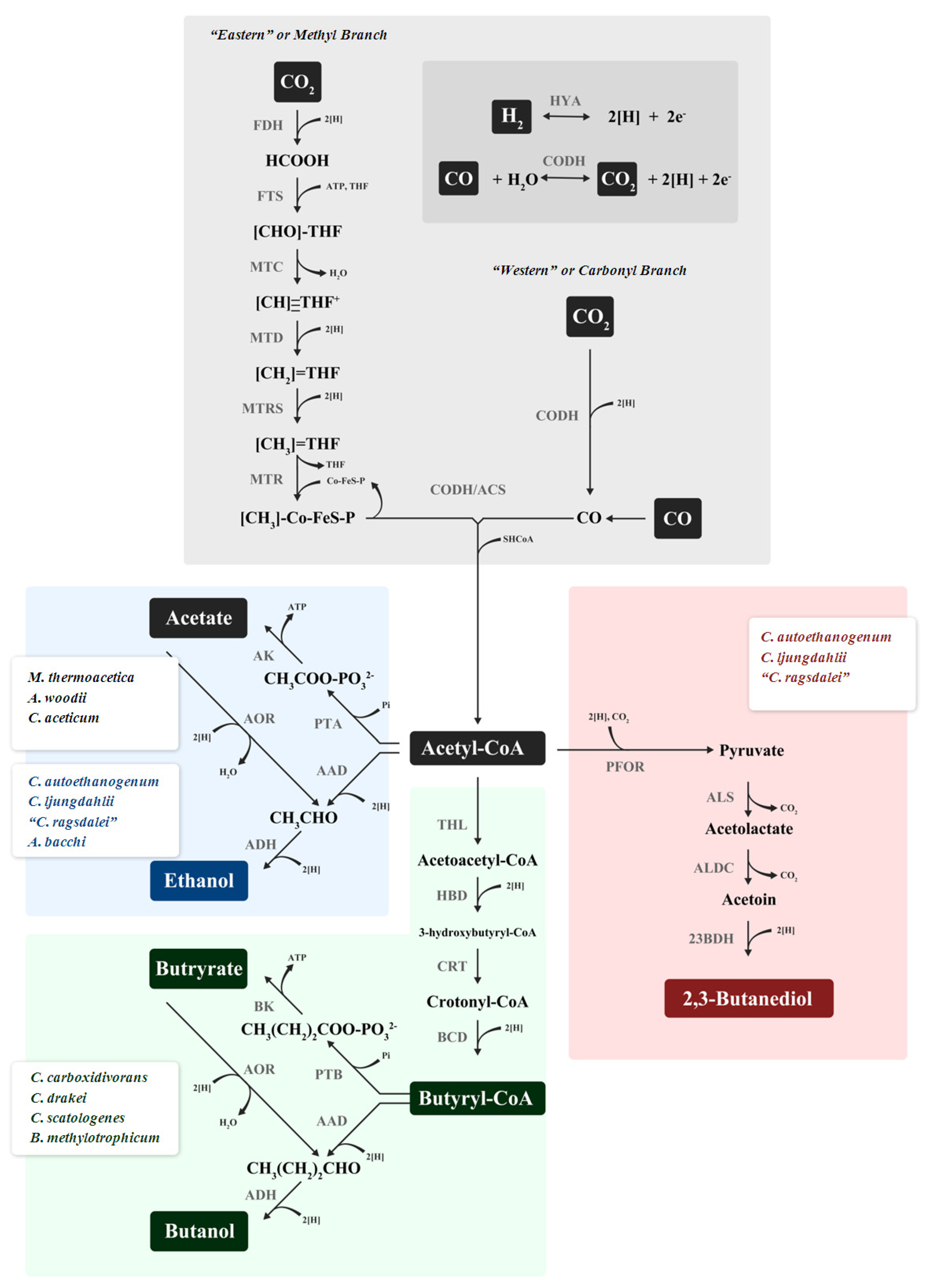
5. Organisms and Products
5.1. Acetate Producers
5.2. Ethanol Production
5.3. Butanol
5.4. 2,3-Butanediol
6. Fermentation and Bioreactor Optimisation
7. Strain Improvement
8. Challenges
8.1. Gasification and Gas Clean-Up
8.2. Fermentation and Bioreactor Design
8.3. Downstream Processing
9. Commercialisation
10. Summary
References
- Stern, N. The Economics of Climate Change: The Stern Review; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2009/28/EC on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Available online: http://europa.eu/legislation_summaries/energy/renewable_energy/en0009_en.htm (accessed on 14 December 2012).
- Energy Independence and Security Act of 2007; 110th United States Congress: Washington, WA, USA, 2007.
- Hertel, T.W.; Tyner, W.E.; Birur, D.K. The global impacts of biofuel mandates. Energy J. 2010, 31, 75–100. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain.Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Bailey, R. Another Inconvenient Truth: How Biofuel Policies Are Deepening Poverty and Accelerating Climate Change; Oxfam International: Oxford, UK, 2008. [Google Scholar]
- Greenpeace European Unit. Europe’s Biofuels Plans Driving Social and Environmental Destruction. Available online: http://www.greenpeace.org/eu-unit/en/News/2010/driving-to-destruction-08-11-10 (accessed on 15 August 2012).
- Graziano da Silva, J. The US Must Take Biofuel Action to Prevent a Food Crisis. Available online: http://on.ft.com/QSyKBJ (accessed on 15 August 2012).
- Hornby, C. U.S. Should Change Biofuel Policy to Avoid Food Crisis: U.N. Available online: http://www.reuters.com/article/2012/08/10/us-food-biofuels-fao-idUSBRE8790K420120810 (accessed on 15 August 2012).
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Reilly, J.M.; Kicklighter, D.W.; Gurgel, A.C.; Cronin, T.W.; Paltsev, S.; Felzer, B.S.; Wang, X.; Sokolov, A.P.; Schlosser, C.A. Indirect emissions from biofuels: how important? Science 2009, 326, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Bioenergy—Chances and Limits; German National Academic of Sciences Leopoldina: Halle, Germany, 2012.
- Mitchell, D.; Kojima, M.; Ward, W. Considering Trade Policies for Liquid Biofuels; Special Report; Energy Sector Management Assistance Program: Washington, DC, USA, 2007. [Google Scholar]
- Carney, B. Can the World Still Feed Itself? Available online: http://on.wsj.com/omahnc (accessed on 20 August 2012).
- Thompson, A. Nestle Chief Calls for End to Using Food in Biofuel Production. Available online: http://uk.reuters.com/article/2012/08/19/uk-nestle-idUKBRE87I03W20120819 (accessed on 20 August 2012).
- Naylor, R.L.; Liska, A.J.; Burke, M.B.; Falcon, W.P.; Gaskell, J.C.; Rozelle, S.D.; Cassman, K.G. The Ripple Effect: Biofuels, Food Security, and the Environment. Environ. Sci. Policy Sustain. Dev. 2007, 49, 30–43. [Google Scholar] [CrossRef]
- Mitchell, D. A Note on Rising Food Prices. World Bank Development Prospects Group: Washington, DC, USA, 2008. [Google Scholar]
- Ajanovic, A. Biofuels versus food production: Does biofuels production increase food prices? Energy 2011, 36, 2070–2076. [Google Scholar] [CrossRef]
- OECD-FAO Agricultural Outlook 2011–2020; Organisation for Economic Co-operation and Development (OECD) and the United Nation’s Food and Agricultural Organization, 2011. Available online: http://www.oecd.org/site/oecd-faoagriculturaloutlook/ (accessed on 14 December 2012).
- Murphy, R.; Woods, J.; Black, M.; McManus, M. Global developments in the competition for land from biofuels. Food Policy 2011, 36, S52–S61. [Google Scholar] [CrossRef]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Perlack, R.; Wright, L.; Turhollow, A.; Graham, R.; Stokes, B.; Erbach, D. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; U.S. Department of Energy: Washington, DC, USA, 2005.
- World Energy Council 2010 Survey of Energy Resources. Available online: http://www.worldenergy.org/documents/ser_2010_report_1.pdf (accessed on 5 August 2012).
- Metz, B.; Davidson, O.; Bosch, P.; Meyer, L. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007; Metz, B., Davidson, O., Bosch, P., Meyer, L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Saddler, J.N.; Mabee, W.E.; Simms, R.; Taylor, M. The Biorefinining Story: Progress in Commercialization of Biomass-to-Ethanol. In Forests in Development: A Vital Balance; Schlichter, T., Montes, L., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 39–51. [Google Scholar]
- Gnansounou, E.; Dauriat, A. Techno-economic analysis of lignocellulosic ethanol: A review. Bioresour. Technol. 2010, 101, 4980–4991. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Maher, M.A.; Jones, C.; Brinker, R.W. Ethanol-the primary renewable liquid fuel. J. Chem. Technol. Biotechnol. 2011, 86, 473–480. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Atiyeh, H.K. Microbial production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 2011, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuels Bioprod. Biorefin. 2011, 5, 93–114. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Tijmensen, M.; Faaij, A.; Hamelinck, C.; van Hardeveld, M. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch Synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Mirwald, J.W.; Inderwildi, O.R. Unraveling the Fischer–Tropsch mechanism: a combined DFT and microkinetic investigation of C-C bond formation on Ru. Phys. Chem. Chem. Phys. 2012, 14, 7028–7031. [Google Scholar] [CrossRef]
- Fischer, F.; Tropsch, H. Process for the production of paraffin-hydrocarbons with more than one carbon atom. U.S. Patent 1746464, 11 February 1930. [Google Scholar]
- Griffin, D.W.; Schultz, M.A. Fuel and chemical products from biomass syngas: A comparison of gas fermentation to thermochemical conversion routes. Environ. Prog. Sustain. Energy 2012, 31, 219–224. [Google Scholar] [CrossRef]
- Van Steen, E.; Claeys, M. Fischer–Tropsch catalysts for the biomass-to-liquid (BTL)-process. Chem. Eng. Technol. 2008, 31, 655–666. [Google Scholar] [CrossRef]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzyme Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Vega, J.L.; Klasson, K.T.; Kimmel, D.E.; Clausen, E.C.; Gaddy, J.L. Sulfur gas tolerance and toxicity of CO-utilizing and methanogenic bacteria. Appl. Biochem. Biotechnol. 1990, 24/25, 329–340. [Google Scholar] [CrossRef]
- Gual, A.; Godard, C.; Castillón, S.; Curulla-Ferré, D.; Claver, C. Colloidal Ru, Co and Fe-nanoparticles. Synthesis and application as nanocatalysts in the Fischer–Tropsch process. Catal. Today 2012, 183, 154–171. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Mabee, W.E.; Gregg, D.J.; Arato, C.; Berlin, A.; Bura, R.; Gilkes, N.; Mirochnik, O.; Pan, X.; Pye, E.K.; Saddler, J.N. Updates on softwood-to-ethanol process development. Appl. Biochem. Biotechnol. 2006, 129-132, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Schiel-Bengelsdorf, B.; Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Junqueira, T.L.; Jesus, C.D.F.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. Improving bioethanol production—Comparison between extractive and low temperature fermentation. Appl. Energy 2012, 98, 548–555. [Google Scholar] [CrossRef]
- Bacovsky, D.; Dallos, M.; Wörgetter, M. Status of 2nd Generation Biofuels Demonstration Facilities in June 2010; IEA Bioenergy Task 39; International Energy Agency (IEA): Paris, France, 2010; pp. 1–126. [Google Scholar]
- LanzaTech Closes US $55.8 Million Series C Round. Available online: http://www.lanzatech.co.nz/sites/default/files/imce_uploads/seriesc_lanzatech_final_for_release.pdf (accessed on 23 July 23, 2012).
- LanzaTech Chinese Steel Miller Commercializing LanzaTech’s Clean Energy Technology. Available online: http://www.lanzatech.co.nz/sites/default/files/imce_uploads/shougangprvf.pdf (accessed on 1 August 2012).
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefin. 2010, 4, 77–93. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (Part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- E4Tech. Review of Technologies for Gasification of Biomass and Wastes; NNFCC Project 09/008 Final Report; NNFCC: York Science Park, UK, 2009. [Google Scholar]
- Köpke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbial. 2011, 77, 5467–5475. [Google Scholar] [CrossRef]
- Skinner, K.A.; Leathers, T.D. Bacterial contaminants of fuel ethanol production. J. Ind. Microbiol. Biotechnol. 2004, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero, J.A.; Montoya, M.I.; Sánchez, O.J.; Giraldo, O.H.; Cardona, C.A. Fuel ethanol production from sugarcane and corn: Comparative analysis for a Colombian case. Energy 2008, 33, 385–399. [Google Scholar] [CrossRef]
- Madson, P. Ethanol distillation: the fundamentals. In The Alcohol Textbook; Jacques, K., Lyons, T., Keisall, D., Eds.; Nottingham University Press: Nottingham, UK, 2003; pp. 319–336. [Google Scholar]
- Kirkels, A.F.; Verbong, G.P.J. Biomass gasification: Still promising? A 30-year global overview. Renew. Sustain. Energy Rev. 2011, 15, 471–481. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.; Prins, W.; Vanswaaij, W.; Vandebeld, B.; Elliott, D.; Neuenschwander, G.; Kruse, A. Biomass gasification in near- and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Babu, S. Thermal gasification of biomass technology developments: end of task report for 1992 to 1994. Biomass Bioenergy 1995, 9, 271–285. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Bronson, B.; Preto, F.; Mehrani, P. Effect of pretreatment on the physical properties of biomass and its relation to fluidized bed gasification. Environ. Prog. Sustain. Energy 2012, 31, 335–339. [Google Scholar] [CrossRef]
- Gómez, C.J.; Mészáros, E.; Jakab, E.; Velo, E.; Puigjaner, L. Thermogravimetry/mass spectrometry study of woody residues and an herbaceous biomass crop using PCA techniques. J. Anal. Appl. Pyrolysis 2007, 80, 416–426. [Google Scholar] [CrossRef]
- Mészáros, E.; Jakab, E.; Várhegyi, G.; Szepesváry, P.; Marosvölgyi, B. Comparative study of the thermal behavior of wood and bark of young shoots obtained from an energy plantation. J. Anal. Appl. Pyrolysis 2004, 72, 317–328. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Conesa, J.A. Effects of the composting and the heating rate on biomass gasification. Energy Fuels 2009, 23, 951–957. [Google Scholar] [CrossRef]
- Barneto, A.G.; Ariza Carmona, J.; Díaz Blanco, M.J. Effect of the previous composting on volatiles production during biomass pyrolysis. J. Phys. Chem. A 2010, 114, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, M.; de Jong, W.; Verkooijen, A.H.M. Fluidized bed gasification as a mature and reliable technology for the production of bio-syngas and applied in the production of liquid transportation fuels—a review. Energies 2011, 4, 389–434. [Google Scholar] [CrossRef]
- Herguido, J.; Corella, J.; Gonzalez-Saiz, J. Steam gasification of lignocellulosic residues in a fluidized bed at a small pilot scale. Effect of the type of feedstock. Ind. Eng. Chem. Res. 1992, 31, 1274–1282. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jand, N.; Foscolo, P.U. Decomposition of wood particles in fluidized beds. Ind. Eng. Chem. Res. 2005, 44, 5079–5089. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Chopra, S.; Jain, A.K. A review of fixed bed gasification systems for biomass. Int. Comm. Agric. Eng. 2007, 9, 1–23. [Google Scholar]
- Bui, T.; Loof, R.; Bhattacharya, S.C. Multi-stage reactor for thermal gasification of wood. Energy 1994, 19, 397–404. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Hlína, M.; Hrabovský, M.; Kopecký, V.; Konrád, M.; Kavka, T.; Skoblja, S. Plasma gasification of wood and production of gas with low content of tar. Czech. J. Phys. 2006, 56, B1179–B1184. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Yoshida, T.; Oshima, Y.; Matsumura, Y. Gasification of biomass model compounds and real biomass in supercritical water. Biomass Bioenergy 2004, 26, 71–78. [Google Scholar] [CrossRef]
- Drake, H.L.; Gössner, A.S.; Daniel, S.L. Old acetogens, new light. Ann. N.Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Ljungdahl, L.G.; Wood, H. Total synthesis of acetate from CO2 by heterotrophic bacteria. Ann. Rev. Microbial. 1969, 23, 515–538. [Google Scholar] [CrossRef]
- Wood, H.G. Life with CO or CO2 and H2 as a source of carbon and energy. FASEB J. 1991, 5, 156–163. [Google Scholar] [PubMed]
- Ljungdahl, L.G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Ann. Rev. Microbial. 1986, 40, 415–450. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Life with carbon monoxide. Crit. Rev. Biochem. Mol. Boil. 2004, 39, 165–195. [Google Scholar] [CrossRef]
- Drake, H.L.; Küsel, K.; Matthies, C.; Wood, H.G.; Ljungdahl, L.G. Acetogenic Prokaryotes. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer New York: New York, NY, USA, 2006; pp. 354–420. [Google Scholar]
- Russell, M.J.; Martin, W. The rocky roots of the acetyl-CoA pathway. Trends biochem. Sci. 2004, 29, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Schopf, W. Earth’s Earliest Biosphere: Its Origin and Evolution; Princeton University Press: Princeton, NJ, USA, 1984; p. 610. [Google Scholar]
- Ragsdale, S.W. The Eastern and Western branches of the Wood/Ljungdahl pathway: How the East and West were won. BioFactors 1997, 6, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Köpke, M.; Held, C.; Hujer, S.; Liesegang, H.; Wiezer, A.; Wollherr, A.; Ehrenreich, A.; Liebl, W.; Gottschalk, G.; Dürre, P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl. Acad. Sci. USA 2010, 107, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.P.; Jones, S.W.; Fast, A.G.; Indurthi, D.C.; Papoutsakis, E.T. Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr. Opin. Biotechnol. 2012, 23, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G. The central metabolic pathway from acetyl-CoA to butyryl-CoA in Clostridium acetobutylicum. FEMS Microbiol. Rev. 1995, 17, 241–249. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Wood, H.G. Interaction of ferredoxin with carbon monoxide dehydrogenase from Clostridium thermoaceticum. J. Boil. Chem. 1992, 267, 897–900. [Google Scholar]
- Drake, H.L.; Hu, S.I.; Wood, H.G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J. Boil. Chem. 1980, 255, 7174–7180. [Google Scholar]
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.; Lemon, B.J.; Peters, J.W. Reversible carbon monoxide binding and inhibition at the active site of the Fe-only hydrogenase. Biochemistry 2000, 39, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Bruschi, M.; Heimdal, J.; Fantucci, P.; De Gioia, L.; Ryde, U. Structural insights into the active-ready form of [FeFe]-hydrogenase and mechanistic details of its inhibition by carbon monoxide. Inorg. Chem. 2007, 46, 7256–7258. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kabe, R.; Nonaka, K.; Ando, T.; Yoon, K.S.; Nakai, H.; Ogo, S. Model study of CO inhibition of [NiFe]hydrogenase. Inorg. Chem. 2011, 50, 8902–8906. [Google Scholar] [CrossRef] [PubMed]
- Seravalli, J.; Zhao, S.; Ragsdale, S.W. Mechanism of transfer of the methyl group from (6S)-methyltetrahydrofolate to the corrinoid/iron-sulfur protein catalyzed by the methyltransferase from Clostridium thermoaceticum: a key step in the Wood-Ljungdahl pathway of acetyl-CoA synthesis. Biochemistry 1999, 38, 5728–5735. [Google Scholar] [CrossRef] [PubMed]
- Bruant, G.; Lévesque, M.J.; Peter, C.; Guiot, S.R.; Masson, L. Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7. PLoS One 2010, 5, 1–12. [Google Scholar] [CrossRef]
- Poehlein, A.; Schmidt, S.; Kaster, A.K.; Goenrich, M.; Vollmers, J.; Thürmer, A.; Bertsch, J.; Schuchmann, K.; Voigt, B.; Hecker, M.; et al. An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 2012, 7, e33439. [Google Scholar] [CrossRef] [PubMed]
- Doukov, T.I.; Iverson, T.M.; Seravalli, J.; Ragsdale, S.W.; Drennan, C.L. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science 2002, 298, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Nickel and the carbon cycle. J. Inorg. Biochem. 2007, 101, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit. Rev. Biochem. Mol. Boil. 1991, 26, 261–300. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Yi, L.; Bender, G.; Gupta, N.; Kung, Y.; Yan, L.; Stich, T.A.; Doukov, T.; Leichert, L.; Jenkins, P.M.; et al. Redox, haem and CO in enzymatic catalysis and regulation. Biochem. Soc. Trans. 2012, 40, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, V. Energy conservation in acetogenic bacteria. Appl. Environ. Microbial. 2003, 69, 6345–6353. [Google Scholar] [CrossRef]
- Das, A.; Ljungdahl, L.G. Electron-transport system in acetogens. In Biochemistry and Physiology of Anaerobic Bacteria; Ljungdahl, L.G., Adams, M.M., Barton, L., Ferry, J.G., Johnson, M., Eds.; Springer Verlag: New York, NY, USA, 2002; pp. 191–204. [Google Scholar]
- Gottwald, M.; Andreesen, J.R.; LeGall, J.; Ljungdahl, L.G. Presence of Cytochrome and Menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J. Bacterial. 1975, 122, 325–328. [Google Scholar]
- Müller, V.; Imkamp, F.; Biegel, E.; Schmidt, S.; Dilling, S. Discovery of a ferredoxin:NAD+-oxidoreductase (Rnf) in Acetobacterium woodii: a novel potential coupling site in acetogens. Ann. N.Y. Acad. Sci. 2008, 1125, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Biegel, E.; Schmidt, S.; González, J.M.; Müller, V. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell. Mol. Life Sci. 2011, 68, 613–634. [Google Scholar] [CrossRef] [PubMed]
- Biegel, E.; Schmidt, S.; Müller, V. Genetic, immunological and biochemical evidence for a Rnf complex in the acetogen Acetobacterium woodii. Environ. Microbial. 2009, 11, 1438–1443. [Google Scholar] [CrossRef]
- Biegel, E.; Müller, V. Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase. Proc. Natl. Acad. Sci. USA 2010, 107, 18138–18142. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, G.; Diekert, G. Thermodynamics of methylenetetrahydrofolate reduction to methyltetrahydrofolate and its implications for the energy metabolism of homoacetogenic bacteria. Arch. Microbiol. 1991, 155, 378–381. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Moll, J.; Thauer, R.K. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J. Bacterial. 2010, 192, 5115–5123. [Google Scholar] [CrossRef]
- Li, F.; Hinderberger, J.; Seedorf, H.; Zhang, J.; Buckel, W.; Thauer, R.K. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J. Bacterial. 2008, 190, 843–850. [Google Scholar] [CrossRef]
- Buckel, W.; Thauer, R.K. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta 2012, in press. [Google Scholar]
- Herrmann, G.; Jayamani, E.; Mai, G.; Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacterial. 2008, 190, 784–791. [Google Scholar] [CrossRef]
- Schuchmann, K.; Mueller, V. A bacterial electron bifurcating hydrogenase. J. Boil. Chem. 2012, 287, 31165–31171. [Google Scholar] [CrossRef]
- Schmidt, S.; Biegel, E.; Müller, V. The ins and outs of Na+ bioenergetics in Acetobacterium woodii. Biochim. Biophys. Acta 2009, 1787, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Meinecke, B.; Bertram, J.; Gottschalk, G. Purification and characterization of the pyruvate-ferredoxin oxidoreductase from Clostridium acetobutylicum. Arch. Microbiol. 1989, 152, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.; Leone, V.; Faraldo-Gómez, J.D.; Müller, V. Promiscuous archaeal ATP synthase concurrently coupled to Na+ and H+ translocation. Proc. Natl. Acad. Sci. USA 2012, 109, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Odelson, D.A.; Breznak, J.A. Volatile Fatty Acid production by the hindgut microbiota of xylophagous termites. Appl. Environ. Microbial. 1983, 45, 1602–1613. [Google Scholar]
- Tholen, A.; Brune, A. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbial. 1999, 65, 4497–4505. [Google Scholar]
- Drake, H.L. Acetogenesis, Acetogenic Bacteria, and the Acetyl-CoA “Wood/Ljungdahl” Pathway: Past and Current Perspectives. In Acetogenesis; Drake, H.L., Ed.; Chapman and Hall: New York, NY, USA, 1994; pp. 3–60. [Google Scholar]
- Sim, J.H.; Kamaruddin, A.H.; Long, W.S.; Najafpour, G. Clostridium aceticum—A potential organism in catalyzing carbon monoxide to acetic acid: Application of response surface methodology. Enzyme Microb. Technol. 2007, 40, 1234–1243. [Google Scholar] [CrossRef]
- Wagner, F.S., Jr. Acetic Acid. Kirk-Othmer Encycl. Chem. Technol. 2002, 1, 115–136. [Google Scholar]
- Global Industry Analysts Inc. Global Market for Acetic Acid to Reach 12.15 Million Tons by 2017, According to New Report by Global Industry Analysts, Inc. Available online: http://www.prweb.com/releases/acetic_acid_acetates/vinyl_acetate_monomer_PTA/prweb9242731.htm (accessed on 12 July 2012).
- Parekh, S.R.; Cheryan, M. Production of acetate by mutant strains of Clostridium thermoaceticum. Appl. Microbiol. Biotechnol. 1991, 36, 384–387. [Google Scholar] [CrossRef]
- Demler, M.; Weuster-Botz, D. Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol. Bioeng. 2011, 108, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.K. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2012, 111, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, G. Bioprocess and Microbe Engineering for Total Carbon Utilization in Biofuel Production. U.S. Patent 0177564, 21 July 2011. [Google Scholar]
- Wieringa, K.T. Over het verdwijnen van waterstof en koolzuur onder anaerobe voorwaarden. Antonie van Leeuwenhoek 1936, 3, 263–273. [Google Scholar] [CrossRef]
- Wieringa, K.T. The formation of acetic acid from carbon dioxide and hydrogen by anaerobic spore-forming bacteria. Antonie van Leeuwenhoek 1939, 6, 251–262. [Google Scholar] [CrossRef]
- Braun, M.; Mayer, F.; Gottschalk, G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch. Microbial. 1981, 128, 288–293. [Google Scholar] [CrossRef]
- Adamse, A. New isolation of Clostridium aceticum (Wieringa). Antonie van Leeuwenhoek 1980, 46, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Kamaruddin, A.H. Optimization of acetic acid production from synthesis gas by chemolithotrophic bacterium—Clostridium aceticum using statistical approach. Bioresour. Technol. 2008, 99, 2724–2735. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Daniel, S.L. Physiology of the thermophilic acetogen Moorella thermoacetica. Res. Microbiol. 2004, 155, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.E.; Peterson, W.H.; McCoy, E.; Johnson, M.J.; Ritter, G.J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J. Bacteriol. 1942, 43, 701–715. [Google Scholar] [PubMed]
- Balk, M.; Weijma, J.; Friedrich, M.W.; Stams, A.J.M. Methanol utilization by a novel thermophilic homoacetogenic bacterium, Moorella mulderi sp. nov., isolated from a bioreactor. Arch. Microbial. 2003, 179, 315–320. [Google Scholar]
- Sakai, S.; Nakashimada, Y.; Yoshimoto, H.; Watanabe, S.; Okada, H.; Nishio, N. Ethanol production from H2 and CO2 by a newly isolated thermophilic bacterium, Moorella sp. HUC22-1. Biotechnol. Lett. 2004, 26, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Henstra, A.M.; Paulo, P.L.; Balk, M.; van Doesburg, W.; Stams, A.J.M. Atypical one-carbon metabolism of an acetogenic and hydrogenogenic Moorella thermoacetica strain. Arch. Microbial. 2009, 191, 123–131. [Google Scholar] [CrossRef]
- Pierce, E.; Xie, G.; Barabote, R.D.; Saunders, E.; Han, C.S.; Detter, J.C.; Richardson, P.; Brettin, T.S.; Das, A.; Ljungdahl, L.G.; et al. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ. Microbial. 2008, 10, 2550–2573. [Google Scholar]
- Wiegel, J.; Carreira, L.H.; Garrison, R.; Rabek, N.E.; Ljungdahl, L.G. Calcium magnesium acetate (CMA) manufacture from glucose by fermentation with thermophilic homoacetogenic bacteria. In Calcium Magnesium Acetate; Wise, D.L., Lavendis, Y.A., Metghalchi, M., Eds.; Elsevier Science Publisher: Amsterdam, The Netherlands, 1991; pp. 359–418. [Google Scholar]
- Reed, W.M.; Bogdan., M.E. Application of cell recycling to continuous fermentative acetic acid production. Biotech. Bioeng. Symp. 1985, 15, 641–647. [Google Scholar]
- Balch, W.E.; Schoberth, S.; Tanner, R.S.; Wolfe, R.S. Acetobacterium, a New Genus of Hydrogen-Oxidizing, Carbon Dioxide-Reducing, Anaerobic Bacteria. Int. J. Syst. Bacteriol. 1977, 27, 355–361. [Google Scholar] [CrossRef]
- Buschhorn, H.; Dürre, P.; Gottschalk, G. Production and Utilization of Ethanol by the Homoacetogen Acetobacterium woodii. Appl. Environ. Microbial. 1989, 55, 1835–1840. [Google Scholar]
- Imkamp, F.; Biegel, E.; Jayamani, E.; Buckel, W.; Müller, V. Dissection of the caffeate respiratory chain in the acetogen Acetobacterium woodii: identification of an Rnf-type NADH dehydrogenase as a potential coupling site. J. Bacterial. 2007, 189, 8145–8153. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Bromley, J.C.; Simpson, S.D. Fermentative production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 2011, 22, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.; Prieto, S.; Harrison, S.B.; Clausen, E.C.; Gaddy, J.L. Biological production of alcohols from coal through indirect liquefaction. Appl. Biochem. Biotechnol. 1988, 18, 363–378. [Google Scholar] [CrossRef]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Bacterial. 1993, 43, 232–236. [Google Scholar] [CrossRef]
- Phillips, J.R.; Klasson, K.T.; Claussen, E.C.; Gaddy, J.L. Biological Production of Ethanol from Coal Synthesis Gas. Appl. Biochem. Biotechnol. 1993, 39, 559–571. [Google Scholar] [CrossRef]
- Huhnke, R.; Lewis, R.; Tanner, R. Isolation and Characterization of Novel Clostridial Species. U.S. Patent 7704723 B2, 27 April 2010. [Google Scholar]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbial. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during “Clostridium ragsdalei” syngas fermentation. Bioresour. Technol. 2011, 102, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Kundiyana, D.K.; Wilkins, M.R.; Maddipati, P.; Huhnke, R.L. Effect of temperature, pH and buffer presence on ethanol production from synthesis gas by “Clostridium ragsdalei”. Bioresour. Technol. 2011, 102, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbial. Biotechnol. 2012, 28, 1553–1561. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Abrini, J.; Naveau, H.; Nyns, E.J. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch. Microbiol. 1994, 161, 345–351. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide to ethanol: effect of pH, gas pressure, reducing agent and yeast extract. Bioresour. Technol. 2012, 114, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Influence of process parameters on growth of Clostridium ljungdahlii and Clostridium autoethanogenum on synthesis gas. Enzyme Microb. Technol. 2009, 44, 281–288. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, J.; Zhang, Y.; Xu, H.; Yuan, Z.; Li, D. Medium optimization for ethanol production with Clostridium autoethanogenum with carbon monoxide as sole carbon source. Bioresour. Technol. 2010, 101, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst. Eng. 2009, 32, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.D.; Caldwell, M.E.; Lawson, P.A.; Huhnke, R.L.; Tanner, R.S. Alkalibaculum bacchi gen. nov., sp. nov., a CO-oxidizing, ethanol-producing acetogen isolated from livestock-impacted soil. Int. J. Syst. Evol. Microbial. 2010, 60, 2483–2489. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Fermentative production of ethanol from syngas using novel moderately alkaliphilic strains of Alkalibaculum bacchi. Bioresour. Technol. 2012, 104, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Köpke, M.; Noack, S.; Dürre, P. The Past, Present, and Future of Biofuels—Biobutanol as Promising Alternative. In Biofuel Production-Recent Developments and Prospects; dos Santos Bernades, M.A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 451–486. [Google Scholar]
- Dürre, P. Biobutanol: an attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiolog. Rev. 1986, 50, 484–524. [Google Scholar]
- Ni, Y.; Sun, Z. Recent progress on industrial fermentative production of acetone-butanol-ethanol by Clostridium acetobutylicum in China. Appl. Microbial. Biotechnol. 2009, 83, 415–423. [Google Scholar] [CrossRef]
- Green, E.M. Fermentative production of butanol—the industrial perspective. Curr. Opin. Biotechnol. 2011, 22, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.C.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbial. 2005, 55, 2085–2091. [Google Scholar] [CrossRef]
- Paul, D.; Austin, F.W.; Arick, T.; Bridges, S.M.; Burgess, S.C.; Dandass, Y.S.; Lawrence, M.L. Genome sequence of the solvent-producing bacterium Clostridium carboxidivorans strain P7T. J. Bacterial. 2010, 192, 5554–5555. [Google Scholar] [CrossRef]
- Hemme, C.L.; Mouttaki, H.; Lee, Y.J.; Zhang, G.; Goodwin, L.; Lucas, S.; Copeland, A.; Lapidus, A.; Glavina del Rio, T.; Tice, H.; et al. Genome Announcement—Sequencing of Multiple Clostridia Genomes Related to Biomass Conversion and Biofuels Production. J. Bacterial. 2010, 192, 6494–6496. [Google Scholar] [CrossRef]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Ukpong, M.N.; Atiyeh, H.K.; De Lorme, M.J.M.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Küsel, K.; Dorsch, T.; Acker, G.; Stackebrandt, E.; Drake, H.L. Clostridium scatologenes strain SL1 isolated as an acetogenic bacterium from acidic sediments. Int. J. Syst. Evol. Microbial. 2000, 50, 537–546. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Lynd, L.H.; Thompson, T.E.; Krzycki, J.A.; Weimer, P.J.; Hegge, P.W. Isolation and characterization of a new, methylotrophic, acidogenic anaerobe, the marburg strain. Curr. Microbiol. 1980, 3, 381–386. [Google Scholar] [CrossRef]
- Worden, R.M.; Grethlein, A.J.; Jain, M.K.; Datta, R. Production of butanol and ethanol from synthesis gas via fermentation. Fuel 1991, 70, 615–619. [Google Scholar] [CrossRef]
- Lynd, L.; Kerby, R.; Zeikus, J.G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J. Bacterial. 1982, 149, 255–263. [Google Scholar]
- Grethlein, A.J.; Worden, R.M.; Jain, M.K.; Datta, R. Evidence for production of n-butanol from carbon monoxide by Butyribacterium methylotrophicum. J. Ferment. Bioeng. 1991, 72, 58–60. [Google Scholar] [CrossRef]
- DSMZ. DSM-3468. Available online: http://www.dsmz.de/catalogues/details/culture/DSM-3468.html (accessed on 20 August 2012).
- Gaddy, J.L.; Clausen, W.C. Clostridium ljungdahlii, an Anaerobic Ethanol and Acetate Producing Microorganism. U.S. Patent 5173429, 22 December 1992. [Google Scholar]
- Huhnke, R.; Lewis, R.; Tanner, R.S. Isolation and Characterization of Novel Clostridial Species. U.S. Patent 2008/0057554, 6 March 2008. [Google Scholar]
- Gaddy, J.; Arora, D.; Ko, C.; Phillips, J.; Basu, R.; Wikstrom, C.; Clausen, E. Methods for Increasing the Production of Ethanol from Microbial Fermentation. U.S. Patent 2012/0122173 A1, 17 May 2012. [Google Scholar]
- Simpson, S.D.; Warner, I.L.; Fung, J.M.Y.; Köpke, M. Optimised fermentation media. U.S. Patent 20110294177, 1 December 2011. [Google Scholar]
- Girbal, L.; Vasconcelos, I.; Saint-Amans, S.; Soucaille, P. How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol. Rev. 1995, 16, 151–162. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Wilkins, M.R.; Delorme, M.J.M.; Atiyeh, H.K.; Huhnke, R.L. Effects of Various Reducing Agents on Syngas Fermentation by “Clostridium ragsdalei”. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2010; Volume 2, pp. 135–144. [Google Scholar]
- Kundiyana, D.K.; Huhnke, R.L.; Maddipati, P.; Atiyeh, H.K.; Wilkins, M.R. Feasibility of incorporating cotton seed extract in Clostridium strain P11 fermentation medium during synthesis gas fermentation. Bioresour. Technol. 2010, 101, 9673–9680. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Nickel-based Enzyme Systems. J. Biolog. Chem. 2009, 284, 18571–18575. [Google Scholar] [CrossRef]
- Grethlein, A.J.; Worden, R.M.; Jain, M.K.; Datta, R. Continuous production of mixed alcohols and acids from carbon monoxide. Appl. Biochem. Biotechnol. 1990, 24-25, 875–884. [Google Scholar] [CrossRef]
- Ungerman, A.J.; Heindel, T.J. Carbon monoxide mass transfer for syngas fermentation in a stirred tank reactor with dual impeller configurations. Biotechnol. Prog. 2007, 23, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, P.C.; Khanal, S.K. Syngas fermentation to biofuel: evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations. Biotechnol. Prog. 2010, 26, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J. Biological Production of Products from Waste Gases. U.S. Patent 6340581 B1, 22 January 2002. [Google Scholar]
- Trevethick, S.; Bromley, J.; Simpson, S.; Khosla, V. Improved Fermentation of Gaseous Substrates. Patent WO 2011/028137 A1, 10 March 2011. [Google Scholar]
- Tsai, S.P.; Datta, R.; Basu, R.; Yoon, S.H. Syngas Conversion System Using Asymmetric Membrane and Anaerobic Microorganism. U.S. Patent 2009/0215163 A1, 29 August 2009. [Google Scholar]
- Tsai, S.P.; Datta, R.; Basu, R.; Yoon, S.H.; Robey, R. Modular Membrane Supported Bioreactor for Conversion of Syngas Components to Liquid Products. U.S. Patent 2009/0029434 A1, 29 January 2009. [Google Scholar]
- Hickey, R.; Datta, R.; Tsai, S.-P.; Basu, R. Membrane Supported Bioreactor for Conversion of Syngas Components to Liquid Products. U.S. Patent 2011/0256597 A1, 20 October 2011. [Google Scholar]
- Hickey, R.; Basu, R.; Datta, R.; Tsai, S. Method of Conversion of Syngas Using Microorganism on Hydrophilic Membrane. U.S. Patent 7923227, 12 April 2011. [Google Scholar]
- Desai, R.P.; Papoutsakis, E.T. Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl. Environ. Microbial. 1999, 65, 936–945. [Google Scholar]
- Tummala, S.B.; Welker, N.E.; Eleftherios, T. Development and Characterization of a Gene Expression Reporter System for Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbial. 1999, 65, 3793–3799. [Google Scholar]
- Girbal, L.; Mortier-Barriere, I.; Raynaud, F.; Rouanet, C.; Croux, C.; Soucaille, P. Development of a sensitive gene expression reporter system and an inducible promoter-repressor system for Clostridium acetobutylicum. Appl. Environ. Microbiol. 2003, 69, 4985–4988. [Google Scholar] [CrossRef] [PubMed]
- Feustel, L.; Nakotte, S.; Durre, P. Characterization and development of two reporter gene systems for Clostridium acetobutylicum. Appl. Environ. Microbiol. 2004, 70, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Hong, W.; Zhang, J.; Li, W.; Feng, Y.; Liu, Y.; Cui, Q. Targeted gene engineering in Clostridium cellulolyticum H10 without methylation. J. Microbial. Methods 2012, 89, 201–208. [Google Scholar] [CrossRef]
- Girbal, L.; Mortier-barrière, I.; Rouanet, C.; Croux, C.; Mortier-barrie, I.; Soucaille, P. Development of a Sensitive Gene Expression Reporter System and an Inducible Promoter-Repressor System for Clostridium acetobutylicum. Appl. Environ. Microbial. 2003, 69, 4985–4988. [Google Scholar] [CrossRef]
- Dong, H.; Tao, W.; Zhang, Y.; Li, Y. Development of an anhydrotetracycline-inducible gene expression system for solvent-producing Clostridium acetobutylicum: A useful tool for strain engineering. Metab. Eng. 2012, 14, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.P.; Jones, S.W.; Papoutsakis, E.T. Inactivation of σE and σG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J. Bacterial. 2011, 193, 1414–1426. [Google Scholar] [CrossRef]
- Argyros, D.A.; Tripathi, S.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbial. 2011, 77, 8288–8294. [Google Scholar] [CrossRef]
- Tripathi, S.; Olson, D.G.; Argyros, D.A.; Miller, B.B.; Barrett, T.F.; Murphy, D.M.; McCool, J.D.; Warner, A.K.; Rajgarhia, V.B.; Lynd, L.R.; et al. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl. Environ. Microbial. 2010, 76, 6591–6599. [Google Scholar] [CrossRef]
- Tracy, B.; Papoutsakis, E. Methods and Compositions for Genetically Engineering Clostridia Species. U.S. Patent 2010/0075424, 25 March 2010. [Google Scholar]
- Cartman, S.; Minton, N. Method of Double Crossover Homologous Recombination in Clostridia. Patent WO/2010/084349, 29 July 2010. [Google Scholar]
- Soucaille, P.; Figge, R.; Croux, C. Process for Chromosomal Integration and DNA Sequence Replacement in Clostridia. Patent WO/2008/040387, 10 April 2008. [Google Scholar]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Carter, G.P.; Minton, N.P. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiology. Methods 2007, 70, 452–464. [Google Scholar] [CrossRef]
- Heap, J.T.; Kuehne, S.; Ehsaan, M.; Cartman, S.T.; Cooksley, C.M.; Scott, J.C.; Minton, N.P. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J. Microbiol. Methods 2010, 80, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Heap, J.T.; Cooksley, C.M.; Cartman, S.T.; Minton, N.P. ClosTron-mediated engineering of Clostridium. Methods Mol. Boil. 2011, 765, 389–407. [Google Scholar]
- Heap, J.T.; Ehsaan, M.; Cooksley, C.M.; Ng, Y.K.; Cartman, S.T.; Winzer, K.; Minton, N.P. Integration of DNA into bacterial chromosomes from plasmids without a counter-selection marker. Nucleic Acids Res. 2012, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lütke-Eversloh, T.; Bahl, H. Metabolic engineering of Clostridium acetobutylicum: Recent advances to improve butanol production. Curr. Opin. Biotechnol. 2011, 22, 634–647. [Google Scholar] [CrossRef] [PubMed]
- William, J.R. Securities and Exchange Commission. Coskata, Inc.: Warrenville, IL, USA. Available online: http://www.sec.gov/Archives/edgar/data/1536893/000119312511343587/d267854ds1.htm (accessed on 9 July 2012).
- Tirado-Acevedo, O. Production of Bioethanol from Synthesis Gas Using Clostridium ljungdahlii. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 17 November 2010. [Google Scholar]
- Koepke, M.; Liew, F. Production of Butanol from Carbon Monoxide by a Recombinant Microorganism. Patent WO/2012/053905, 26 April 2012. [Google Scholar]
- Lederle, S.M. Heterofermentative Acetonproduktion. Ph.D. Thesis, Ulm University, Ulm, Germany, 7 July 2010. [Google Scholar]
- May, A.; Fischer, R.J.; Maria Thum, S.; Schaffer, S.; Verseck, S.; Dürre, P.; Bahl, H. A modified pathway for the production of acetone in Escherichia coli. Metab. Eng. 2012, in press. [Google Scholar]
- Peralta-Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Trawick, J.D.; Burk, M.J.; Burgard, A.P. Microorganisms and Methods for Conversion of Syngas and Other Carbon Sources to Useful Products. Patent WO/2010/071697, 26 June 2010. [Google Scholar]
- Burk, M.; Schilling, C.H.; Burgard, A.; Trawick, J.D. Methods and Organisms for Utilizing Synthesis Gas or Other Gaseous Carbon Sources and Methanol. Patent WO/2009/094485, 7 July 2009. [Google Scholar]
- Papoutsakis, E.T.; Al-Hinai, M.A.; Jones, S.W.; Indurthi, D.C.; Mitchell, D.K.; Fast, A. Recombinant Clostridia That Fix CO2 and CO and Uses Thereof. U.S. Patent 2012/0064587 A1, 15 March 2012. [Google Scholar]
- Kaster, A.K.; Goenrich, M.; Seedorf, H.; Liesegang, H.; Wollherr, A.; Gottschalk, G.; Thauer, R.K. More than 200 genes required for methane formation from H2 and CO2 and energy conservation are present in Methanothermobacter marburgensis and Methanothermobacter thermautotrophicus. Archaea 2011, 2011, 973848. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Ko, H.J.; Kim, D.; Choi, D.G.; Park, S.; Kim, S.; Chang, I.S.; Choi, I.G. Complete Genome Sequence of a Carbon Monoxide-Utilizing Acetogen, Eubacterium limosum KIST612. J. Bacterial. 2011, 193, 307–308. [Google Scholar] [CrossRef]
- Elsevier B.V. SciVerse® Scopus®. Available online: http://www.scopus.com (accessed on 20 July 2012).
- Thomson Reuters. Thomson Innovation. Available online: http://thomsoninnovation.com (accessed on 20 July 2012).
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of biomass-generated producer gas to ethanol. Biotechnol. Bioeng. 2004, 86, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Lewis, R.S. Fermentation of biomass-generated synthesis gas: Effects of nitric oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Rabou, L.P. L.M.; Zwart, R.W.R.; Vreugdenhil, B.J.; Bos, L. Tar in Biomass Producer Gas, the Energy research Centre of the Netherlands (ECN) Experience: An Enduring Challenge. Energy Fuels 2009, 23, 6189–6198. [Google Scholar] [CrossRef]
- Brogren, C.; Karlsson, H.T.; Bjerle, I. Absorption of NO in an alkaline solution of KMnO4. Chem. Eng. Technol. 1997, 20, 396–402. [Google Scholar] [CrossRef]
- Chu, H.; Chien, T.W.; Li, S.Y. Simultaneous absorption of SO2 and NO from flue gas with KMnO4/NaOH solutions. Sci. Total Environ. 2001, 275, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sada, E.; Kumazawa, H.; Kudo, I.; Kondo, T. Absorption of NO in aqueous mixed solutions of NaClO2 and NaOH. Chem. Eng. Sci. 1978, 33, 315–318. [Google Scholar] [CrossRef]
- Mojtahedi, W.; Ylitalo, M.; Maunula, T.; Abbasian, J. Catalytic decomposition of ammonia in fuel gas produced in pilot-scale pressurized fluidized-bed gasifier. Fuel Process. Technol. 1995, 45, 221–236. [Google Scholar] [CrossRef]
- Xu, C.C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Grethlein, A.J.; Soni, B.K.; Worden, R.M.; Jain, M.K. Influence of hydrogen sulfide on the growth and metabolism of butyribacterium methylotrophicum and clostridium acetobutylicum. Appl. Biochem. Biotechnol. 1992, 34–35, 233–246. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Bain, R.L.; Davis, R.E.; Dutta, A.; Feik, C.J.; Gaston, K.R.; Jablonski, W.; Phillips, S.D.; Nimlos, M.R. Pilot-Scale Gasification of Corn Stover, Switchgrass, Wheat Straw, and Wood: 1. Parametric Study and Comparison with Literature. Ind. Eng. Chem. Res. 2010, 49, 1859–1871. [Google Scholar] [CrossRef]
- Imlay, J. Iron-sulphur clusters and the problem with oxygen. Mol. Microbial. 2006, 59, 1073–1082. [Google Scholar] [CrossRef]
- Uyeda, K.; Rabinowitz, J.C. Pyruvate-ferredoxin oxidoreductase. IV. Studies on the reaction mechanism. J. Biol. Chem. 1971, 246, 3120–3125. [Google Scholar] [PubMed]
- Karnholz, A.; Küsel, K.; Gössner, A.; Schramm, A.; Drake, H.L. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl. Environ. Microbiol. 2002, 68, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Watamura, Y.; Ono, M.; Watanabe, T.; Takeda, K.; Niimura, Y. Adaptive responses to oxygen stress in obligatory anaerobes Clostridium acetobutylicum and Clostridium aminovalericum. Appl. Environ. Microbiol. 2005, 71, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Ishikura, J.; Watamura, Y.; Niimura, Y. Identification of O2-induced peptides in an obligatory anaerobe, Clostridium acetobutylicum. FEBS Lett. 2004, 571, 21–25. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Keele, B.B.; Fridovich, I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 1971, 68, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dong, H.; Zhang, Y.; Li, Y. Engineering the robustness of Clostridium acetobutylicum by introducing glutathione biosynthetic capability. Metab. Eng. 2011, 13, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, H.; Den Uil, H.; Calis, H.P. Green Diesel from Biomass via Fischer–Tropsch Synthesis: New Insights in Gas Cleaning and Process Design. In Proceedings of Pyrolysis and Gasification of Biomass and Waste, Expert Meeting, Strasbourg, France, 30 September–1 October 2002.
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb. Cell Fact. 2005, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Takeda, K.; Kobayashi, G.; Sonomoto, K. High production of acetone-butanol-ethanol with high cell density culture by cell-recycling and bleeding. J. Biotechnol. 2005, 120, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.; Ackerson, C.; Clausen, E.; Gaddy, J. Biological conversion of synthesis gas into fuels. Int. J. Hydrog. Energy 1992, 17, 281–288. [Google Scholar] [CrossRef]
- Jones, D.T. Bacteriophages of Clostridium. In Handbook on Clostridia; Dürre, P., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 699–719. [Google Scholar]
- Jones, D.T.; Shirley, M.; Wu, X.; Keis, S. Bacteriophage infections in the industrial acetone butanol (AB) fermentation process. J. Mol. Microbiol. Biotechnol. 2000, 2, 21–26. [Google Scholar] [PubMed]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefin. 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Xiu, Z.L.; Zeng, A.P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.L. Clostridium strain which produces acetic acid from waste gases. U.S. Patent 5593886, 14 January 1997. [Google Scholar]
- Gaddy, J.L. Biological production of acetic acid from waste gases with Clostridium ljungdahlii. U.S. Patent 5807722, 15 September 1998. [Google Scholar]
- INEOS Bio. INEOS Bio: Advanced bioethanol production from ligno-cellulose residues and waste materials. Available online: http://www.biee.org/downloads/?dir=&download=INEOS+Bio+presentation+for+EI+April+2011.pdf (accessed on 22 July 2012).
- Klasson, K.T.; Elmore, B.B.; Vega, J.L.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Biological production of liquid and gaseous fuels from synthesis gas. Appl. Biochem. Biotechnol. 1990, 24–25, 857–873. [Google Scholar] [CrossRef]
- INEOS. Cars to run on fuel from household waste within two years. Available online: http://www.ineos.com/new_item.php?id_press=223 (accessed on 22 July 2012).
- Wald, M. Yet Another Route to Cellulosic Ethanol. Available online: http://nyti.ms/POnkOJ (accessed on 16 July 2012).
- Williams, P. Ineos Bio Takes Advanced Biofuel Technology Commercial. Available online: http://biomassmagazine.com/articles/6841/ineos-bio-takes-advanced-biofuel-technology-commercial (accessed on 16 July 2012).
- INEOS Bio. INEOS Bio JV Breaks Ground on 1st Advanced Waste-to-Fuel Commercial Biorefinery in U.S. Available online: http://www.ineosbio.com/76-Press_releases-15.htm (accessed on 16 July 2012).
- INEOS Bio. INEOS Bio Names AMEC as its Global License Support Engineering Firm for its Waste-to-Bioenergy Technology. Available online: http://www.ineosbio.com/76-Press_releases-33.htm (accessed on 16 July 2012).
- U.S. Department of Energy. INEOS Bio Commercializes bioenergy technology in Florida. Available online: http://www1.eere.energy.gov/biomass/pdfs/ibr_arraprojects_ineos.pdf (accessed on 1 August 2012).
- INEOS Bio. Pioneering waste-to–biofuel technology attracts cross-Party support in UK. Available online: http://www.ineosbio.com/76-Press_releases-16.htm (accessed on 16 July 2012).
- Zahn, J.A.; Saxena, J. Novel Ethanologenic Species Clostridium Coskatii. U.S. Patent 2011/0229947 A1, 22 September 2011. [Google Scholar]
- Coskata Inc Coskata, Inc.’s Semi-Commercial Facility Demonstrates Two Years of Successful Operation. Available online: http://www.coskata.com/company/media.asp?story=504B571C-0916-474E-BFFA-ACB326EFDB68 (accessed on 23 July 2012).
- Lane, J. Coskata switches focus from biomass to natural gas; to raise $100M in natgas-oriented private placement. Available online: http://www.biofuelsdigest.com/bdigest/2012/07/20/coskata-switches-from-biomass-to-natural-gas-to-raise-100m-in-natgas-oriented-private-placement/ (accessed on 23 July 2012).
- Heijstra, B.; Kern, E.; Koepke, M.; Segovia, S.; Liew, F. Novel bacteria and methods of use thereof. Patent WO/2012/015317, 2 February 2012. [Google Scholar]
- LanzaTech. INVISTA and LanzaTech Sign Joint Development Agreement for Bio-Based Butadiene. Available online: http://www.lanzatech.com/sites/default/files/imce_uploads/news_release_-_invista_announces_lanzatech_partnership_-_embargoed_final_0.pdf (accessed on 25 August 2012).
- LanzaTech. LanzaTech’s commercialisation goes transtasman. Available online: http://www.lanzatech.co.nz/sites/default/files/imce_uploads/lanzatech_signs_with_bluescope_steel_march_2012.pdf (accessed on 23 July 2012).
- Herndon, A. Range Fuels Sells Government-Backed Biofuel Plant To LanzaTech. Available online: http://www.bloomberg.com/news/2012-01-04/range-fuels-sells-government-backed-biofuel-plant-to-lanzatech.html (accessed on 23 July 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372-5417. https://doi.org/10.3390/en5125372
Daniell J, Köpke M, Simpson SD. Commercial Biomass Syngas Fermentation. Energies. 2012; 5(12):5372-5417. https://doi.org/10.3390/en5125372
Chicago/Turabian StyleDaniell, James, Michael Köpke, and Séan Dennis Simpson. 2012. "Commercial Biomass Syngas Fermentation" Energies 5, no. 12: 5372-5417. https://doi.org/10.3390/en5125372




