Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass
Abstract
:1. Introduction
- Microalgae can be cultivated in brackish water on non-arable land, and therefore may not incur land use change, minimizing associated environmental impacts [6]. Utilizing marine biomass, which can be grown in a variety of marine environments including fresh water and salt water, avoids the problem of land use change from arable to bioenergy crops [1,8]. Utilizing the marine environment ensures a large cultivation area, limiting competition with other land uses and resources [3].
- Microalgae production can utilize the carbon dioxide component of flue gas, reducing the carbon emissions from power plants [2]. Marine macroalgae have a high rate of carbon dioxide fixation from the atmosphere and water at 8–10 t ha−1 yr−1, comparable to temperate woodlands [3,4]. As such, they have a high potential for carbon dioxide remediation.
2. European Union (EU) Sustainability Criteria
- The directive lays out certain GHG emissions reductions to be achieved from the use of biofuels. In the case of biofuels and produced by installations that were in operation on 23 January 2008, GHG emissions savings must be at least 35% from 2013. This figure rises to 50% in 2017, and further to 60% in 2018 for biofuels produced in installations in which production started on or after January 2017.
- The raw materials sourced for biofuel production, from within the EU or from third countries, should not be obtained from land with high biodiversity value, land with a high carbon stock, or land that was peatland in 2008 [9].
3. Microalgae
| Plant source | Biodiesel (L ha−1 yr−1) | Area required to produce global oil demand (hectares × 106) | Area required as percent global land mass | Area as percent global arable land |
|---|---|---|---|---|
| Cotton | 325 | 15,002 | 100.7 | 756.9 |
| Soybean | 446 | 10,932 | 73.4 | 551.6 |
| Mustard seed | 572 | 8,524 | 57.2 | 430.1 |
| Sunflower | 952 | 5,121 | 34.4 | 258.4 |
| Rapeseed/canola | 1,190 | 4,097 | 27.5 | 206.7 |
| Jatropha | 1,892 | 2,577 | 17.3 | 130 |
| Oil palm | 5,950 | 819 | 5.5 | 41.3 |
| Microalgae (10 g m−2 day−1 at 30% TAG) | 12,000 | 406 | 2.7 | 20.5 |
| Microalgae (50 g m−2 day−1 at 50% TAG) | 98,500 | 49 | 0.3 | 2.5 |
3.1. Cultivation and Harvest
3.2. Processing
3.3. Biochemical Processing
3.3.1. Transesterification
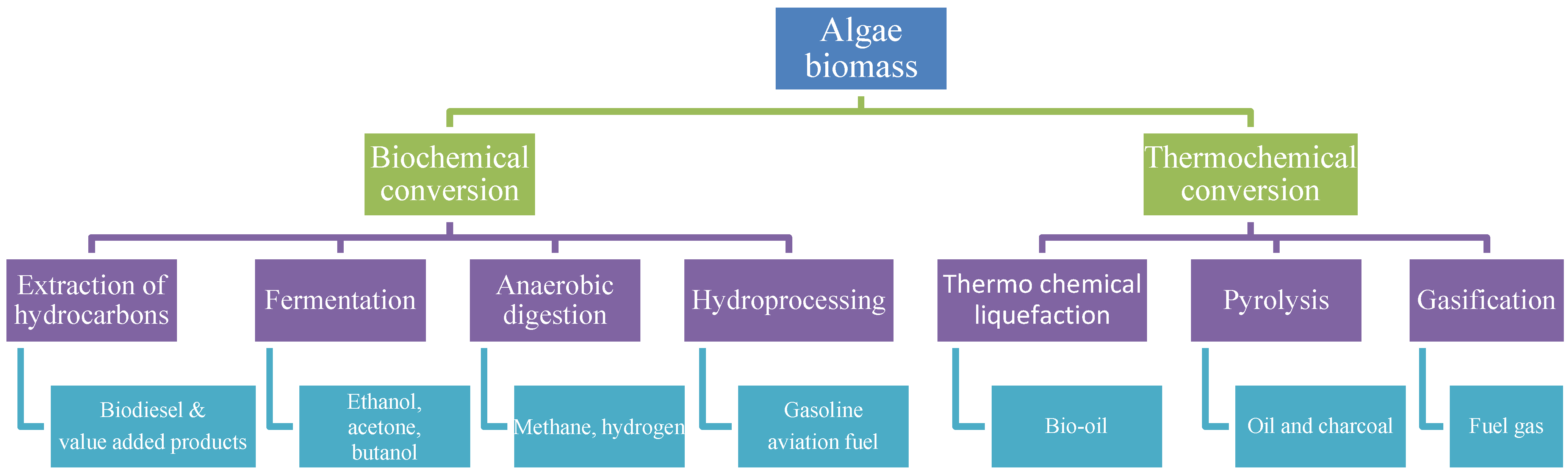
3.3.2. Fermentation
3.3.3. Anaerobic Digestion
3.3.4. Hydroprocessing
3.4. Thermochemical Processing
3.4.1. Hydrothermal Liquefaction
3.4.2. Gasification
3.4.3. Pyrolysis
3.5. Properties
| Parameter | Algae 1 | Algae 2 | Unit |
|---|---|---|---|
| Free-fatty acids | 0.45 | 1.75 | % weight |
| Cloud point | −5.2 | 3.9 | °C |
| Cold filter plugging point | −7 | 2 | °C |
| Free Glycerin | 0.009 | 0.014 | mass% |
| Total Glycerin | 0.091 | 0.102 | mass% |
| Monoglycerides | 0.265 | 0.292 | mass% |
| Diglycerides | 0.078 | 0.070 | mass% |
| Triglycerides | 0.020 | 0.019 | mass% |
| Water & sediment | <0.005 | <0.005 | % volume |
| Acid number | 0.022 | 0.003 | mg KOH/g |
| Visual inspection | 1 | 1 | Haze |
| Relative density at 60 F | 0.8780 | 0.8780 | N/A |
| Oxidative stability (110 °C) | 8.5 | 11 | h |
| Flash point (closed cup) | >160 | >160 | °C |
| Moisture | 0.037 | 0.026 | mass% |
| Cold soak filtration | 85 | 84 | s |
| Sulfur | 5.1 | 0.6 | ppm |
| Calcium | <0.1 | 0.7 | ppm (µg/g) |
| Magnesium | 0.3 | 1.1 | ppm (µg/g) |
| Phosphorus | <0.1 | <0.1 | mass% |
| Carbon residue | 0.007 | 0.042 | mass% |
| Sulfated ash | <0.005 | <0.005 | mass% |
| Kinematic viscosity at 40 °C | 4.519 | 4.624 | mm2/s |
| Copper corrosion (3 h at 50 °C) | 1a | 1a | N/A |
3.6. Commercial Biofuel—Worldwide Production
| Technology | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|
| Fermentation | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 945.38 | 945.38 |
| Hydroprocessing | 0.57 | 61.88 | 162.69 | 166.47 | 166.47 | 166.47 | 166.47 |
| Transesterification | 1.87 | 2.06 | 14.42 | 14.42 | 14.42 | 14.42 | 14.42 |
| Other | 0.38 | 0.76 | 0.76 | 1.66 | 1.66 | 1.66 | 1.66 |
| Total | 3.20 | 65.08 | 178.25 | 182.94 | 182.94 | 1127.94 | 1127.94 |
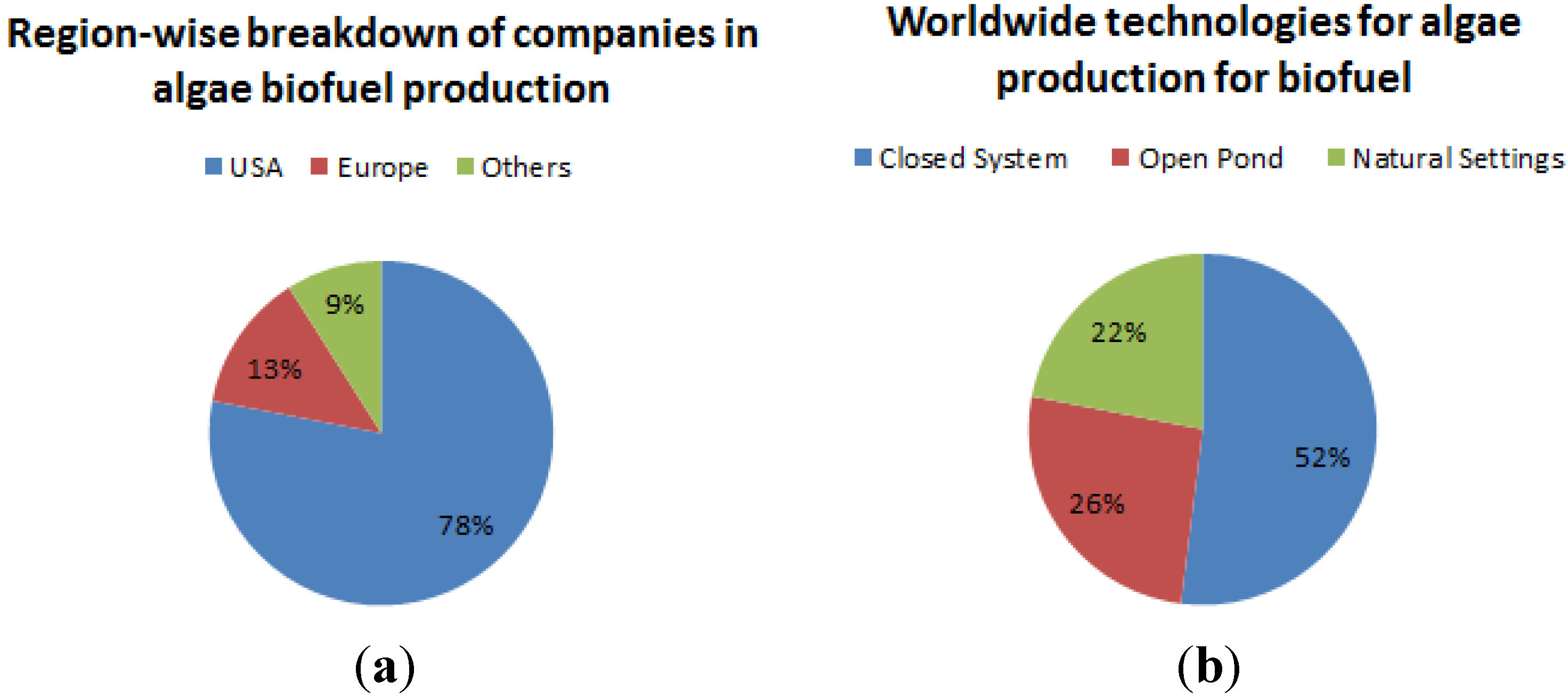
3.7. Resource Potential—Ireland
4. Macroalgae
| Seaweed | Class | Carbohydrate composition | Total carbohydrates (%) | Lipid (%) | Protein (%) | Ash (%) | Sugars released by hydrolysis | Sugar composition |
|---|---|---|---|---|---|---|---|---|
| Gelidium amansii | Red | Agar, Carrageenan, Cellulose | 75.2–83.6 | 0.6–1.1 | 12.2–18.5 | 3.3–5.7 | 34.6–67.5 | Glucose. Galactose |
| Laminaria japonica | Brown | Laminarin, Mannitol, Alginate, Fucoidan, Cellulose | 51.9–59.5 | 1.5–1.8 | 8.1–14.8 | 30.9–31.5 | 9.6–37.6 | Glucose, Mannitol |
| Sargassum fulvellum | 39.6 | 1.4 | 13 | 46 | 9.6 | |||
| Ulva lactuca | Green | Starch, Cellulose | 54.3 | 6.2 | 20.6 | 18.9 | 19.4 | Glucose |
| Ulva pertusa | 65.2 | 2.6 | 7.0 | 25.2 | 59.6 |
4.1. Cultivation and Harvest

4.2. Processing
4.2.1. Fermentation
4.2.2. Anaerobic Digestion
4.2.3. Hydrothermal Liquefaction
4.2.4. Pyrolysis
4.2.5. Transesterification
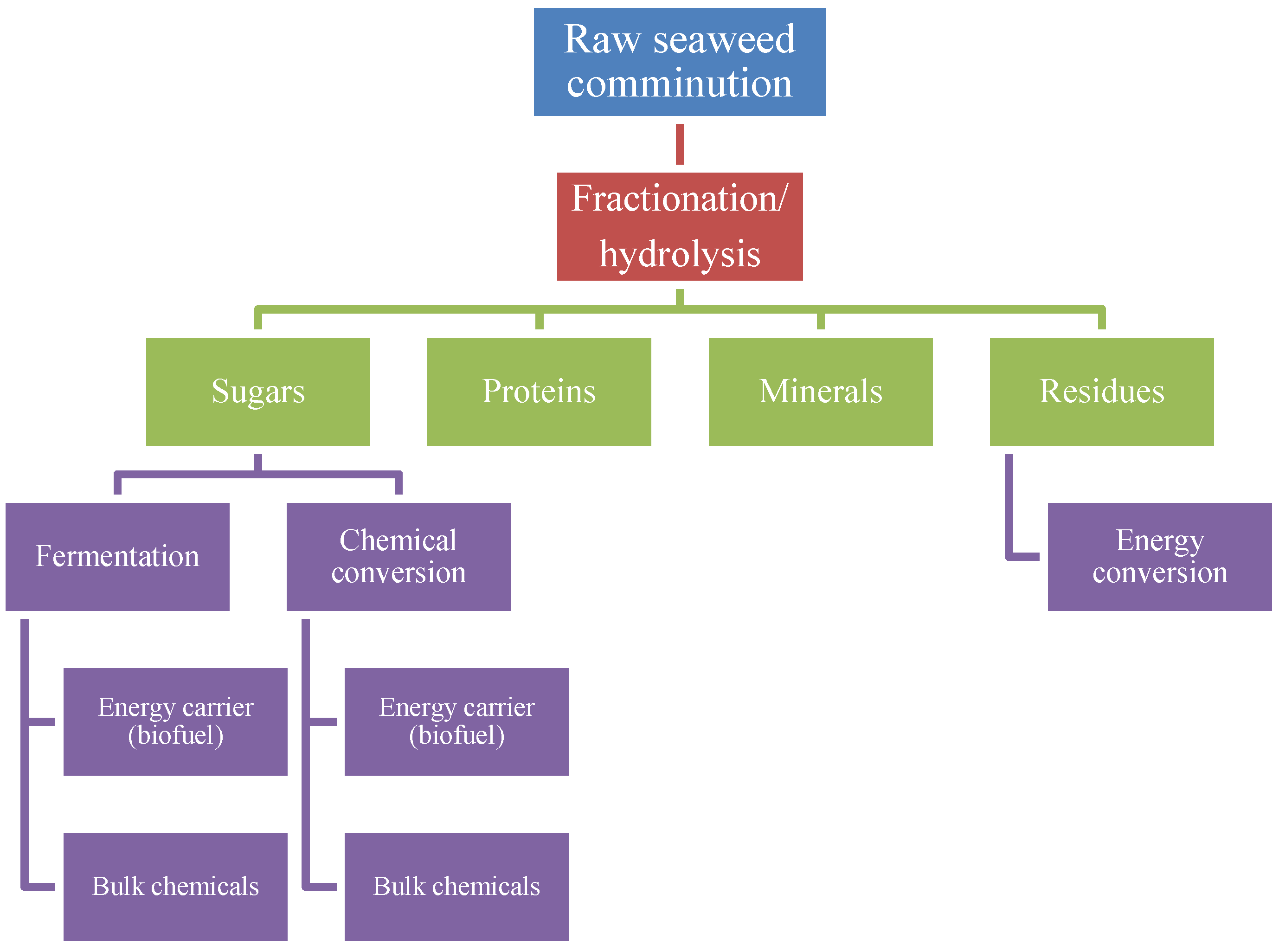
4.3. Biorefinery Concept
4.4. Properties
4.5. Commercial Biofuel from Macroalgae
| Properties | Units | Algal biodiesel | ASTM limits |
|---|---|---|---|
| Flash point | °C | 166 | >160 min |
| Kinematic viscosity at 40 °C | mm2 s−1 | 4.35 | 1.9–6.0 |
| Water and sediment | vol.% | 0.005 | 0.050 max |
| Density at 15 °C | kg m−3 | 878.47 | n/a |
| Cetane number | - | 58.5 | 47 min |
| Cloud point | °C | 3 | n/a |
| Acid value | mg KOH g−1 | 0.43 | 0.8 |
| Free glycerine | mass% | 0.0034 | 0.020 |
| Total glycerin | mass% | 0.123 | 0.240 |
| Sulfated ash | mass% | 0.0024 | 0.020 |
| Pour point | °C | −2 | n/a |
| Carbon residue | mass% | 0.01 | 0.050 |
| Sulfur | mass% | 0.00056 | 0.05 |
| Copper strip corrosion | - | 1 | No. 3 max |
| Distillation temperature | °C | 346 | 360 |
| Phosphorous | mass% | 0.0004 | 0.001 |
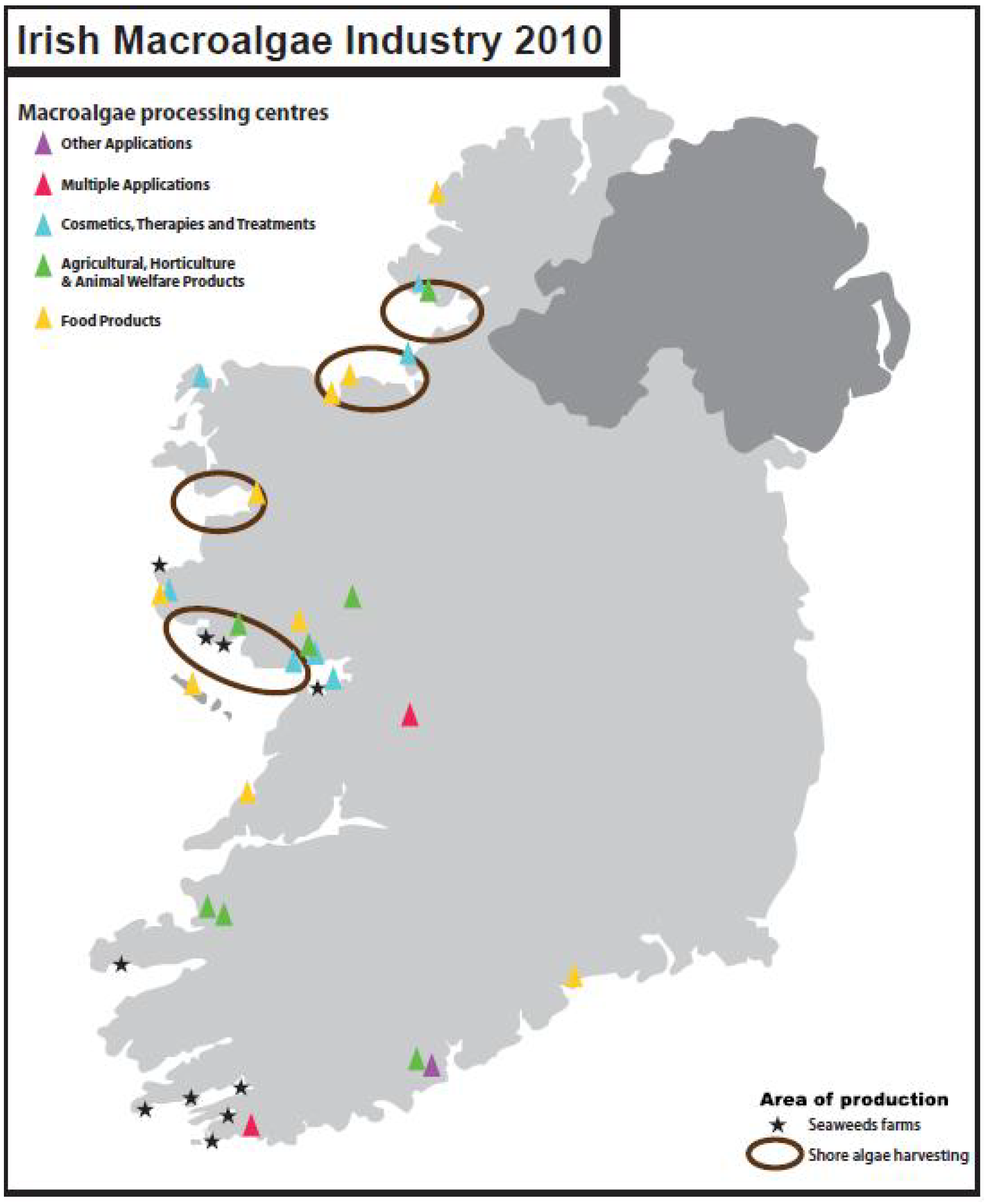
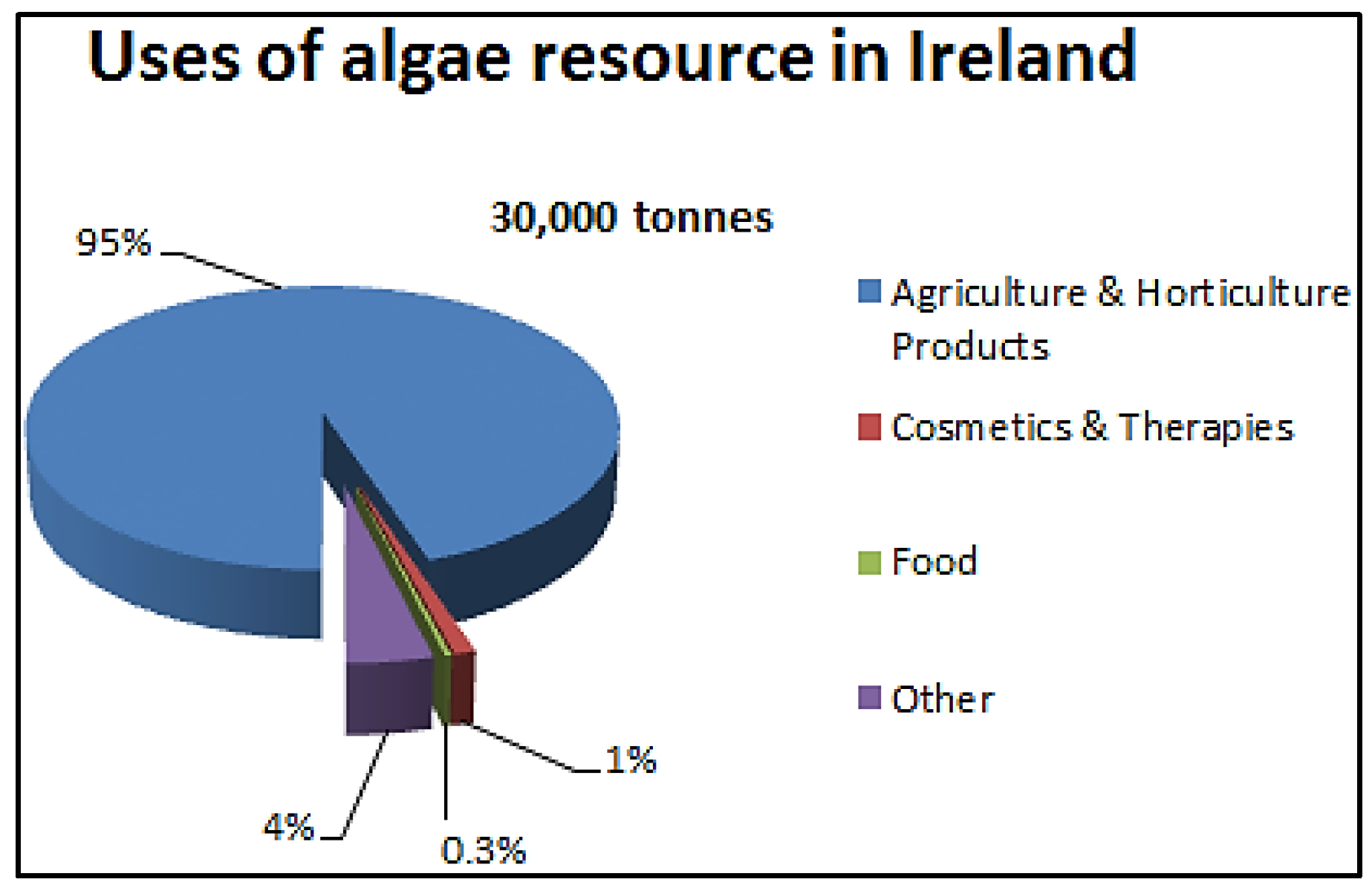
4.6. Macroalgae in Ireland
5. Life Cycle Assessment (LCA) of Micro and Macroalgae Systems
5.1. Microalgae
5.2. Macroalgae
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Reith, J.H.; van Hal, J.W.; Lenstra, W.J. Large-Scale Carbon Recycling via Cultivation and Biorefinery of Seaweeds for Production of Biobased Chemicals and Fuels. In Proceedings of the Conference on Carbon Dioxide as Feedstock for Chemistry and Polymers, Essen, Germany, 10–11 October 2012.
- Skjermo, J. Macroalgae Activities in Norway; Nordic Algae Workshop: Grenaa, Denmark, 2012. [Google Scholar]
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production: A Feasibility Study with Recommendations for Further Research; Marine Estate Research Report; The Crown Estate, the Scottish Association for Marine Science: Scotland, UK, 2008. [Google Scholar]
- European Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, L40, 16–47. [Google Scholar]
- Benemann, J.R.; Oswald, W.J. Systems and Economic Analysis of Microalgae Ponds for Conversion of CO2 to Biomass; University of California: Berkeley, CA, USA, 1996. [Google Scholar]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland; Sustainable Energy Ireland: Dublin, Ireland, 2009. [Google Scholar]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Mechanism and challenges in commercialisation of algal biofuels. Bioresour. Technol. 2011, 102, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Kanda, H.; Li, P. Simple extraction method of green crude from natural blue-green microalgae by dimethyl ether. Fuel 2011, 90, 1264–1266. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Sarada, R.; Vidyashankar, S.; VenuGopal, K.S.; Kumudha, A. Cultivation of Micro-Algae for Lipids and Hydrocarbons, and Utilization of Spent Biomass for Livestock Feed and for Bio-Active Constituents. In Biofuel Co-Products for Livestock Feed—Opportunities and Challenges; Makkar, H.P.S., Ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012; Chapter 24; pp. 423–446. [Google Scholar]
- Singh, N.K.; Dhar, D.W. Microalgae as second generation biofuel. A review. Agron. Sustain. Dev. 2011, 31, 605–629. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Bezergianni, S.; Dimitriadis, A.; Kalogianni, A.; Pilavachi, P.A. Hydrotreating of waste cooking oil for biodiesel production. Part I: Effect of temperature on product yields and heteroatom removal. Bioresour. Technol. 2010, 101, 6651–6656. [Google Scholar] [CrossRef] [PubMed]
- Kanes, S.; Forster, D. The Choice of Next-Generation Biofuels (Algae Excerpt); Equity Research Industry Report; Scotia Capital: New York, NY, USA, 2009. [Google Scholar]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae biofuel potentials (Review). Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar] [CrossRef]
- Jet Fuel from Microalgal Lipids; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Guzman, A.; Torres, J.E.; Prada, L.P.; Nuñez, M.L. Hydroprocessing of crude palm oil at pilot plant scale. Catal. Today 2010, 156, 38–43. [Google Scholar] [CrossRef]
- Chernova, N.I.; Korobkova, T.P.; Kiseleva, S.V. Use of biomass for producing liquid fuel: Current state and innovations. Therm. Eng. 2010, 57, 937–945. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar]
- Brown, T.M.; Duan, P.; Savage, P.E. Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Jena, U.; Das, K.C.; Kastner, J.R. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef] [PubMed]
- Jena, U.; Das, K.C. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- López Barreiro, D.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Biller, P.; Riley, R.; Ross, A.B. Catalytic hydrothermal processing of microalgae: Decomposition and upgrading of lipids. Bioresour. Technol. 2011, 102, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Hirano, A.; Hon-Nami, K.; Kunito, S.; Hada, M.; Ogushi, Y. Temperature effect on continuous gasification of microalgal biomass: Theoretical yield of methanol production and its energy balance. Catal. Today 1998, 45, 399–404. [Google Scholar] [CrossRef]
- Sánchez Mirón, A.; Cerón Garcı́a, M.-C.; Garcı́a Camacho, F.; Molina Grima, E.; Chisti, Y. Growth and biochemical characterization of microalgal biomass produced in bubble column and airlift photobioreactors: Studies in fed-batch culture. Enzym. Microb. Technol. 2002, 31, 1015–1023. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Yang, C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Demirbaş, A. Oily products from mosses and algae via pyrolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2006, 28, 933–940. [Google Scholar] [CrossRef]
- Sanford, S.D.; White, J.M.; Shah, P.S.; Wee, C.; Valverde, M.A.; Meier, G.R. Feedstock and Biodiesel Characteristics Report; Renewable Energy Group, Inc.: Ames, IA, USA, 2009. [Google Scholar]
- Biofuels Digest Advanced Biofuels & Chemicals Project Database Release Q3 2012 (07/27/12-Revision 1.1). Available online: http://www.biofuelsdigest.com/bdigest/wp-content/uploads/2012/07/ABPD-Q312.xls (accessed on 20 March 2013).
- European Commission: Community Research and Development Information Service (CORDIS): Projects: Direct Ethanol from MicroAlgae (DEMA). Available online: http://cordis.europa.eu/projects/rcn/106280_en.html (accessed on 9 October 2013).
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; Pacific Northwest National Laboratory: Richland, WA, USA, 2010.
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Roberts, T.; Upham, P. Prospects for the use of macro-algae for fuel in Ireland and the UK: An overview of marine management issues. Mar. Policy 2012, 36, 1047–1053. [Google Scholar] [CrossRef]
- The Seaweed Site: Information on Marine Algae. Available online: http://www.seaweed.ie/algae/phaeophyta.php (accessed on 11 March 2013).
- Hughes, A.D.; Black, K.D.; Campbell, I.; Heymans, J.J.; Orr, K.K.; Stanley, M.S.; Kelly, M.S. Comments on “Prospects for the use of macroalgae for fuel in Ireland and UK: An overview of marine management issues”. Mar. Policy 2013, 38, 554–556. [Google Scholar] [CrossRef]
- Streefland, M.; Rous, J.-F.; Zachleder, V.; Casteleyn, G.; Vernon, P.-A.; Acien, G.F.; Garofalo, R. Report on Biofuel Production Processes from Micro-, Microalgae and Other Aquatic Biomass; AquaFUELs: Brussels, Belgium, 2010. [Google Scholar]
- Seaweed Industry in Europe; Netalgae: Cork, Ireland, 2012.
- Fishery and Aquaculture Statistics—Aquaculture Production; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2010.
- Critchley, A.T.; Ohno, M.; Largo, D.B. World seaweed resources. An authoritative reference system. Bot. Mar. 2006, 49, 456–457. [Google Scholar]
- Buck, B.H.; Buchholz, C.M. The offshore-ring: A new system design for the open ocean aquaculture of macroalgae. J. Appl. Phycol. 2004, 16, 355–368. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An Engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Nagendra Gandhi, N.; Renganathan, S. Production of algal biodiesel from marine macroalgae Enteromorpha compressa by two step process: Optimization and kinetic study. Bioresour. Technol. 2013, 128, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Streefland, M.; Wiffels, R.; van Es, M.; Boussiba, S.; Leu, S.; Zachleder, V.; Verween, A.; Vieira, V.V.; Acien, G.F.; Molina-Grima, E.; et al. Technological Assessment Including Downstream AddedValue Products; AquaFUELs: Brussels, Belgium, 2011. [Google Scholar]
- Ystanes, L.; Waage Fougner, M. Seaweed to Biofuels—Future Perspectives by Industry Actor; Marine Agronomy Group: Hilo, HI, USA, 2012. [Google Scholar]
- Seaweed Energy Solutions (SES) Awarded Contract by Statoil; Seaweed Energy Solutions AS: Trondheim, Norway, 2012.
- Statoil & Bio Architecture Lab Partner for Macroalgae-to-Ethanol. Available online: http://www.algaeindustrymagazine.com/statoil-bio-architecture-lab-partner-for-macroalgae-to-ethanol/ (accessed on 6 March 2013).
- Irish Macroalgae Industry; Netalgae: County Cork, Ireland, 2011.
- Welcome to the BioMara Project—BioMara. Available online: http://www.biomara.org/ (accessed on 11 October 2013).
- Vanegas, C.H.; Bartlett, J. Green energy from marine algae: Biogas production and composition from the anaerobic digestion of Irish seaweed species. Environ. Technol. 2013, 34, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.C. Reducing the Environmental Impact of Seacage Fish Farming through Cultivation of Seaweed. Ph.D. Thesis, The Open University, Buckinghamshire, UK, 2006. [Google Scholar]
- O’Sullivan, G.; McDonagh, N.; Pedreschi, D. Irish Participation in EU FP7 Funded Competitive Marine Research Projects during the Period 2007–2008; Marine Institute: Galway, Ireland, 2009. [Google Scholar]
- INTERREG IVB NWE Programme—Investing in Opportunities. Available online: http://www.nweurope.eu/index.php?act=project_detail&id=5376 (accessed on 9 October 2013).
- EnAlgae Home Website. Available online: http://www.enalgae.eu/index.htm (accessed on 11 October 2013).
- EnAlgae. EnAlgae Work Packages. Available online: http://www.enalgae.eu/enalgae-work-packages.htm (accessed on 11 October 2013).
- Farrelly, D.; Fagan, C.; McDonnell, K. Effect of Carbon Dioxide Concentrations on the Carbon Mitigation Potential of Microalgae. In Biosystems Engineering Research Review 17; Cummins, E., Curran, T., Eds.; University College Dublin: Dublin, Ireland, 2012; pp. 123–126. [Google Scholar]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies 2013, 6, 6391-6412. https://doi.org/10.3390/en6126391
Murphy F, Devlin G, Deverell R, McDonnell K. Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies. 2013; 6(12):6391-6412. https://doi.org/10.3390/en6126391
Chicago/Turabian StyleMurphy, Fionnuala, Ger Devlin, Rory Deverell, and Kevin McDonnell. 2013. "Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass" Energies 6, no. 12: 6391-6412. https://doi.org/10.3390/en6126391
APA StyleMurphy, F., Devlin, G., Deverell, R., & McDonnell, K. (2013). Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies, 6(12), 6391-6412. https://doi.org/10.3390/en6126391





