Use of Isomerization and Hydroisomerization Reactions to Improve the Cold Flow Properties of Vegetable Oil Based Biodiesel
Abstract
:1. Introduction
2. Methods
2.1. Catalyst Preparation
2.2. Hydrolysis
2.3. Isomerization
2.4. Hydroisomerization
2.5. Esterification
2.6. Gas Chromatograph/Mass Spectrometer (GC/MS)
2.7 Cloud and Pour Point
2.8. Carbon Deposition, CO Adsorption and Viscosity
2.9. Acid Number
| Sample | Hydrolysis | Esterification | |||
| Acid number (mg KOH/g oil) | Conversion (%) | Acid Number (mg KOH/g fatty acid) | Conversion (%) | ||
| Palm oil | Original | 197.3 | 95.2 | 3.71 | 98.21 |
| Reacted | 2.98 | 98.56 | |||
| Coconut oil | Original | 249.6 | 96.9 | 4.02 | 98.64 |
| Reacted | 2.94 | 98.84 | |||
| Rapeseed oil | Original | 189.3 | 94.7 | 1.72 | 99.14 |
| Reacted | 3.26 | 98.37 | |||
| Corn oil | Original | 192.3 | 96.2 | 2.42 | 98.47 |
| Reacted | 3.01 | 98.50 | |||
| Soybean oil | Original | 190.4 | 95.25 | 1.98 | 98.75 |
| Reacted | 2.85 | 98.96 | |||
| Animal fat | Original | 187.9 | 94.9 | 2.22 | 98.69 |
| Reacted | 2.04 | 98.84 | |||
| Lard | Original | 185.2 | 92.4 | 3.51 | 98.25 |
| Reacted | 3.01 | 98.50 | |||
| Olive oil | Original | 189.6 | 94.9 | 1.79 | 99.08 |
| Reacted | 3.02 | 98.47 | |||
2.10. Surface Area
2.11. Oligomer Determination
3. Results
3.1. Cloud Point Analysis
| Sample | Unsaturated FAME (wt %) | Saturated Long Chain FAME (>C16 chain length) (wt %) | Saturated Medium Chain FAME (C10–C14 chain length) (wt %) | ||||
| Poly | Mono | SC | BC | SC | BC | ||
| Palm oil | Original | 10 | 40 | 49 | 0 | 1 | 0 |
| Reacted | 0 | 18 | 33 | 36 | 2 | 2 | |
| Coconut oil | Original | 2 | 6 | 12 | 0 | 71 | 0 |
| Reacted | 0 | 0 | 13 | 9 | 34 | 39 | |
| Rapeseed oil | Original | 32 | 62 | 6 | 0 | 0 | 0 |
| Reacted | 2 | 4 | 57 | 26 | 3 | 0 | |
| Corn oil | Original | 59 | 28 | 13 | 0 | 0 | 0 |
| Reacted | 0 | 4 | 48 | 40 | 0 | 2 | |
| Soybean oil | Original | 61 | 24 | 15 | 0 | 0 | 0 |
| Reacted | 2 | 3 | 43 | 21 | 5 | 14 | |
| Beef fat | Original | 4 | 43 | 43 | 0 | 3 | 0 |
| Reacted | 0 | 12 | 62 | 17 | 0 | 0 | |
| Lard | Original | 10 | 44 | 40 | 0 | 2 | 0 |
| Reacted | 0 | 3 | 59 | 25 | 2 | 2 | |
| Olive oil | Original | 11 | 71 | 16 | 0 | 0 | 0 |
| Reacted | 0 | 0 | 74 | 21 | 0 | 0 | |
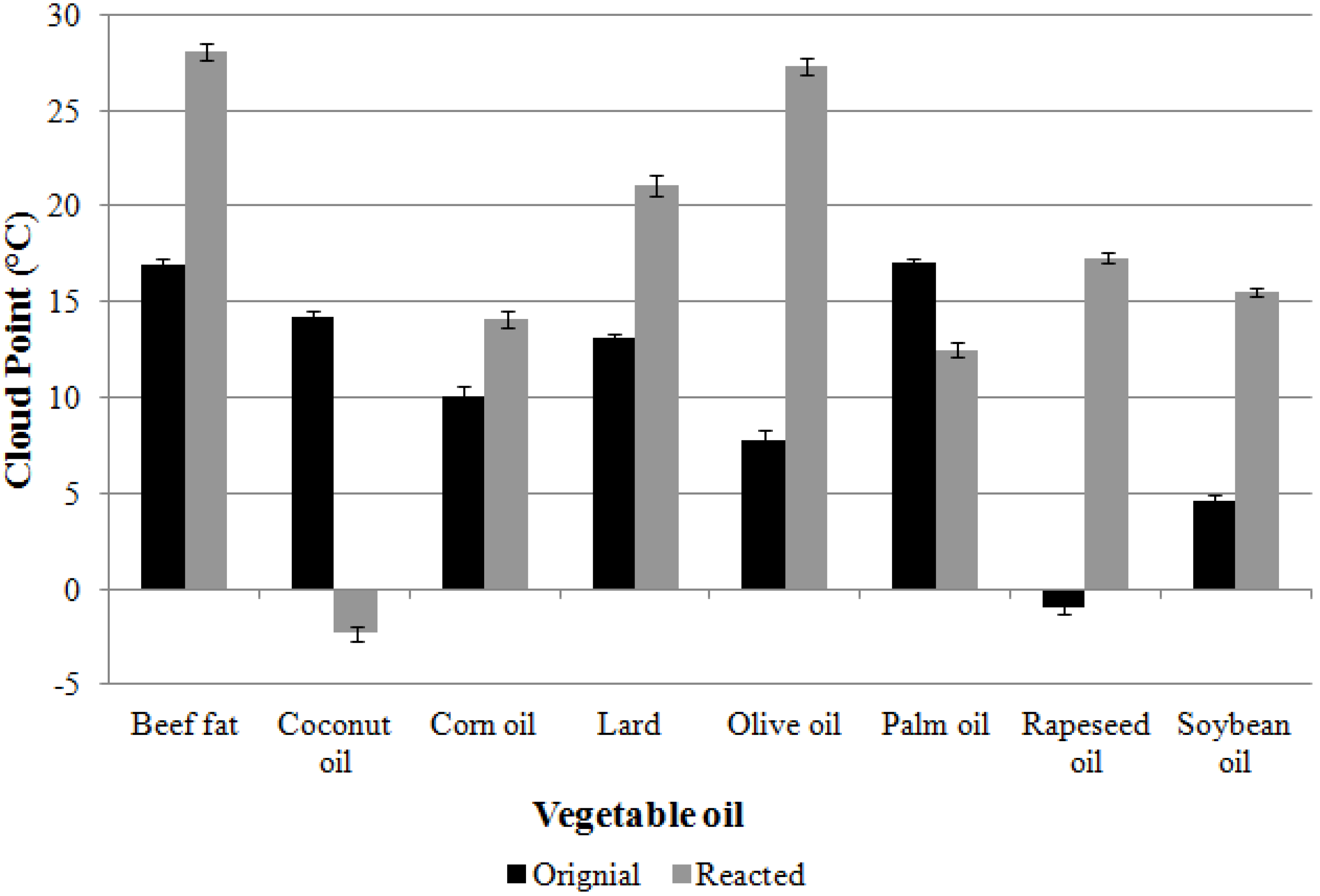
| Sample | Cloud Point (°C) | Pour Point (°C) | Viscosity (mm2/s) (@40 °C) | Density (kg/m3) (@15 °C) | |
|---|---|---|---|---|---|
| Palm oil | Original | 17.5 | 15 | 4.42 | 882 |
| Reacted | 12.8 | 9 | 4.08 | 864 | |
| Coconut oil | Original | 14.2 | 9 | 4.58 | 850 |
| Reacted | −2.3 | −3 | 3.57 | 824 | |
| Rapeseed oil | Original | −1.0 | −15 | 4.53 | 874 |
| Reacted | 17.3 | 18 | 6.95 | 902 | |
| Corn oil | Original | 11.5 | 9 | 5.01 | 880 |
| Reacted | 15.9 | 12 | 6.52 | 894 | |
| Soybean oil | Original | 4.6 | 0 | 4.62 | 882 |
| Reacted | 15.5 | 9 | 6.68 | 886 | |
| Animal fat | Original | 16.9 | 15 | 5.84 | 892 |
| Reacted | 28.1 | 24 | 6.99 | 905 | |
| Lard | Original | 13.1 | 0 | 5.02 | 873 |
| Reacted | 21.1 | 18 | 6.04 | 865 | |
| Olive oil | Original | 7.8 | −3 | 4.21 | 875 |
| Reacted | 27.3 | 27 | 7.03 | 903 | |
3.2. Catalyst Recycle
| Catalyst Sample | Yield of BCFA (%) | Cloud point of reacted ester (°C) | Surface area (m2/g) | Carbon deposition (% w/w) | CO adsorption (% w/w) |
|---|---|---|---|---|---|
| Isomerization (original) | 22 | 2.3 | 556 | 0 | N/A |
| R1 | 14 | −3.8 | 65 | 8 | N/A |
| R2 | 11 | −7.2 | 49 | 9 | N/A |
| R1 c | 17 | 0.8 | 297 | 0 | N/A |
| R1 h | 12 | −4.1 | 125 | 3 | N/A |
| Hydroisomerization (original) | 44 | 20.1 | 374 | 0 | 1.1 |
| R1 c | 44 | 20.4 | 294 | 0 | 1 |
| R1 | 27 | 23.8 | 50 | 17 | ≅0 |
| R1 h | 40 | 21.2 | 143 | 3 | 0.25 |
| R2 c | 41 | 21.9 | 219 | 0 | 0.95 |
| R2 | 32 | 24.0 | 42 | 10 | ≅0 |
3.3. Reaction By-Products
3.4. Energy Use
- (1)
- Hydroisomerization improvement:Hydrolysis → Hydroisomerization → Esterification
- (2)
- Standard process:Transesterification
| Production Stage | Improvement (kJ/25 g oil) | Standard (kJ/25 g oil) |
|---|---|---|
| Hydrolysis | ||
| Heating | 14.08 | N/A |
| Loss | 4.60 | N/A |
| Gas compression | 0.01 | N/A |
| Mixing | 0.11 | N/A |
| Hydroisomerization | ||
| Heating | 14.30 | N/A |
| Loss | 42.05 | N/A |
| Gas compression | 0.01 | N/A |
| Mixing | 0.88 | N/A |
| Esterification/Transesterification | ||
| Heating | 6.24 | 3.04 |
| Loss | 0.79 | 0.89 |
| Gas compression | 0 | 0 |
| Mixing | 0.11 | 0.11 |
| Total Energy Use | 83.19 | 4.04 |
4. Conclusions
Acknowledgements
References
- Friedlingstein, P.; Houghton, R.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.; Canadell, J.; Raupach, M.; Ciais, P.; Le Quéré, C. Update on CO2 emissions. Nat. Geosci. 2010, 3, 811–812. [Google Scholar] [CrossRef]
- Lammertsma, E.I.; de Boer, H.J.; Dekker, S.C.; Dilcher, D.L.; Lotter, A.F.; Wagner-Cremer, F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA 2011, 108, 4035–4040. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Reinhardt, G.; Stelzer, T. Life cycle analysis of biofuels under different environmental aspects. Biomass Bioenergy 1997, 12, 121–134. [Google Scholar] [CrossRef]
- Beer, T.; Grant, T.; Williams, D.; Watson, H. Fuel-cycle greenhouse gas emissions from alternative fuels in Australian heavy vehicles. Atmos. Environ. 2002, 36, 753–763. [Google Scholar] [CrossRef]
- Amonette, J.E.; Woolf, D.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Mitigation of climate change with biomass: Technical potentials and factors affecting implementation. In Proceedings of 17th International Palm Oil Conference and Exopalma, Cartagena, Colombia, 27 September 2012.
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G.; Dunn, R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Ono, Y. A survey of the mechanism in catalytic isomerization of alkanes. Catal. Today 2003, 81, 3–16. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Synergistic effects of skeletal isomerization on oleic and palmitic acid mixtures for the reduction in cloud point of their methyl esters. Energy Fuels 2012, 26, 4514–4520. [Google Scholar] [CrossRef]
- Ismail, A.; van de Voort, F.; Emo, G.; Sedman, J. Rapid quantitative determination of free fatty acids in fats and oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1993, 70, 335–341. [Google Scholar] [CrossRef]
- Doumenq, P.; Guiliano, M.; Bertrand, J.; Mille, G. GC/FT-IR analysis of fatty acid methyl esters. Appl. Spectrosc. 1990, 44, 1355–1359. [Google Scholar] [CrossRef]
- Claude, M.C.; Martens, J.A. Monomethyl-branching of long n-alkanes in the range from decane to tetracosane on Pt/H-ZSM-22 bifunctional catalyst. J. Catal. 2000, 190, 39–48. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Moser, B.R. Comparative oxidative stability of fatty acid alkyl esters by accelerated methods. J. Am. Oil Chem. Soc. 2009, 86, 699–706. [Google Scholar] [CrossRef]
- Lee, I.; Johnson, L.A.; Hammond, E.G. Use of branched-chain esters to reduce the crystallization temperature of biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 1155–1160. [Google Scholar] [CrossRef]
- Yori, J.C.; D’Amato, M.A.; Grau, J.M.; Pieck, C.L.; Vera, C.R. Depression of the cloud point of biodiesel by reaction over solid acids. Energy Fuels 2006, 20, 2721–2726. [Google Scholar] [CrossRef]
- Ngo, H.L.; Nuñez, A.; Lin, W.; Foglia, T.A. Zeolite-catalyzed isomerization of oleic acid to branched-chain isomers. Eur. J. Lipid Sci. Technol. 2007, 109, 214–224. [Google Scholar] [CrossRef]
- Villegas, J.; Kumar, N.; Heikkilä, T.; Lehto, V.P.; Salmi, T.; Murzin, D.Y. Isomerization of n-butane to isobutane over Pt-modified Beta and ZSM-5 zeolite catalysts: Catalyst deactivation and regeneration. Chem. Eng. J. 2006, 120, 83–89. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Optimizing reaction conditions for the isomerization of fatty acids and fatty acid methyl esters to their branch chain products. J. Am. Oil Chem. Soc. 2011, 1, 1–11. [Google Scholar] [CrossRef]
- Horsepower required to compress air. The Engineering Toolbox Website. Available online: http://www.engineeringtoolbox.com/horsepower-compressed-air-d_1363.html (accessed on 13 November 2012).
- Souza, M.S.; Aguieiras, E.C.G.; da Silva, M.A.P.; Langone, M.A.P. Biodiesel synthesis via esterification of feedstock with high content of free fatty acids. Appl. Biochem. Biotechnol. 2009, 154, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; He, B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reaume, S.J.; Ellis, N. Use of Isomerization and Hydroisomerization Reactions to Improve the Cold Flow Properties of Vegetable Oil Based Biodiesel. Energies 2013, 6, 619-633. https://doi.org/10.3390/en6020619
Reaume SJ, Ellis N. Use of Isomerization and Hydroisomerization Reactions to Improve the Cold Flow Properties of Vegetable Oil Based Biodiesel. Energies. 2013; 6(2):619-633. https://doi.org/10.3390/en6020619
Chicago/Turabian StyleReaume, Stephen J., and Naoko Ellis. 2013. "Use of Isomerization and Hydroisomerization Reactions to Improve the Cold Flow Properties of Vegetable Oil Based Biodiesel" Energies 6, no. 2: 619-633. https://doi.org/10.3390/en6020619




