Understanding the Mechanism of Cypress Liquefaction in Hot-Compressed Water through Characterization of Solid Residues
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Apparatus and Experimental Procedure
2.3. Analysis
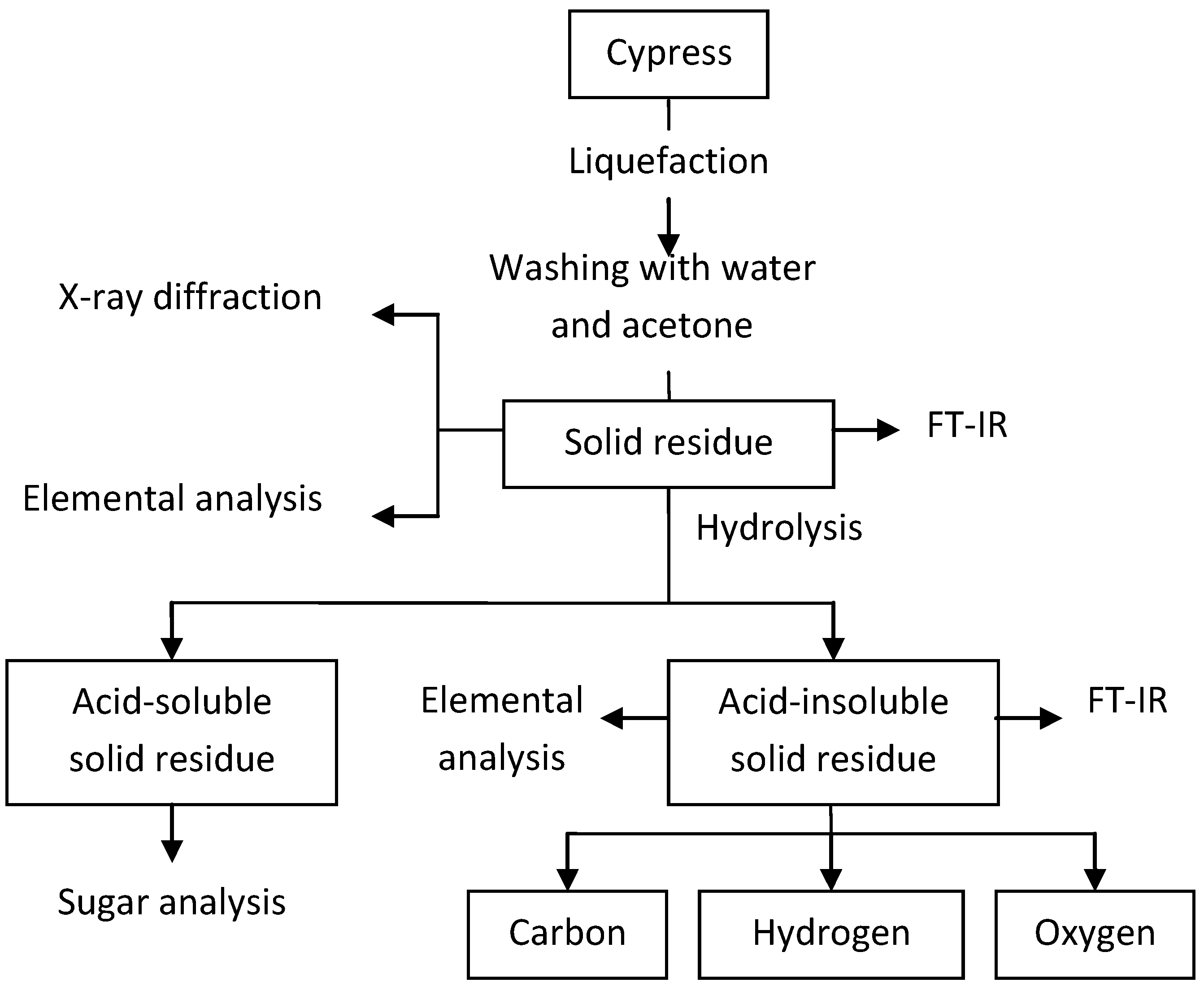
3. Results and Discussion
3.1. The Yield of Solid Residue
3.1.1. Effect of Temperature on Yield of Solid Residue
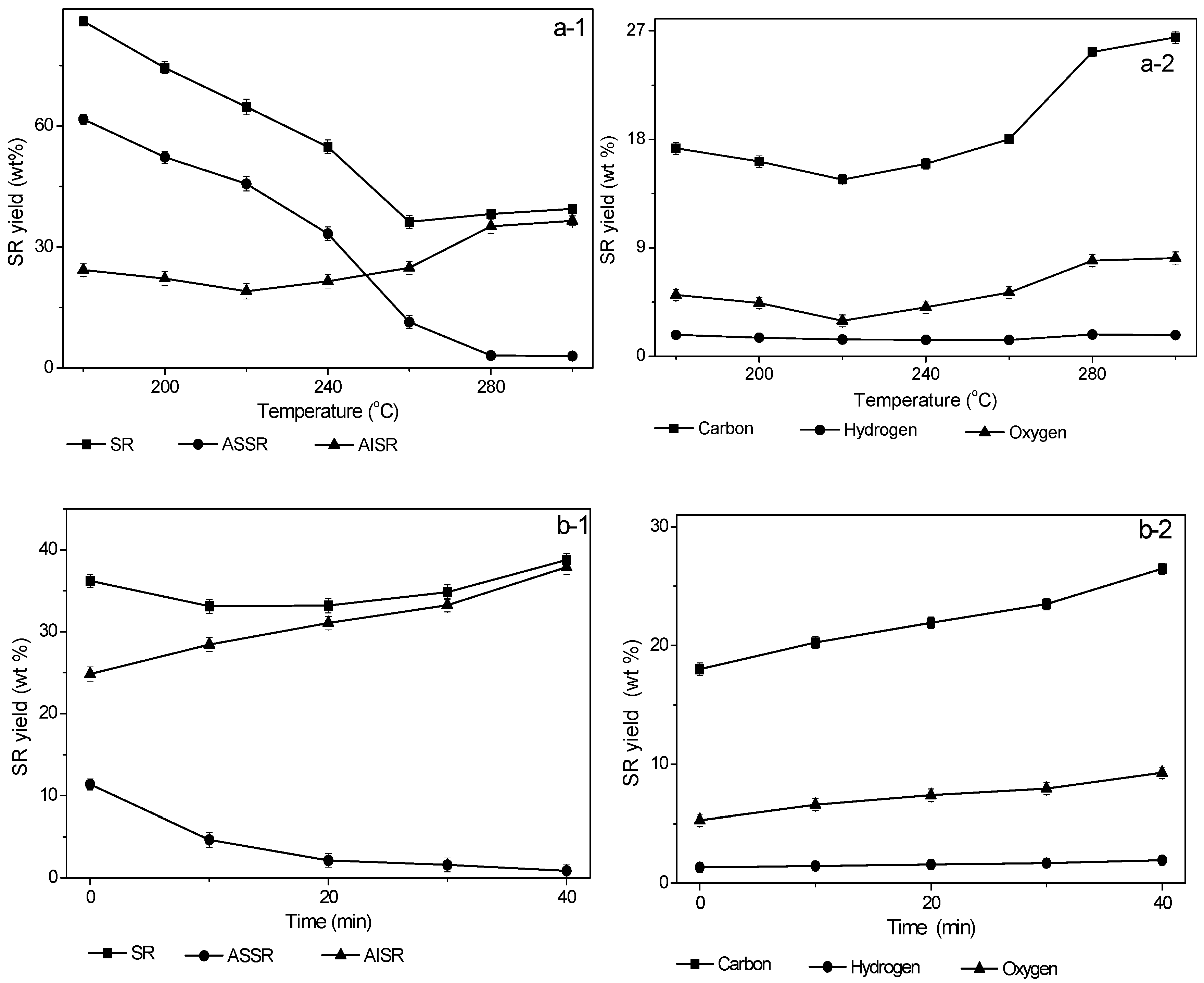
3.1.2. Effect of Retention Time on Yield of Solid Residue
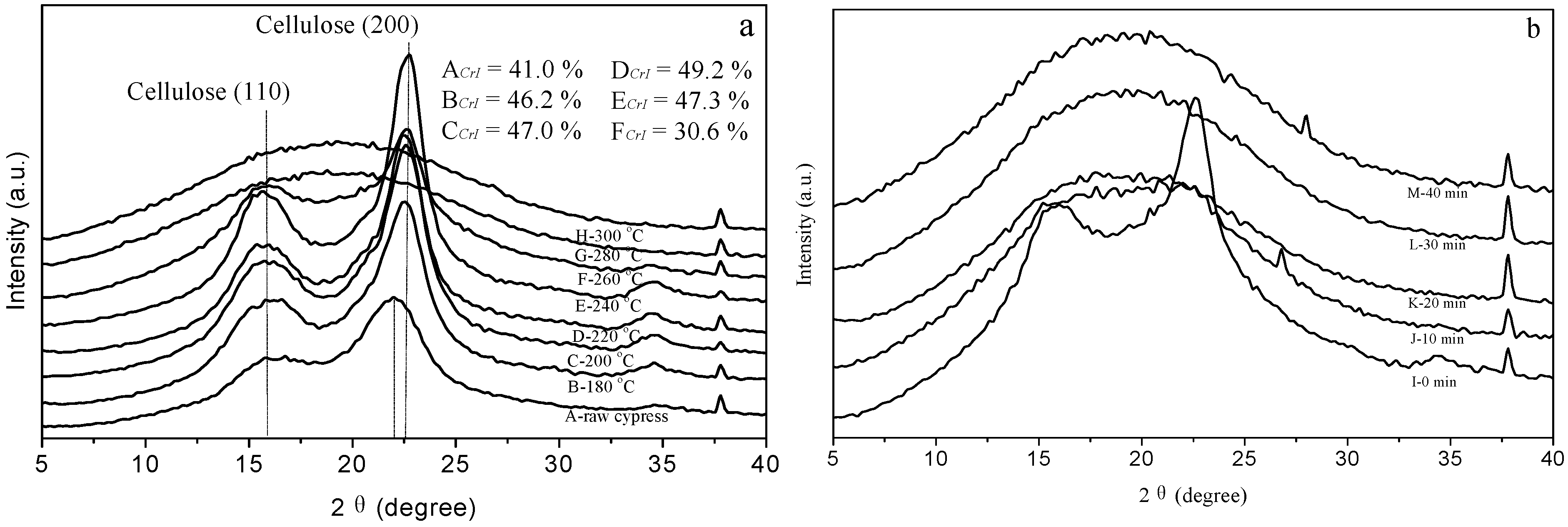
3.2. Crystallinity of Solid Residue
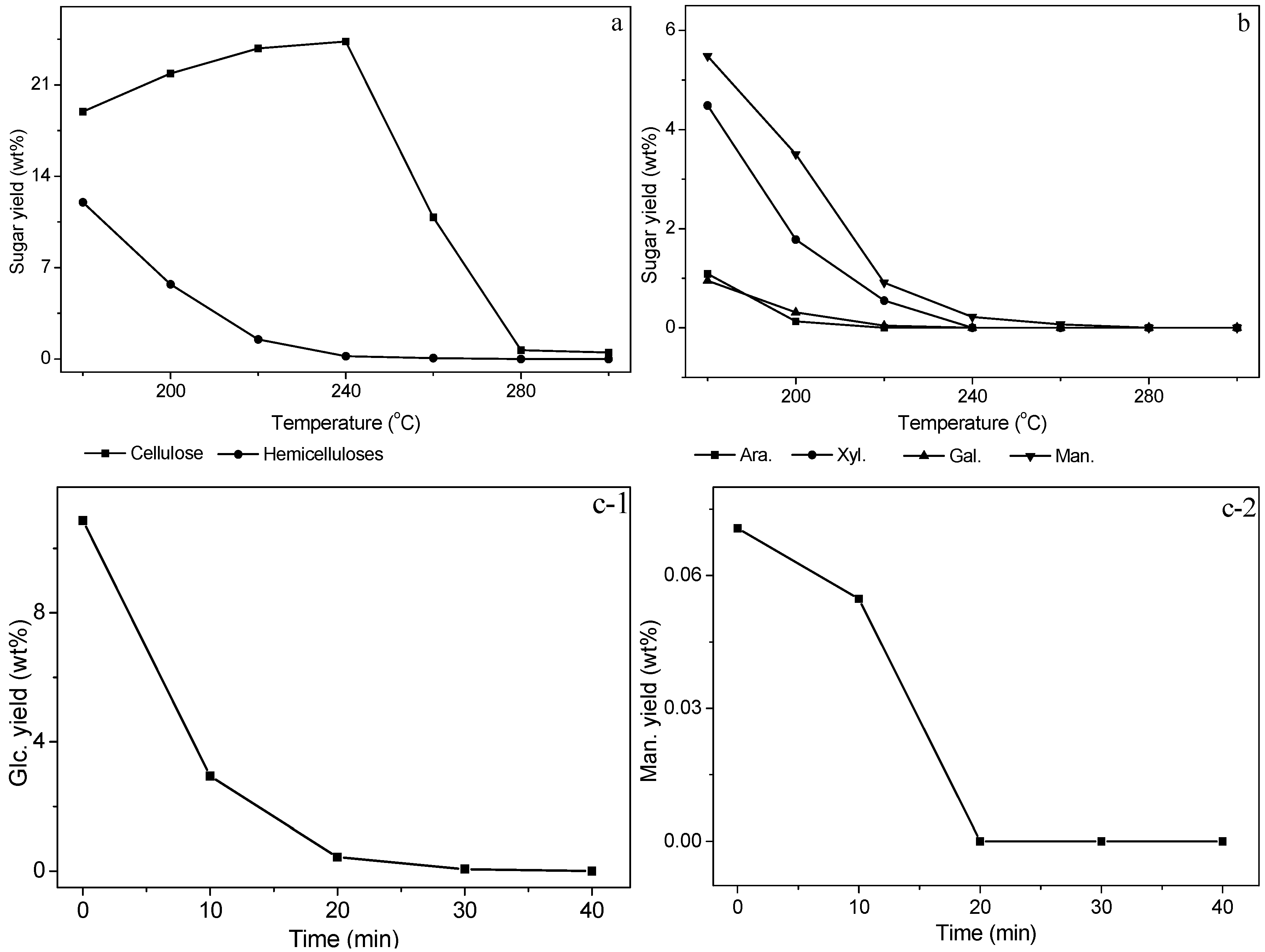
3.3. Sugar Analysis of Solid Residue
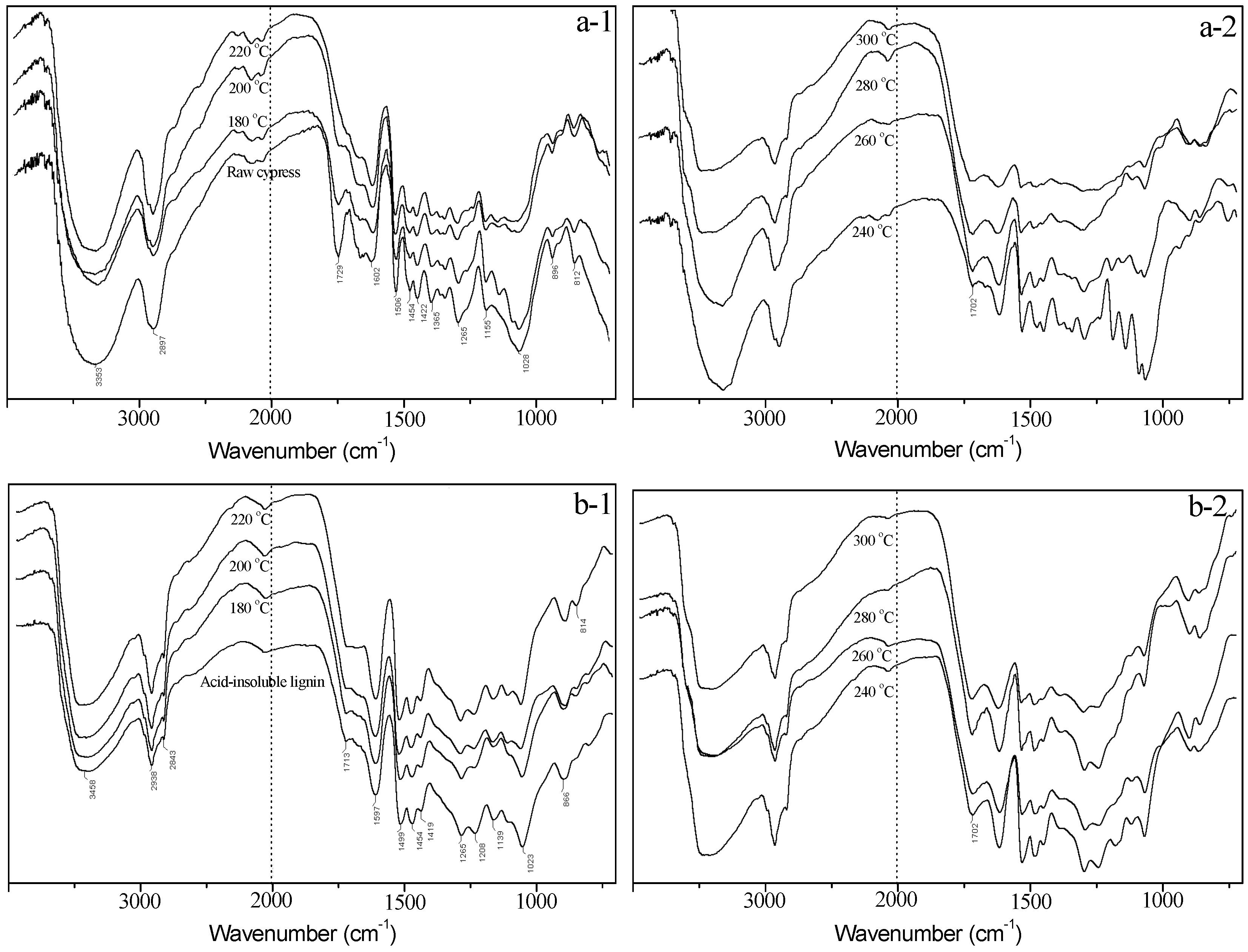
3.4. FT-IR Analysis
3.4.1. FT-IR Analysis of Solid Residue
3.4.2. FT-IR Analysis of Acid-insoluble Solid Residue
3.5. Elemental Analysis and Higher Heating Value of Solid Residues
| Samples | Elemental components (wt %) | O/C | H/C | HHV (MJ/kg) | |||
|---|---|---|---|---|---|---|---|
| C | H | O | N | ||||
| 180 °C | 49.1 | 6.1 | 44.6 | <0.3 | 0.90 | 0.12 | 17.4 |
| 200 °C | 50.1 | 6.0 | 43.8 | <0.3 | 0.87 | 0.12 | 17.6 |
| 220 °C | 51.2 | 6.1 | 42.6 | <0.3 | 0.83 | 0.12 | 18.4 |
| 240 °C | 53.7 | 5.9 | 40.2 | <0.3 | 0.75 | 0.11 | 19.4 |
| 260 °C | 62.5 | 5.5 | 31.6 | 0.42 | 0.51 | 0.088 | 23.4 |
| 280 °C | 69.4 | 5.1 | 25.2 | 0.43 | 0.36 | 0.073 | 26.2 |
| 300 °C | 70.1 | 4.9 | 24.7 | 0.41 | 0.35 | 0.070 | 26.3 |
| 10 min | 66.9 | 5.2 | 27.5 | 0.45 | 0.41 | 0.078 | 25.1 |
| 20 min | 68.7 | 5.1 | 25.8 | 0.42 | 0.38 | 0.074 | 25.9 |
| 30 min | 69.3 | 5.1 | 25.3 | 0.44 | 0.37 | 0.073 | 26.2 |
| 40 min | 69.2 | 5.1 | 25.2 | 0.46 | 0.36 | 0.073 | 26.2 |
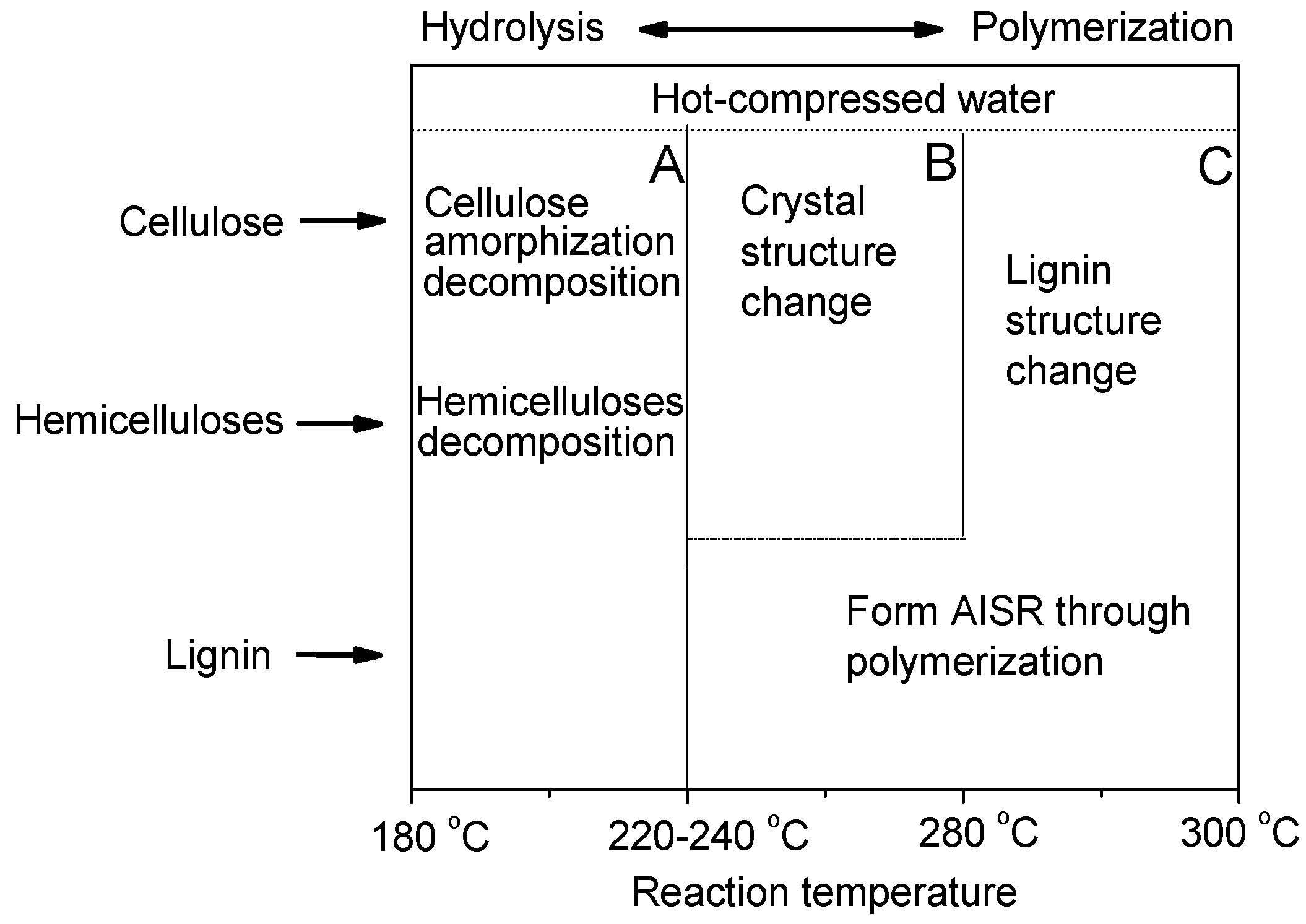
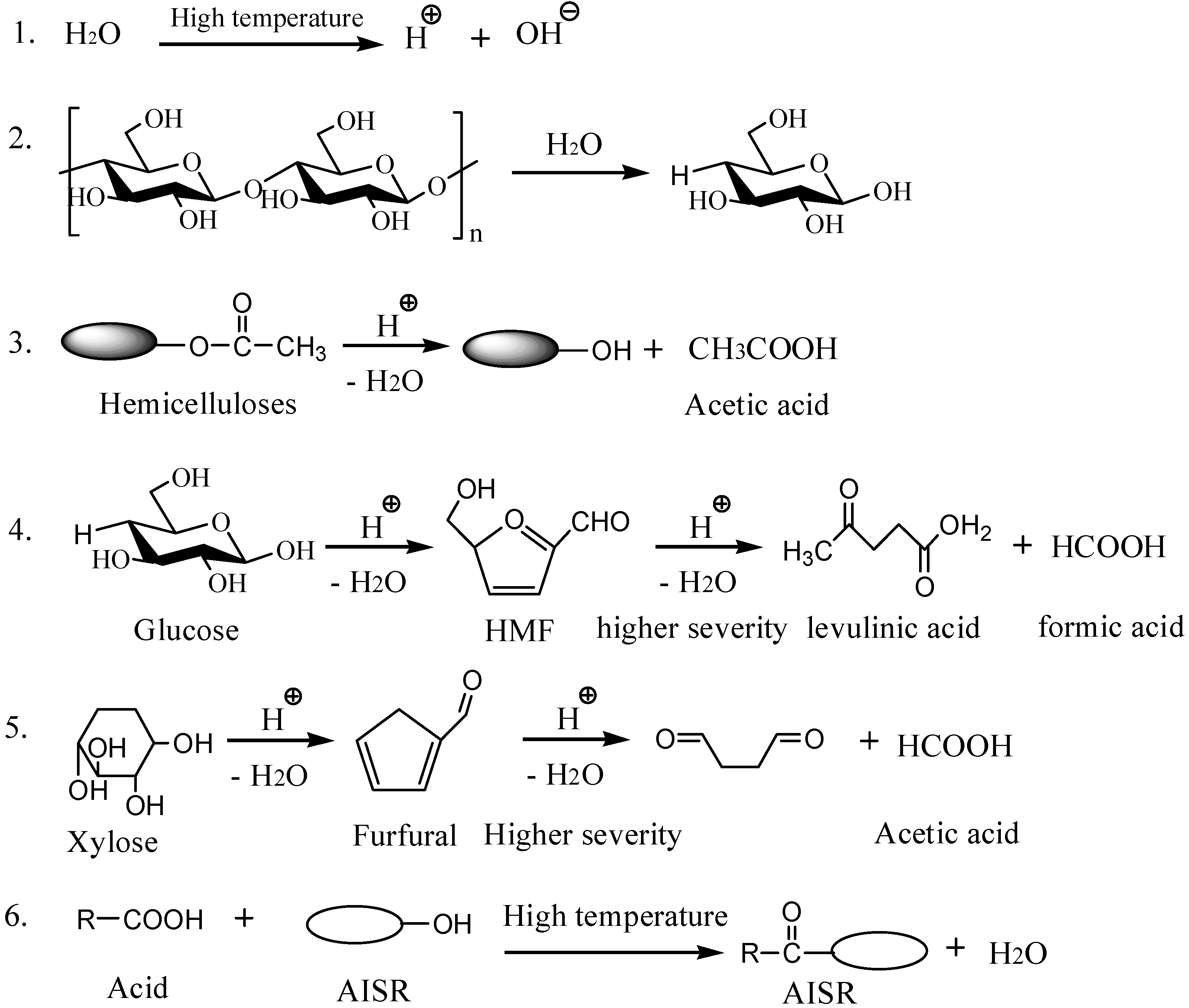
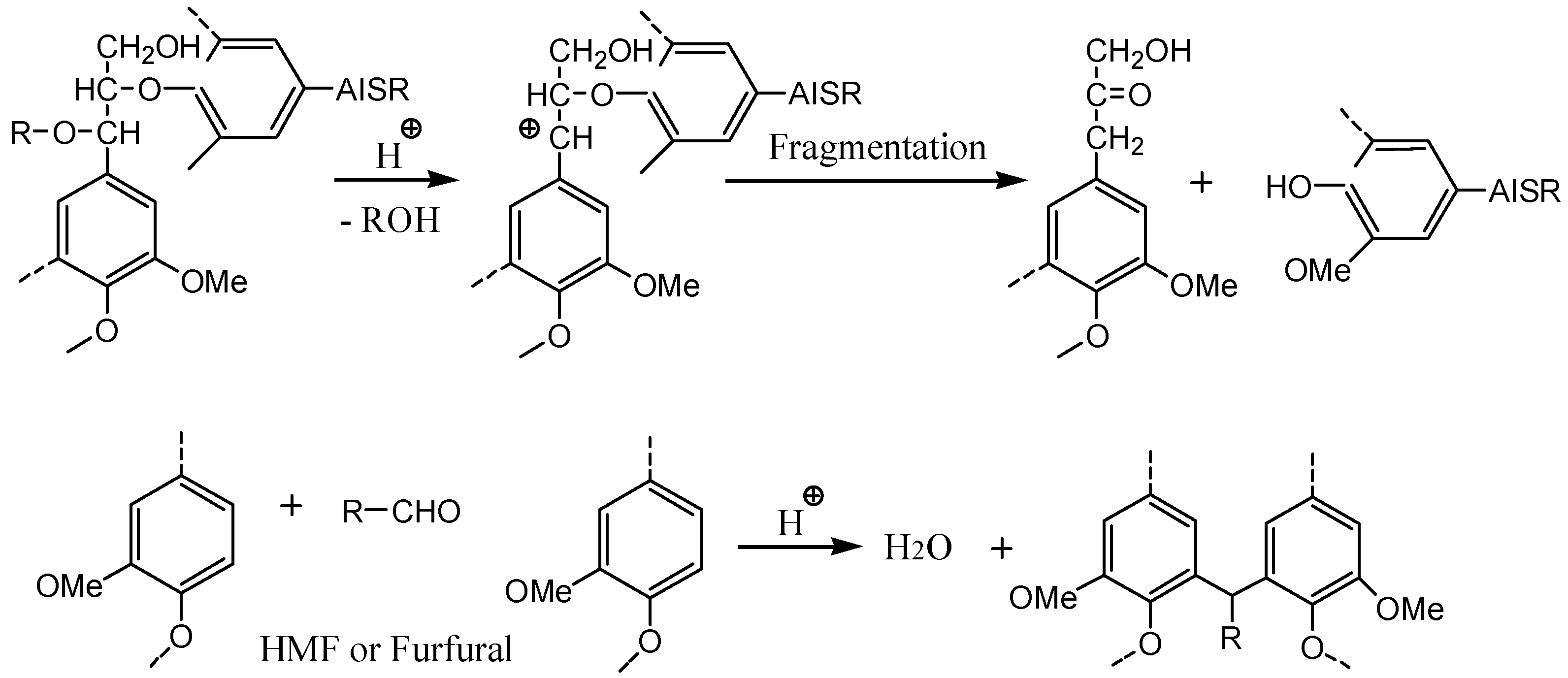
3.6. Investigation of Presumed Hydrothermal Liquefaction Mechanism
4. Conclusions
Acknowledgments
References
- Zhang, X. Study on biomass pyrolysis kinetics. J. Eng. Gas. Turbines Power 2006, 128, 493–496. [Google Scholar] [CrossRef]
- Zhang, B.; Keitz, M.; Valentas, K. Thermochemical liquefaction of high-diversity grassland perennials. J. Anal. Appl. Pyrol. 2009, 84, 18–24. [Google Scholar] [CrossRef]
- Fan, S.P.; Zakaria, S.; Chia, C.H.; Jamaluddin, F.; Nabihah, S.; Liew, T.K.; Pua, F.L. Comparative studies of products obtained from solvolysis liquefaction of oil palm empty fruit bunch fibres using different solvents. Bioresour. Technol. 2011, 102, 3521–3526. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Yao, Y.; Yoshioka, M.; Shiraishi, N. Liquefaction mechanism of cellulose in the presence of phenol under acid catalysis. Carbohydr. Polym. 2004, 57, 123–129. [Google Scholar] [CrossRef]
- Fang, Z.; Fang, C. Complete dissolution and hydrolysis of wood in hot water. AIChE J. 2008, 54, 2751–2758. [Google Scholar] [CrossRef]
- Fang, Z.; Minowa, T.; Smith, R.L.; Ogi, T.; Kozinski, J.A. Liquefaction and gasification of cellulose with Na2CO3 and Ni in subcritical water at 350 °C. Ind. Eng. Chem. Res. 2004, 43, 2454–2463. [Google Scholar] [CrossRef]
- Fang, Z.; Sato, T.; Smith, R.L.; Inomata, H.; Arai, K.; Kozinski, J.A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Yuliansyah, A.T.; Hirajima, T.; Kumagai, S.; Sasaki, K. Production of solid biofuel from agricultural wastes of the palm oil industry by hydrothermal treatment. Waste Biomass Valor. 2010, 1, 395–405. [Google Scholar] [CrossRef]
- Liu, W.W.; Hu, C.; Yang, Y.; Zhu, L.; Tong, D. Effect of the interference instant of zeolite HY catalyst on the pyrolysis of pubescens. Chin. J. Chem. Eng. 2010, 18, 351–354. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass conversion in water at 330–410 and 30–50 MPa. Identification of key compounds for indicating differenct chemical reaction pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, X.; Wang, F.; Yu, G. Pyrolysis pretreatment of biomass for entrained-flow gasification. Appl. Energy 2010, 87, 149–155. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atnospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Duan, P.; Savage, P.E. Characterization of product fractions from hydrothermal liquefaction of nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Lu, W.; Wang, C.; Yang, Z. The preparation of high caloric fuel (HCF) from water hyacinth by deoxy-liquefaction. Bioresour. Technol. 2009, 100, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Shahbazi, A.; Shirley, V.B.; Wang, L. Swine manure/crude glycerol co-liquefaction: Physical properties and chemical analysis of bio-oil product. Bioresour. Technol. 2010, 102, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Xie, X.A.; Li, M.F.; Sun, R.C. Hydrothermal liquefaction of cypress: Effects of reaction conditions on 5-lump distribution and composition. J. Anal. Appl. Pyrol. 2010, 94, 177–183. [Google Scholar] [CrossRef]
- Segal, L.; Creely, L.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Shirley, V.; Cheng, D. Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J. Anal. Appl. Pyrol. 2010, 88, 73–79. [Google Scholar] [CrossRef]
- Sun, P.; Heng, M.; Sun, S.H.; Chen, J. Analysis of liquid and solid products from liquefaction of paulownia in hot-compressed water. Energy Convers. Manag. 2011, 52, 924–933. [Google Scholar] [CrossRef]
- Hui, L.; Yuang, X.; Zeng, G.; Huang, D.; Huang, H.; Tong, J.; You, Q.; Zhang, J.; Zhou, M. The formation of bio-oil from sludge by deoxy-liquefaction in supercritical ethanol. Bioresour. Technol. 2010, 101, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Sakaki, T.; Kokusho, T.; Shibata, M.; Uemura, Y.; Hatate, Y. Decomposition behavior of plant biomass in hot-compressed water. Ind. Eng. Chem. Res. 2000, 39, 3688–3693. [Google Scholar] [CrossRef]
- Akhtar, J.; Kuang, S.K.; Amin, N.S. Liquefaction of empty palmfruit bunch (EPFB) in alkaline hot compressed water. Renew. Energy 2010, 35, 1220–1227. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Xu, F.; Geng, Z.C.; Liu, C.F.; Ren, J.L.; Sun, J.X.; Sun, R.C. Structural characterization of residual lignins isolated with cyanamide-activated hydrogen peroxide from various organosolvs pretreated wheat straw. J. Appl. Polym. Sci. 2008, 109, 555–564. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, F.; Sun, X.F.; Sun, R.C.; Wu, S.B. Comparative study of lignins from ultrasonic irradiated sugar-cane bagasse. Polym. Int. 2004, 53, 1711–1721. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Wang, S.Q.; Zhu, W. Characterization of lignins from wheat straw by alkaline peroxide treatment. Polym. Degrad. Stabil. 2000, 67, 101–109. [Google Scholar] [CrossRef]
- Wang, C.; Pan, J.; Li, J.; Yang, Z. Comparative studies of products produced from four different biomass samples via deoxy-liquefaction. Bioresour. Technol. 2008, 99, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, M.; Jin, B.; Huang, Y. High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 2006, 20, 715–720. [Google Scholar] [CrossRef]
- Kuchonthara, P.; Bhattacharya, S.; Tsutsumi, A. Combination of thermochemical recuperative coal gasification cycle and fuel cell for power generation. Fuel 2005, 84, 1019–1021. [Google Scholar] [CrossRef]
- Foston, M.; Ragauskas, A.J. Changes in lignocellulosic supramolecular and ultrastructure during dilute acid pretreatment of populus and switchgrass. Biomass Bioenergy 2010, 34, 1885–1895. [Google Scholar] [CrossRef]
- Stephens, C.; Whitmore, P.; Morris, H.; Bier, M. Hydrolysis of the amorphous cellulose in cotton-based paper. Biomacromolecules 2008, 9, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Emelsy, A.; Heywood, R. On the kinetics of degradation of cellulose. Cellulose 1997, 4, 1–5. [Google Scholar] [CrossRef]
- Li, J.B.; Henriksson, G.; Gellerstedt, G. Carbohyhydrate reactions during high-temperature steam treatment of aspen wood. Appl. Biochem. Biotechnol. 2005, 125, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Henriksson, G.; Gellerstedt, G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour. Technol. 2007, 98, 2329–2335. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, H.-M.; Li, M.-F.; Yang, S.; Sun, R.-C. Understanding the Mechanism of Cypress Liquefaction in Hot-Compressed Water through Characterization of Solid Residues. Energies 2013, 6, 1590-1603. https://doi.org/10.3390/en6031590
Liu H-M, Li M-F, Yang S, Sun R-C. Understanding the Mechanism of Cypress Liquefaction in Hot-Compressed Water through Characterization of Solid Residues. Energies. 2013; 6(3):1590-1603. https://doi.org/10.3390/en6031590
Chicago/Turabian StyleLiu, Hua-Min, Ming-Fei Li, Sheng Yang, and Run-Cang Sun. 2013. "Understanding the Mechanism of Cypress Liquefaction in Hot-Compressed Water through Characterization of Solid Residues" Energies 6, no. 3: 1590-1603. https://doi.org/10.3390/en6031590




