Life-Cycle Energy Use and Greenhouse Gas Emissions Analysis for Bio-Liquid Jet Fuel from Open Pond-Based Micro-Algae under China Conditions
Abstract
:1. Introduction
1.1. China is Facing Rapidly Increasing Energy Demand and Oil Imports
1.2. Liquid Fuel Shortage in China Due to Transportation Increasing
1.3. China is Developing Alternative Fuels on a Large Scale
1.4. Life-Cycle Analysis is a Useful Tool for Policy Decision Making
1.5. Algae-Based Biofuel: Energy Positive or Not?
1.6. About This Study
2. Method and Data
2.1. Model Description
2.2. Life-Cycle Stages Covered and System Boundary
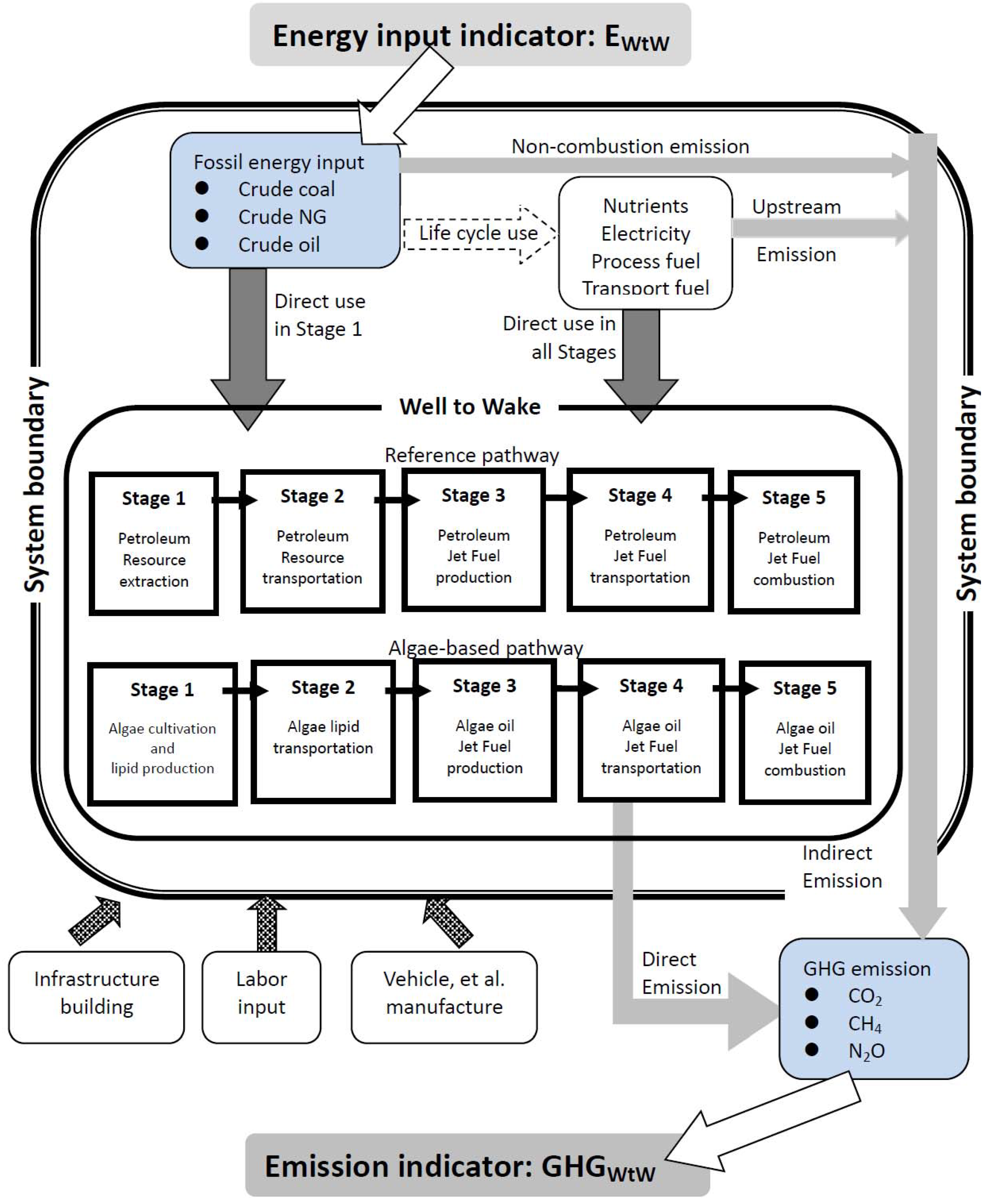
2.3. Reference Pathway
| Item | Number | Unit |
|---|---|---|
| Life-cycle fossil energy consumption | 1.26 | MJ/MJ |
| Including: Coal | 0.07 | MJ/MJ |
| Natural gas | 0.06 | MJ/MJ |
| Petroleum | 1.13 | MJ/MJ |
| Life-cycle GHG emissions | 93.5 | g CO2, e/MJ |
2.4. Baseline Algal BJF Pathway
| Item | Properties | Note |
|---|---|---|
| Type of algae cultivation system | Open raceway ponds | Selected because of its lower cost and energy consumption than the bioreactor system [26] |
| Scale of algae farm and CO2 source | A nearby power plant | Based on primary bench-scale data and process modeling in Kadam (2002) [39] which considered a coal power plant with algae biomass using flue gas from the power plants. |
| CO2 content of flue gas | 20 vol % | CAERC report [36] |
| Algae productivity | 25 g/m2/day | Davis et al. (2011) [38] |
| Lipid content | 25% | Davis et al. (2011) [38] |
| Type of algae oil extraction system | Wet extraction | Extraction must be done onsite proximal to the algae growth pond [40] |
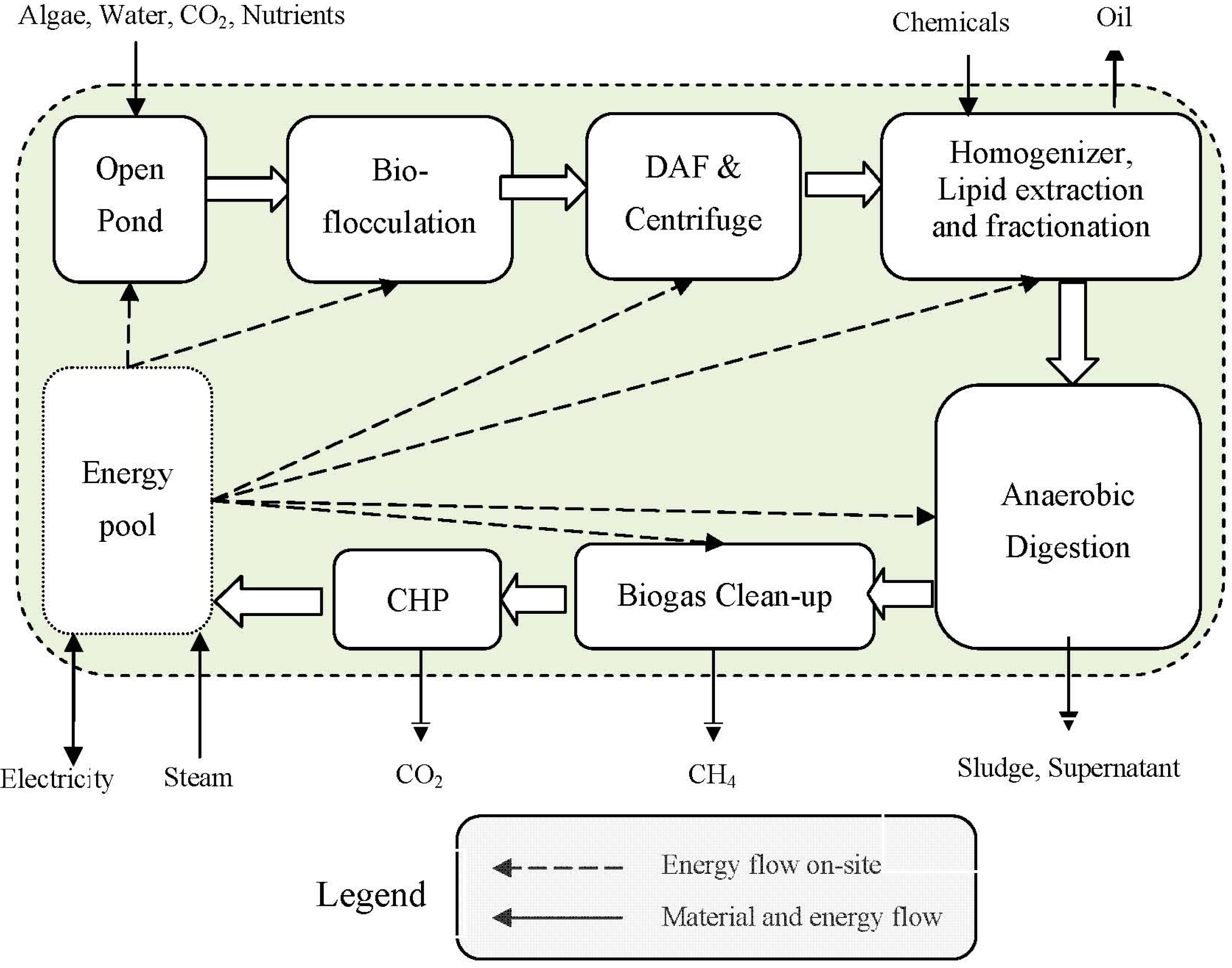
2.5. Energy and GHG Intensity of Process Fuels and Nutrients
| Item | EFLC | By sort | GHGLC | Upstream emission | ||||
| EFLC,Coal | EFLC,NG | EFLC,Petrol | CO2,up | CH4,up | N2Oup | |||
| Units | MJ/MJ | g CO2,e/MJ | g/MJ | g/MJ | mg/MJ | |||
| Coal | 1.172 | 1.061 | 0.001 | 0.110 | 104.5 | 5.733 | 0.425 | 0.172 |
| Natural gas | 1.196 | 0.081 | 1.015 | 0.065 | 72.73 | 13.544 | 0.110 | 0.161 |
| Diesel | 1.319 | 0.156 | 0.027 | 1.119 | 102.4 | 28.287 | 0.078 | 0.441 |
| Gasoline | 1.331 | 0.164 | 0.049 | 1.130 | 98.86 | 30.506 | 0.086 | 0.472 |
| Electricity | 2.924 | 2.572 | 0.021 | 0.330 | 289.6 | 273.308 | 1.010 | 3.917 |
| Item | Unit | N | P | K | Steam (from coal) | H2 (from coal) |
|---|---|---|---|---|---|---|
| Lifecycle fossil Energy | MJ/kg | 55.17 | 7.98 | 8.90 | 1.56 | 1.78 |
| Of which, Coal | MJ/kg | 41.52 | 3.01 | 5.71 | 1.41 | 1.76 |
| Natural gas | MJ/kg | 7.83 | 4.25 | 1.14 | 0.00 | 0.00 |
| Petroleum | MJ/kg | 5.55 | 0.58 | 2.01 | 0.15 | 0.02 |
| Lifecycle GHG | g CO2,e/kg | 5148 | 587 | 811 | 139 | 163 |
3. Results and Discussion
3.1. Detailed Analysis of All Stages
3.1.1. Algae Farming
3.1.2. Algae Harvesting
3.1.3. Oil Extraction
3.1.4. AD, Biogas Cleaning and CHP
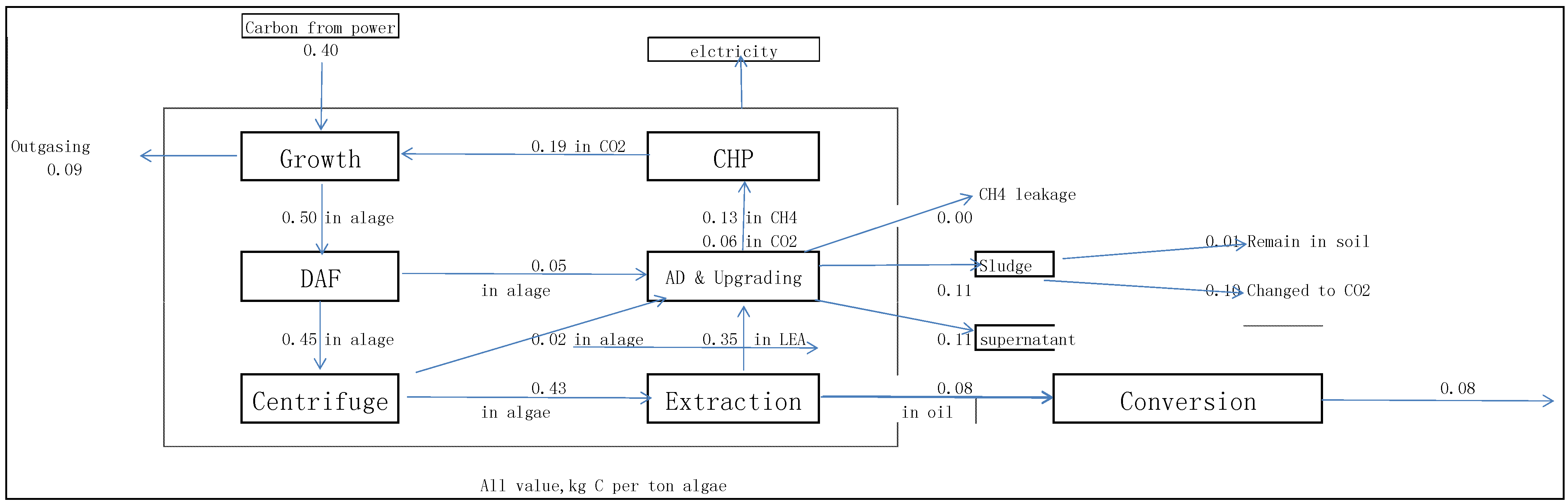
3.2. Carbon Balance
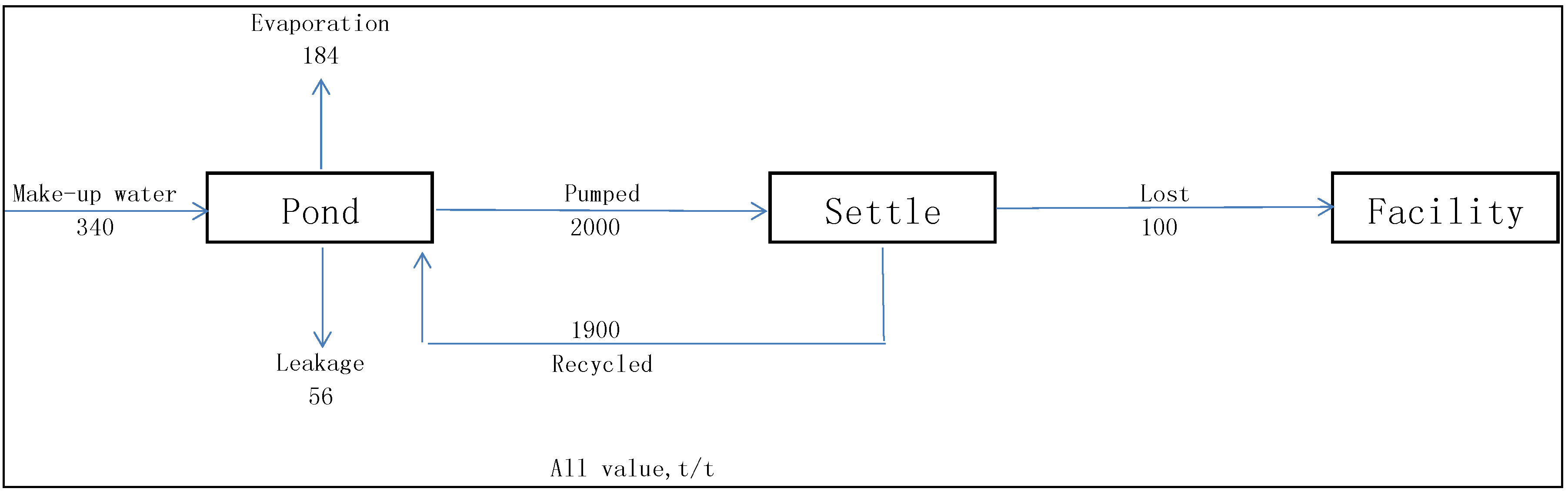
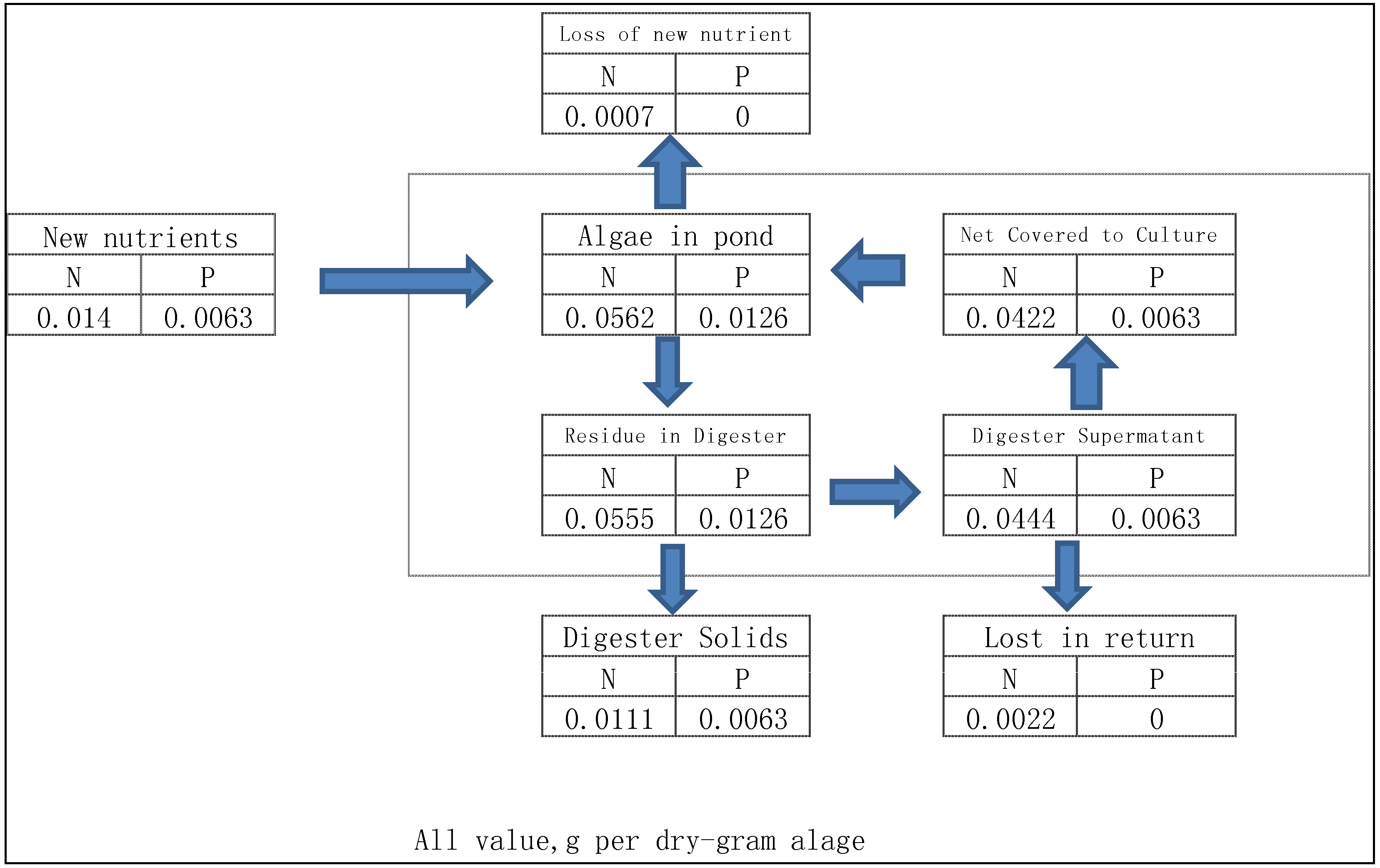
3.3. Nutrients Balance
3.4. Non-CO2 GHG Emissions
3.5. Direct Energy Demand
| Item | Unit | Direct energy demand | CO2 demand | |||||
|---|---|---|---|---|---|---|---|---|
| Process | Process input | Thermal (MJ) | Electrical (kWh) | Thermal (MJ) | Electrical (kWh) | per kg algae | per kg of algae lipid | |
| Growth | – | per kg algae | per kg algae | per kg of algae lipid | per kg of algae lipid | 2.16 | 11.80 | |
| water circulation | 2.00 | kW per ha | – | 0.096 | – | 0.53 | – | – |
| water pumping | 3.90 | t per kg algae | – | 0.195 | – | 1.07 | – | – |
| water replenishment | 0.60 | cm per day | – | 0.116 | – | 0.63 | – | – |
| Off-site CO2 transport to onsite | – | – | – | 0.017 | – | 0.09 | 1.45 | 7.95 |
| Off-site CO2 transfer into pond | – | – | – | 0.031 | – | 0.17 | 1.45 | 7.95 |
| Recovered CO2 transfer into pond | – | – | – | 0.015 | – | 0.08 | 0.70 | 3.85 |
| Harvest | 1.17 | kg algae/kg dewatered algae | – | per kg dewatered algae | – | per kg of algae lipid | – | – |
| 1.16 | 5.41 | |||||||
| Lipid extraction | 4.68 | kg dewatered algae/kg lipids | per kg lipids | per kg lipids | per kg of algae lipid | per kg of algae lipid | – | – |
| 6.12 | 0.77 | 6.12 | 0.77 | |||||
| Anaerobic digester | 4.47 | kg feed/kg lipids | per kg feed | per kg feed | per kg of algae lipid | per kg of algae lipid | – | – |
| 2.45 | 0.14 | 10.95 | 0.61 | |||||
| Biogas cleanup | 0.297 | cubic meter/kg feed | – | per cubic meter | – | per kg of algae lipid | – | – |
| 0.25 | 0.33 | |||||||
| Total direct demand on site | – | – | – | – | 17.07 | 9.69 | – | – |
| Recovered on site (CHP) | 76% | CHP efficiency | per MJ CH4 | per MJ CH4 | per kg of algae lipid | per kg of algae lipid | – | – |
| 35.8 | MJ/cubic meter | 0.43 | 0.09 | 20.45 | 4.36 | |||
| Imported externally | – | – | – | – | −3.38 | 5.33 | – | – |
3.6. Jet Fuel Production
3.7. Co-Products, Oil and Fuel Transportation
| Product/Intermediate | Mode | Energy Intensity (MJ/t km, backhaul of the vehicle includes when appropriate) | Fuel mix (%) | Distance (km) |
|---|---|---|---|---|
| Digestate solids transported to fields | Medium heavy-duty truck | 1.36 | Diesel (100%) | 100 |
| Algae oil transported to fuel production | Railway | 0.07 | Residue oil (100%) | 500 |
| Fuel transported to terminal | Railway | 0.07 | Residue oil (100%) | 1000 |
| Fuel distributed to airport | Heavy heavy-duty truck | 0.68 | Diesel (100%) | 50 |
3.8. Energy and GHG Emissions Results
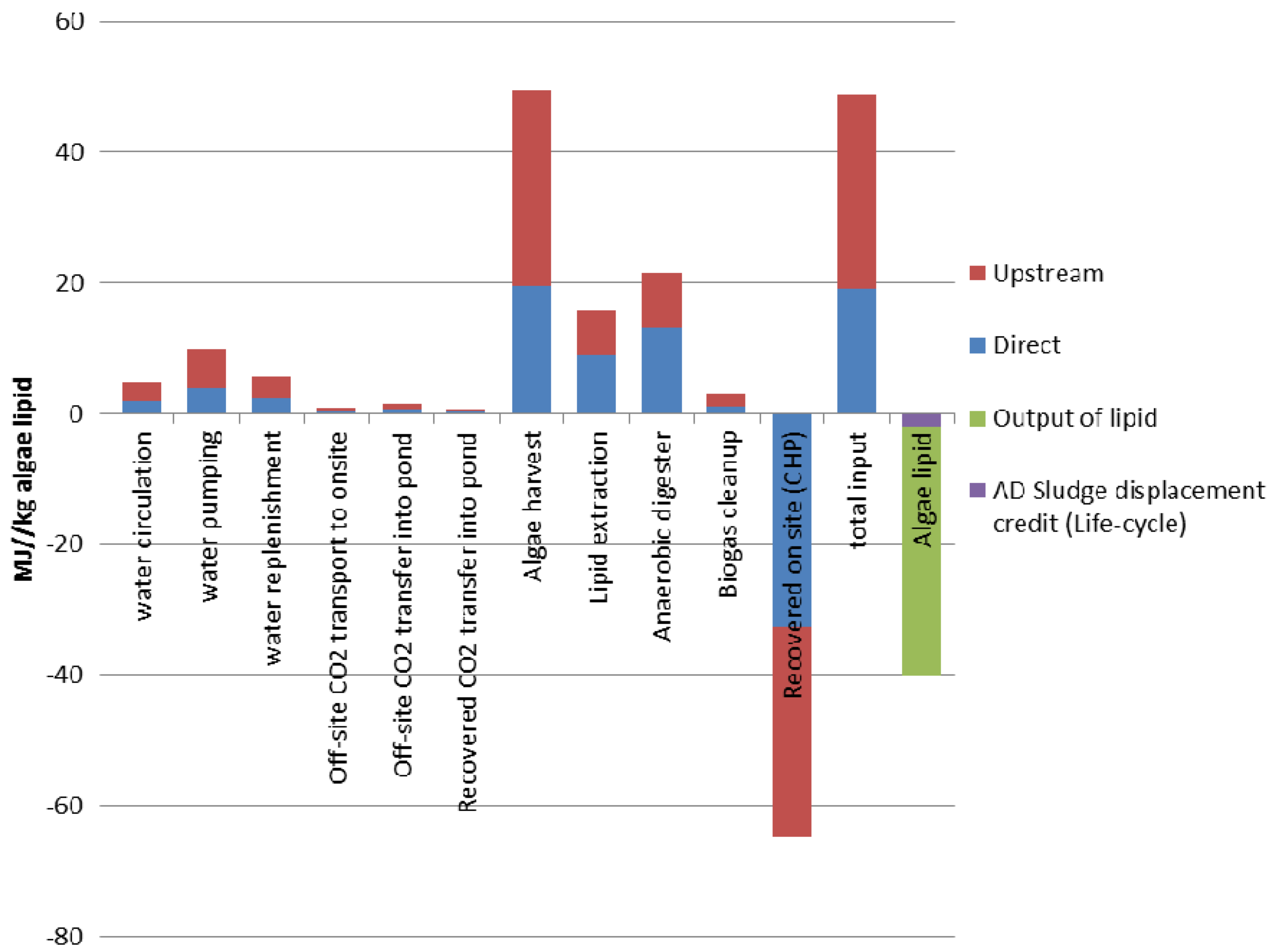
3.8.1. Energy Returns on Investment for Algae Oil
3.8.2. Life-Cycle Results for Algae Oil-Based Jet Fuel
| Item | Unit | per MJ BJF | per MJ CJF | Ratio of BJF to CJF |
|---|---|---|---|---|
| Life-cycle fossil Energy | MJ/MJ | 1.76 | 1.26 | 1.39 |
| Of which, Coal | MJ/MJ | 1.48 | 0.07 | 20.07 |
| Natural gas | MJ/MJ | 0.10 | 0.06 | 1.73 |
| Petroleum | MJ/MJ | 0.18 | 1.13 | 0.16 |
| Life-cycle GHG | gCO2,e/MJ | 159 | 93 | 1.70 |
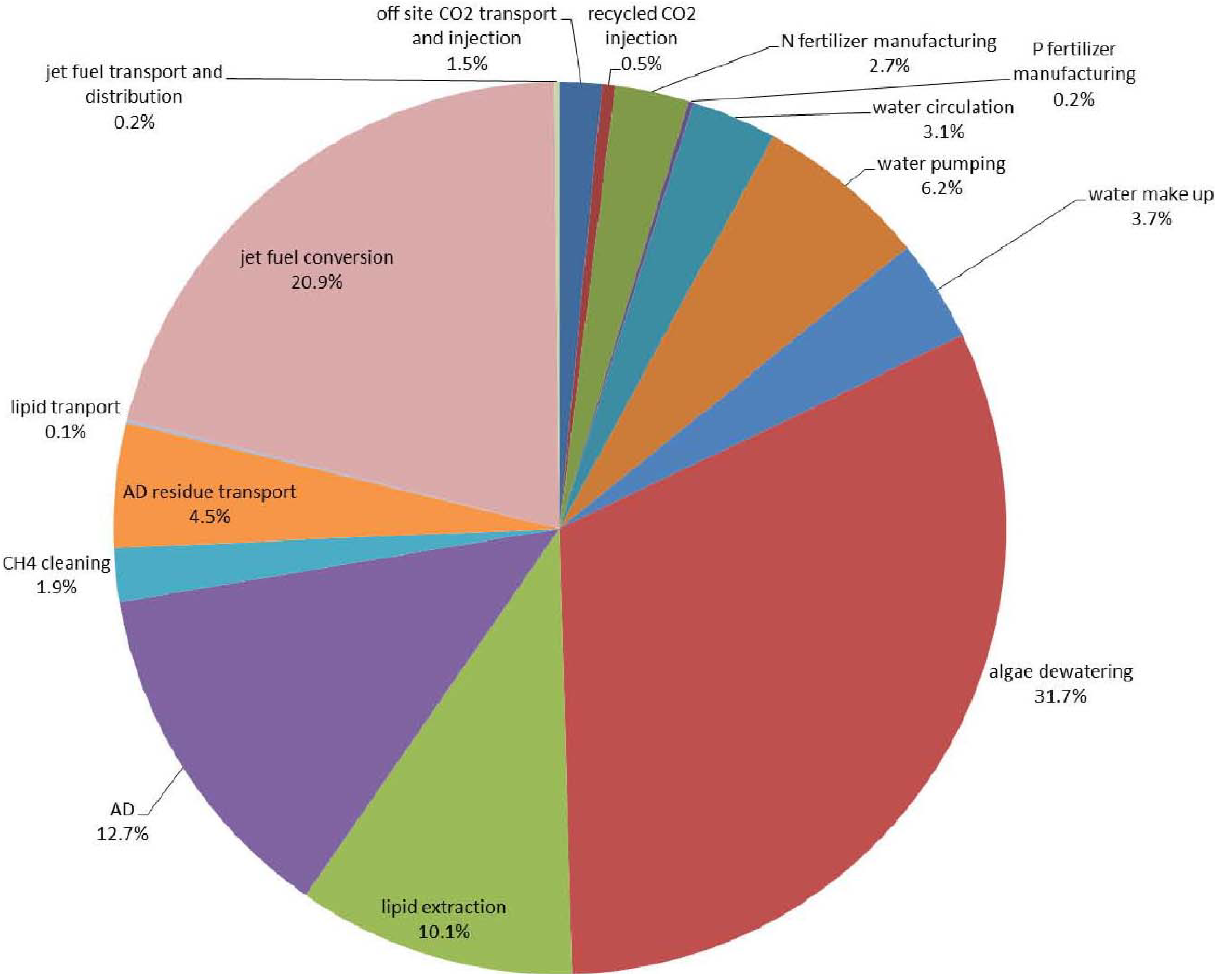
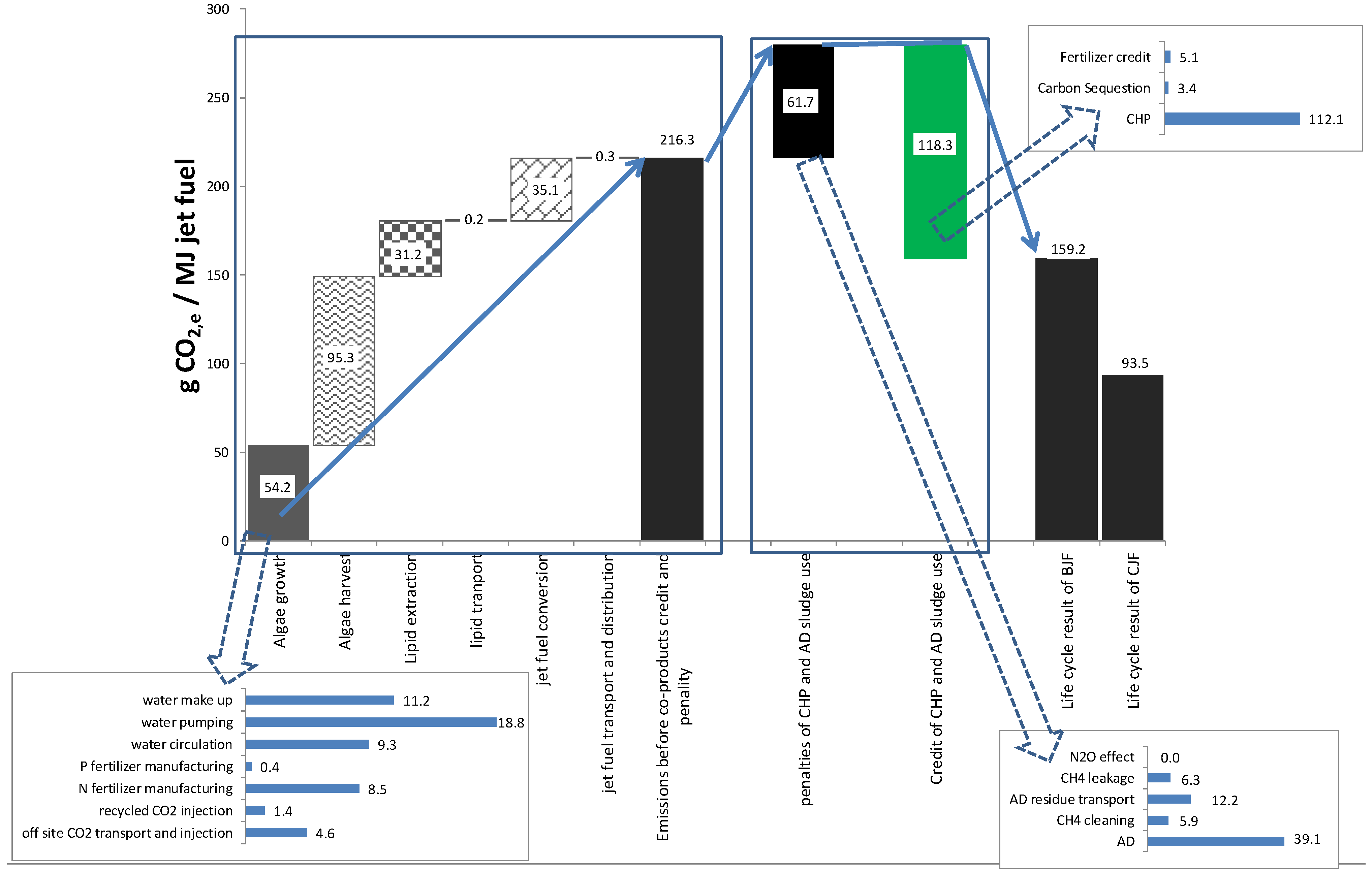
3.9. Sensitivity Analysis
| Parameter | Unit | Cases | ||
|---|---|---|---|---|
| Baseline case | Low case | High case | ||
| Lipid content | wt% | 25 | 50 | 1.25 |
| Fresh water energy use | – | China current situation | Not counted in | Similar to US situation in [34] |
| CO2 acquire energy use | – | Flue gas (20 vol%) | Not counted in | As pure CO2: 140 kWh/t [54] |
| Algae productivity | g/m2/day | 2.5 | 5 | 1.25 |
| Energy use for algae harvest | kWh per kg algae | About 1.2 | About half that in base case | About 10 time that in base case |
| Energy use for lipid extraction | per kg lipid | 6 MJ of heat and 0.5 kWh of electricity | 2 MJ of heat and 0.1 kWh of electricity | 12 MJ of heat and 1.0 kWh of electricity |
| CH4 yield | L/g-TS | 0.3 | 0.4 | 0.2 |
| CHP electrical efficiency | % | 33 | 38 | 28 |
| Fraction of N recovered to culture | % | 75 | 65 | 85 |
| – | – | Baseline case | Alternative case | |
| Water pumping to/from pond | kWh per kg algae | 195 (calculated by authors) | 1450(situation in [34]) | |
| Recovered energy by CHP | – | Yes | No | |
| Embodied energy in pond construction materials | – | No | Yes. Energy embodied in materials is about 30% that of biomass produced [34]. | |
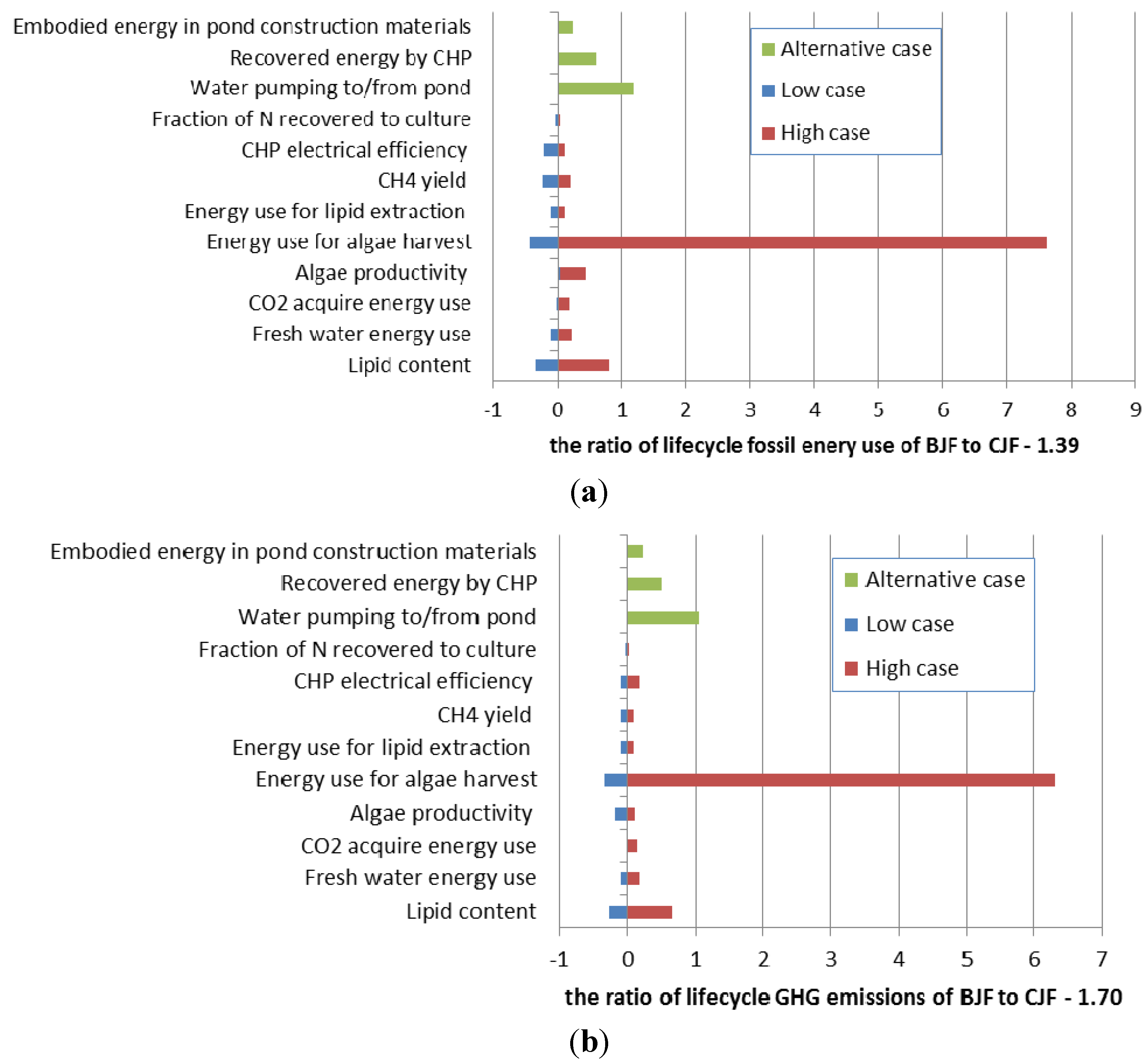
3.10. Better Scenario Discussion
- It is obvious that the overall GHG emissions of this pathway can be greatly improved if the electricity and heat generation can be sourced from low-carbon fuels. Some potential improvements in the life cycle of algae-based fuel pathway are also assumed to be achieved in this future case. For example, more renewable electricity and cleaner power will be available in the future to change the coal-dominant power system in China currently;
- Because energy consumption during algae harvesting has a significant impact on the final result, it is also assumed that great efforts will be made to decrease the energy-intensity of algae harvesting to half that of the current level. This parameter in the future case will be reduced to only 10% of the assumed value in the baseline scenario;
- In addition, biogas yield from the LEA flow into the digester can be increased and thereby improve the LCA results further. This parameter in the future case will be increased by 33% from the assumed level in the baseline scenario;
- Based on the fact that the contributions of the stirring, pumping, water replenishment, algae dewatering, and even algae oil extraction to the overall GHG emissions (per unit jet fuel product) depend on the lipid content of the algae. The lipid content is assumed be increased from 25% to 50%, to substantially reduce emissions for this pathway.
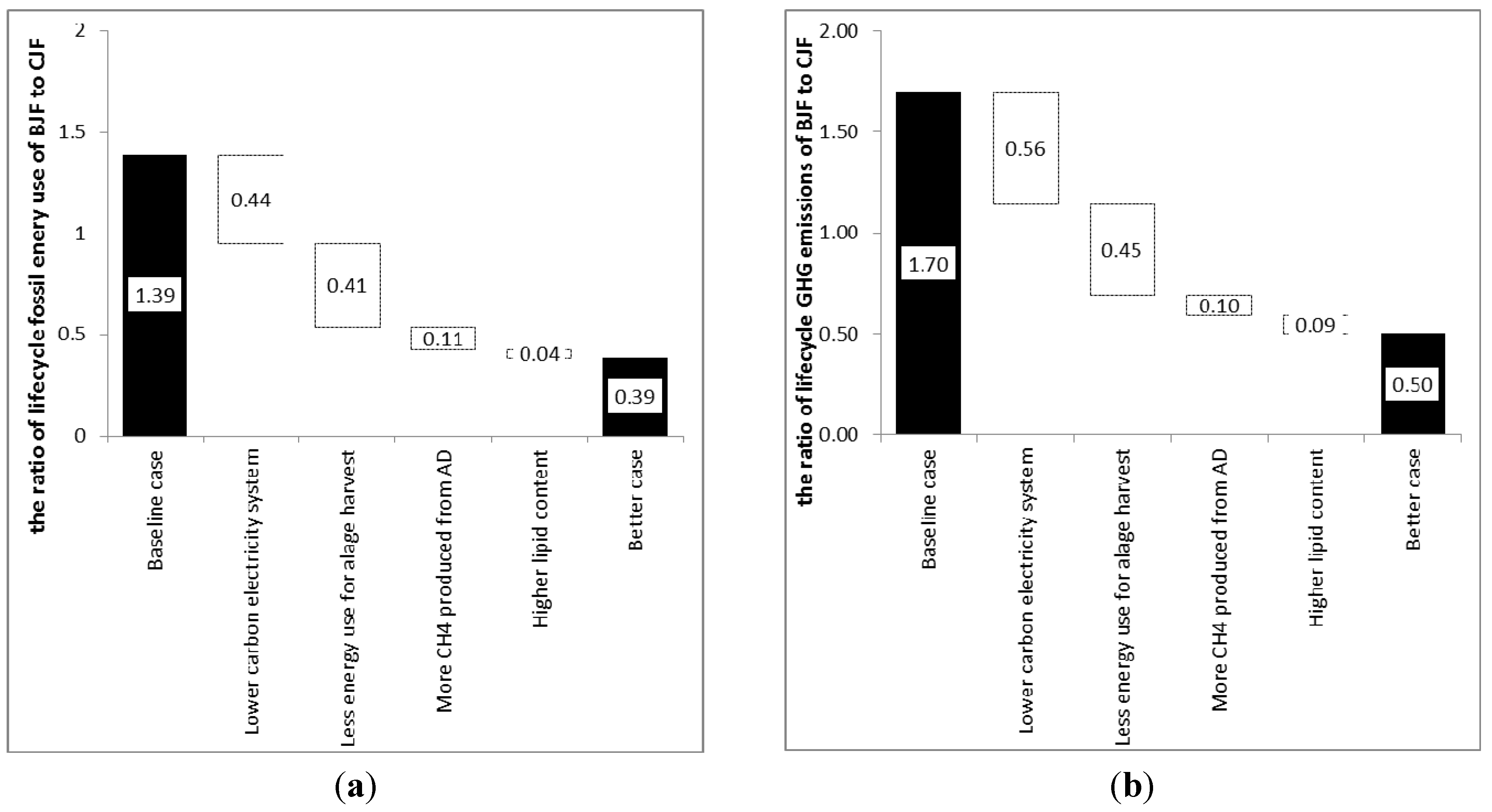
3.11. Comparative Study
| Source | Location of the study | EROI (direct) for algae oil | EROI (Life-cycle) for algae oil | The ratio of lifecycle fossil energy use for algae-based jet fuel to CJF | The ratio of lifecycle GHG emissions for algae-based jet fuel to CJF |
|---|---|---|---|---|---|
| This study | China | base case: 2.0 | base case: 0.82 | base case: 1.36 with a range from 1.01 to 7.68; Better case: 0.39 | Base case: 1.66 with a range from 1.23 to 9.67; Better case: 0.50 |
| Lardon et al. 2009 [24] | Mediterranean | – | – | 1.0 (biodiesel to Petroleum diesel) | 1.1 (biodiesel to Petroleum diesel) |
| Clarens et al. 2010[25] | Virginia, Iowa, California in USA | – | – | 1.1 (biodiesel to Petroleum diesel) | 0.61 (biodiesel to Petroleum diesel) |
| Jorquera et al. 2010 [56] | Unspecified location | – | – | 2.8 (biodiesel to Petroleum diesel) | – |
| Sander and Murthy, 2010 [57] | U.S. nationwide data, Unspecified location | – | – | 0.2 (biodiesel to Petroleum diesel) | 0.50 (biodiesel to Petroleum diesel) |
| Stephenson et al. 2010 [58] | United Kingdom | – | – | 0.3 (biodiesel to Petroleum diesel) | 0.64 (biodiesel to Petroleum diesel) |
| Campbell et al. 2011 [26] | Coastal Australia | – | – | 1.05 (biodiesel to Petroleum diesel) | 0.56 (biodiesel to Petroleum diesel) |
| Liu et al. 2011 [59] | – | – | 1.6~4.0 (biodiesel) | – | – |
| ANL [28] | USA | – | – | 0.45 (biodiesel to low sulfur diesel) | 0.55 (biodiesel to low sulfur diesel) |
| Handler et al. 2012 [55] | Unspecified location | – | 0.1~2.3 | – | Three cases: 0.61,1.44, 5.38 |
| Stratton et al. 2010 [32] | – | – | – | – | 0.6 with a range from 0.2 to 2.3 |
| Vasudevan et al. (2012) [41] | – | – | – | (biodiesel to Petroleum diesel) wet extraction: about 0.5; Dry extraction: about 3.00. | |
4. Concluding Remarks
- Algae strain selection is one of the key bottlenecks for high lipid content algae cultivation in an open and wide system. Moreover, some strains should be selected to maximize the final algae lipid productivity based on the trade-off between algae biomass productivity and lipid content;
- Innovative design and technology integration should be introduced into this new pathway to decrease energy use and costs as some traditional processes (e.g., centrifugation and drying) are very energy-intensive.
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (IEA). World Energy Outlook 2011; IEA: Paris, France, 2011. [Google Scholar]
- National Bureau of Statistics of China. China Statistics 2012; China Statistics Press: Beijing, China, 2012.
- British Petroleum (BP). BP Statistical Review of World Energy 2012. Available online: http://bp.com/statisticalreview (accessed on 26 November 2012).
- Wang, Q.Y. China Energy Data 2011; [in Chinese]; China Automotive Energy Research Center (CAERC): Beijing, China, 2011. [Google Scholar]
- China Energy Research Society. China Energy Development Report 2013; [in Chinese]; China Electric Power Press: Beijing, China, 2013. [Google Scholar]
- China Automotive Energy Research Center (CAERC). China Automotive Energy Outlook 2012; [in Chinese]; Scientific Press: Beijing, China, 2012. [Google Scholar]
- Yan, X.; Crookes, R.J. Reduction potentials of energy demand and GHG emissions in China’s road transport sector. Energy Policy 2009, 37, 658–668. [Google Scholar] [CrossRef]
- Yan, X.; Crookes, R.J. Energy demand and emissions from road transportation vehicles in China. Prog. Energy Combust. Sci. 2010, 36, 651–676. [Google Scholar] [CrossRef]
- Delluchi, M.A. A Lifecycle Emissions Model (LEM); Institute of Transportation Studies: Davis, CA, USA, 2003. [Google Scholar]
- Wang, M.Q.; Santini, D. Magnitude and value of electric vehicles emissions reduction for six driving cycles in four US cities with varying air quality problems. Transp. Res. Rec. 1993, 1416, 33–42. [Google Scholar] [CrossRef]
- Delluchi, M.A. A Revised Model of Emissions of GHG from the Use of Transportation Fuels and Electricity; Institute of Transportation Studies: Davis, CA, USA, 1997. [Google Scholar]
- Wang, M.Q. Development and Use of GREET 1.6 Fuel-Cycle Model for Transportation Fuels and Vehicle Technologies; Center for Transportation Research, Argonne National Laboratory: Argonne, IL, USA, 2001.
- Wang, M.; Wu, Y.; Elgowainy, A. Operating Manual for GREET Version 1.7. Available online: http://greet.anl.gov/publications.html (accessed on 26 November 2012).
- Wang, M.Q.; Weber, T. Well-to-Wheels Energy Use and GHG Emissions of Advanced Fuel/Vehicles System—North American Analysis; Center for Transportation Research, Argonne National Laboratory: Argonne, IL, USA, 2001.
- China Automotive Technology and Research Center (CATARC) and General Motor. Well-to-Wheels Analysis of Energy Consumption and GHG Emissions of Multi Vehicle Fuel in Future China; CATARC: Beijing, China, 2007. [Google Scholar]
- Nguyen, T.L.T.; Gheewala, S.H.; Garivait, S. Full chain energy analysis of fuel EtOH from cane molasses in Thailand. Appl. Energy 2008, 85, 722–734. [Google Scholar] [CrossRef]
- Yan, X.; Boies, A.M. Quantifying the uncertainties in life cycle greenhouse gas emissions for UK wheat ethanol. Environ. Res. Lett. 2013, 8, 015024:1–015024:12. [Google Scholar] [CrossRef]
- Yan, X.; Tan, D.K.Y.; Inderwildi, O.R.; Smith, J.A.C.; King, D.A. Life cycle energy and greenhouse gas analysis for agave-derived bioethanol. Energy Environ. Sci. 2011, 4, 3110–3121. [Google Scholar] [CrossRef]
- Shirvani, T.; Yan, X.; Inderwildi, O.R.; Edwards, P.; King, D.A. Life cycle energy and greenhouse gas analysis for algae-derived biodiesel. Energy Environ. Sci. 2011, 4, 3773–3778. [Google Scholar] [CrossRef]
- Allen, D.T.; Allport, C.; Atkins, K. Framework and Guidance for Estimating Greenhouse Gas Footprints of Aviation Fuels; Air Force Research Laboratory: Wright-Patterson, OH, USA, 2009. [Google Scholar]
- Lardon, L.; Helias, A.; Sialve, B.; Stayer, J.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Beer, T.; Batten, D. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol. 2011, 102, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Collet, P.; Hélias, A.; Lardon, L.; Ras, M.; Goy, R.A.; Steyer, J.P. Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour. Technol. 2011, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.D.; Han, J.; Wang, M.Q. Life-Cycle Analysis of Algal Lipid Fuels with the GREET Model; Energy Systems Division, Argonne National Laboratory: Argonne, IL, USA, 2011.
- National Research Council of the National Academies (NRC). Sustainable Development of Algal Biofuels in the United States; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Yan, X.; Crookes, R.J. Life cycle analysis of energy use and greenhouse gas emissions for road transportation fuels in China. Renew. Sustain. Energy Rev. 2009, 13, 2505–2514. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, P.; Yuan, X.; Zheng, Y. Life cycle assessment of biodiesel from soybean, jatropha and microalgae in China conditions. Renew. Sustain. Energy Rev. 2011, 15, 5081–5091. [Google Scholar] [CrossRef]
- Stratton, R.W.; Wong, H.M.; Hileman, J.I. Life Cycle Greenhouse Gas Emissions from Alternative Jet Fuels; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Ou, X.; Zhang, X.; Chang, S. Energy consumption and GHG emissions of six biofuel pathways by LCA in (the) People’s Republic of China. Appl. Energy 2009, 86, S197–S208. [Google Scholar] [CrossRef]
- Ou, X.; Chang, S.; Zhang, X. Alternative fuel buses currently in use in China: Life-cycle fossil energy use, GHG emissions and policy recommendations. Energy Policy 2010, 38, 406–418. [Google Scholar] [CrossRef]
- Li, X.; Ou, X.; Zhang, X. Life-cycle fossil energy consumption and greenhouse gas emission intensity of dominant secondary energy pathways of China in 2010. Energy 2013, 50, 15–23. [Google Scholar] [CrossRef]
- Murphy, C.F.; Allen, D.T. Energy-Water Nexus for Mass Cultivation of Algae. Environ. Sci. Technol. 2011, 45, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- UOP A Honeywell Company. Green Jet Fuel. Available online: http://www.uop.com/processing-solutions/biofuels/green-jet-fuel/ (accessed on 26 November 2012).
- Hu, X.; Li, Z.; Fu, X. Liquid Biofuels: From Fossil to Biomass; [in Chinese]; Chemical Industry Press: Beijing, China, 2012. [Google Scholar]
- China Automotive Energy Research Center (CAERC). Aviation Fuel Life Cycle Analysis in China; CAERC: Beijing, China, 2013. [Google Scholar]
- Wang, M.; Lee, H.; Molburg, J. Allocation of energy use in petroleum refineries to petroleum products: Implications for life-cycle energy use and emission inventory of petroleum transportation fuels. Int. J. Life Cycle Assess. 2004, 9, 34–44. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Kadam, K.L. Environmental implications of power generation via coal-microalgae cofiring. Energy 2002, 27, 905–922. [Google Scholar] [CrossRef]
- Vasudevan, V.; Stratton, R.W.; Pearlson, M.N.; Jersey, G.R.; Beyene, A.G.; Weissman, J.C.; Rubino, M.; Hileman, J.I. Environmental performance of algal biofuel technology options. Environ. Sci. Technol. 2012, 46, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, T.J. Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institute: Berkeley, CA, USA, 2010. [Google Scholar]
- Harris, R.W. Process Design and Cost Estimating Algorithms for the Computer Assisted Procedure for Design and Evaluation of Wastewater Treatment Systems; U.S. Environmental Protection Agency: Washington, DC, USA, 1982.
- Ren, G.; Guo, J. Chang in pan evaporation and influential factors over China: 1965–2000. [in Chinese]. J. Nat. Resour. 2006, 21, 31–44. [Google Scholar]
- Hu, G.; Ou, X.; Zhang, Q.; Karplus, V.J. Analysis on energy-water nexus by Sankey diagram: The case of Beijing. Desalin. Water Treat. 2013, 51, 4183–4193. [Google Scholar] [CrossRef]
- Mohn, F.H. Experiences and Strategies in the Recovery of Biomass from Mass Cultures of Microalgae. In Algae Biomass; Elsevier: Oxford, UK, 1980; pp. 547–571. [Google Scholar]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of Microalgal Cultures: A Major Bottleneck to Algae-Based Fuels. J. Renew. Sustain. Energy 2010, 2, 012701:1–012701:15. [Google Scholar] [CrossRef]
- Benemann, J.R.; Oswald, W.J. Systems and Economic Analysis of Microalgae Ponds for Conversion of CO2 to Biomass; Pittsburgh Energy Technology Center: Pittsburgh, PA, USA, 1996.
- Environmental Protection Agency (EPA). Emerging Technologies for Bio Solids Management; EPA: Washington, DC, USA, 2006.
- Klein-Marcuschamer, D.; Turner, C.; Allen, M.; Gray, P.; Dietzgen, R.G.; Gresshoff, P.M.; Hankamer, B.; Heimann, K.; Scott, P.T.; Stephens, E.; et al. Technoeconomic analysis of renewable aviation fuel from microalgae, Pongamia pinnata, and sugarcane. Biofuels Bioprod. Biorefin. 2013, 7, 416–428. [Google Scholar] [CrossRef]
- Ras, M. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies: Developments and Innovations; IEA: Paris, France, 2009. [Google Scholar]
- Flesch, T.K.; Raymond, L.; Desjardins, D.W. Fugitive CH4 Emissions from an Agricultural Biodigester. Biomass Bioenergy 2011, 9, 3927–3935. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, X. Life-Cycle Analysis of Automotive Energy Pathways in China; [in Chinese]; Tsinghua University Press: Beijing, China, 2011. [Google Scholar]
- Jaramillo, P.; Samaras, C.; Wakeley, H.; Meisterling, K. Greenhouse gas implications of using coal for transportation: Life cycle assessment of coal-to-liquids, plug-in hybrids, and hydrogen pathways. Energy Policy 2009, 37, 2689–2695. [Google Scholar] [CrossRef]
- Handler, R.M.; Canter, C.E.; Kalnes, T.N.; Lupton, F.S.; Kholiqov, O.; Shonnard, D.R.; Blowers, P. Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: Analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res. 2012, 1, 83–92. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruau, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: A comparison of raceways and air-lift tubular bioreactors. Energy Fuels 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Liu, X.; Clarens, A.F.; Colosi, L.M. Algae biodiesel has potential despite inconclusive results to date. Bioresour. Technol. 2012, 104, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Dragnet, J.-O.; White, S. The Story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Vaccari, D.A.; Strigul, N. Extrapolating phosphorus production to estimate resource reserves. Chemosphere 2011, 84, 792–797. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ou, X.; Yan, X.; Zhang, X.; Zhang, X. Life-Cycle Energy Use and Greenhouse Gas Emissions Analysis for Bio-Liquid Jet Fuel from Open Pond-Based Micro-Algae under China Conditions. Energies 2013, 6, 4897-4923. https://doi.org/10.3390/en6094897
Ou X, Yan X, Zhang X, Zhang X. Life-Cycle Energy Use and Greenhouse Gas Emissions Analysis for Bio-Liquid Jet Fuel from Open Pond-Based Micro-Algae under China Conditions. Energies. 2013; 6(9):4897-4923. https://doi.org/10.3390/en6094897
Chicago/Turabian StyleOu, Xunmin, Xiaoyu Yan, Xu Zhang, and Xiliang Zhang. 2013. "Life-Cycle Energy Use and Greenhouse Gas Emissions Analysis for Bio-Liquid Jet Fuel from Open Pond-Based Micro-Algae under China Conditions" Energies 6, no. 9: 4897-4923. https://doi.org/10.3390/en6094897





