Performance Analysis of Cold Energy Recovery from CO2 Injection in Ship-Based Carbon Capture and Storage (CCS)
Abstract
:1. Introduction
2. Description of Injection System
2.1. System 1: Conventional Injection System

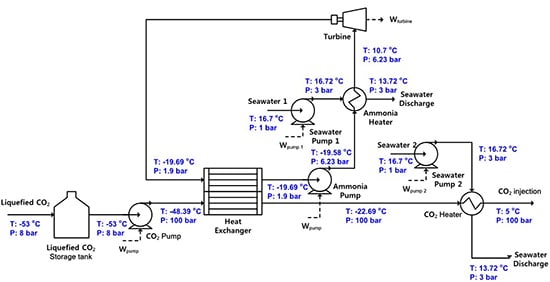
2.2. System 2: New Injection System with a Rankine Cycle


| Working fluid | Boiling point (°C) | Produced work per mass (kJ/kg) |
|---|---|---|
| C3H8 (Propane) | −42 | 65.4 |
| C4H10 (i-Butane) | −13 | 23.9 |
| NH3 (Ammonia) | −33 | 121.9 |
3. Comparison Approaches
3.1. Basis of Simulation
| Item | Note |
|---|---|
| Location | Ulleung Basin in the East Sea of Korea |
| Composition of CO2 | Pure CO2 |
| Inlet conditions | 8 bar, −53 °C |
| Outlet conditions | 100 bar, 5 °C |
| Flow rate | 580 ton/h |
| Seawater temperature | 16.6 °C |
| Discharge pressure of seawater pump | 3 bar |
| Discharge seawater temperature | ±3 °C |
| Equation of state | Peng-Robinson |
| Turbine efficiency | 80% |
| Pump efficiency | 75% |
| Working fluid | NH3 |
3.2. Specific Net Power Consumption and Thermal Efficiency
3.3. Exergy Efficiency

3.4. Life-Cycle Cost
| Item | Value |
|---|---|
| Injection time | 24 h/ship |
| The number of voyages | 72/year |
| MDO consumption rate | 180 g/kWh |
| MDO price | 1,000 $/ton |
| Life time | 20 years |
4. Results and Discussion
4.1. Specific Net Power Consumption

4.2. Exergy Efficiency

4.3. Life-Cycle Cost

4.4. Sensitivity Analysis
4.4.1. Discharge Pressure



4.4.2. Seawater Temperature


4.5. Discussion

5. Conclusions
Acknowledgments
Nomenclature
| CCS | Carbon capture and storage | LCC | Life cycle cost |
| LNG | Liquefied natural gas | CAPEX | Capital expenditures |
| OPEX | Operational expenditures | MDO | Marine diesel oil |
| Ẇ | Power [kW] | m | Mass [ton] |
| ẇ | Specific power [kW/ton] | ṅ | Mole flow rate [kgmol/hr] |
| Η | Efficiency | Heat transfer rate [kW] | |
| Ėx | Exergy [kJ/hr] | ex | Specific exergy [kJ/kgmol] |
| h | Specific enthalpy [kJ/kgmol] | s | Specific entropy [kJ/kgmol∙K] |
| T | Temperature [K] |
Subscripts
| loss | Loss | net | Net power consumption |
| input | Input into a system | output | Output from a system |
| in | Inlet | out | Outlet |
| turbine, i | i-th turbine | pump_CO2, i | i-th pump of CO2 |
| pump_WF, i | i-th pump of working fluid | pump_SW, i | i-th pump of sea water |
| p | Pump | t | Turbine |
| th | Thermal | ex | Exergy |
| i | Integer number (1, 2, 3, ∙∙∙) | j | Integer number (1, 2, 3, ∙∙∙) |
| CO2 | Carbon dioxide | 0 | Ambient condition (1 atm, 298 K) |
| SW | Sea water |
References
- Working Group 3. Greenhouse Gas Emissions Accelerate Despite Reduction Efforts. Available online: http://www.ipcc.ch/pdf/ar5/pr_wg3/20140413_pr_pc_wg3_en.pdf (accessed on 25 September 2014).
- Abellera, C.; Short, C. The Costs of CCS and Other Low-carbon Technologies; No 2; Global CCS Institute: Melbourne Australia, 2011; pp. 1–12. [Google Scholar]
- Hosa, A.; Esentia, M.; Stewart, J.; Haszeldine, S. Injection of CO2 into saline formations: Benchmarking worldwide projects. Chem. Eng. Res. Des. 2011, 89, 1855–1864. [Google Scholar] [CrossRef]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2009, 4, 659–667. [Google Scholar] [CrossRef]
- Nimtz, M.; Klatt, M.; Wiese, B.; Kuhn, M.; Krautz, H.J. Modelling of the CO2 process- and transport chain in CCS systems—Examination of transport and storage process. Chem. Erde 2010, 70, 185–192. [Google Scholar] [CrossRef]
- Krogh, E.; Nilsen, R.; Henningsen, R. Liquefied CO2 injection modelling. Energy Procedia 2012, 23, 527–555. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeiger, G.; Liljemark, S.; Le Gallo, Y. Dynamics CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar]
- Patel, D.; Mak, J.; Rivera, D.; Angtuaco, J. LNG vaporizer selection based on site ambient conditions. In Proceedings of the LNG 17, Houston, TX, USA, 16–19 April 2013.
- Choi, I.H.; Lee, S.I.; Seo, Y.T.; Chang, D.J. Analysis and optimization of cascade Rankine cycle for LNG cold energy recovery. Energy 2013, 61, 179–195. [Google Scholar] [CrossRef]
- Shi, X.; Che, D. A combined power cycle utilizing low-temperature waste heat and LNG cold energy. Energy Convers. Manag. 2008, 50, 567–575. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Che, D. Thermodynamic optimization of the operating parameters for a combined power cycle utilizing low-temperature waste heat and LNG cold energy. Appl. Therm. Eng. 2013, 59, 490–497. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Silva, O.; Carrera, J.; Olivella, S. Liquid CO2 injection for geological storage in deep saline aquifers. Int. J. Greenh. Gas Control 2013, 14, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Chiyoda Corp. Preliminary Feasibility Study on CO2 Carrier for Ship-based CCS. Available online: http://www.globalccsinstitute.com/publications/preliminary-feasibility-study-co2-carrier-ship-based-ccs (accessed on 18 July 2013).
- Aspelund, A.; Mølnvik, M.J.; de Koeijer, G. Ship transport of CO2 technical solutions and analysis of costs, energy utilization, exergy efficiency and co2 emissions. Chem. Eng. Res. Des. 2006, 84, 847–855. [Google Scholar] [CrossRef]
- Zero Emissions Platform (ZEP). The Costs of CO2 Transport. Available online: http://www.zeroemissionsplatform.eu/library/publication/167-zep-cost-report-transport.html (accessed on 18 July 2013).
- Liu, H.; You, L. Characteristics and applications of the cold heat exergy of liquefied natural gas. Energy Convers. Manag. 1999, 40, 1515–1525. [Google Scholar] [CrossRef]
- Venkatarathnam, G. Fundamental principles and processes. In Cryogenic Mixed Refrigerant Processes; Timmerhaus, K.D., Rizzuto, C., Mendelssohn, K., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Lee, S.I.; Choi, I.H.; Chang, D.J. Multi-objective optimization of VOC recovery and reuse in crude oil loading. Appl. Energy 2013, 108, 439–447. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.; Seo, Y.; Huh, C.; Chang, D. Performance Analysis of Cold Energy Recovery from CO2 Injection in Ship-Based Carbon Capture and Storage (CCS). Energies 2014, 7, 7266-7281. https://doi.org/10.3390/en7117266
You H, Seo Y, Huh C, Chang D. Performance Analysis of Cold Energy Recovery from CO2 Injection in Ship-Based Carbon Capture and Storage (CCS). Energies. 2014; 7(11):7266-7281. https://doi.org/10.3390/en7117266
Chicago/Turabian StyleYou, Hwalong, Youngkyun Seo, Cheol Huh, and Daejun Chang. 2014. "Performance Analysis of Cold Energy Recovery from CO2 Injection in Ship-Based Carbon Capture and Storage (CCS)" Energies 7, no. 11: 7266-7281. https://doi.org/10.3390/en7117266