Influence of Coal Blending on Ash Fusibility in Reducing Atmosphere
Abstract
:1. Introduction
2. Experimental Section
2.1. Coal and Ash Samples
2.2. AFT Tests
| Samples | G | D |
|---|---|---|
| Proximate Analysis (wt %, ad) | ||
| Moisture | 6.93 | 13.43 |
| Ash | 24.02 | 5.11 |
| Volatile matter | 26.62 | 27.01 |
| Fixed carbon | 42.43 | 54.39 |
| Ultimate Analysis (wt %, ad) | ||
| C | 54.58 | 64.47 |
| H | 3.31 | 3.11 |
| O | 9.43 | 12.64 |
| N | 1.13 | 0.69 |
| S | 0.6 | 0.49 |
| Ash Composition (wt %, normalized) | ||
| Na2O | 0.16 | 4.08 |
| MgO | 1.24 | 9.58 |
| Al2O3 | 37.82 | 9.46 |
| SiO2 | 52.52 | 16.78 |
| SO3 | 0.51 | 20.38 |
| K2O | 0.11 | 0.65 |
| CaO | 2.81 | 39.06 |
| Fe2O3 | 3.34 | 3.68 |
2.3. Heat Treatment Experiments
2.4. XRD and SEM/EDX Analysis
2.5. Thermodynamic Calculation with FactSage
2.5.1. Ternary Phase Diagram and Liquidus Temperature Calculation
2.5.2. Slag Content and Eutectic Analysis
3. Results and Discussion
3.1. Ash Fusion Characteristics
| Samples | DT (°C) | ST (°C) | HT (°C) | FT (°C) |
|---|---|---|---|---|
| G100 | 1490 | >1500 | >1500 | >1500 |
| D100 | 1322 | 1371 | 1390 | 1437 |
| G10D90 | 1243 | 1258 | 1271 | 1291 |
| G20D80 | 1211 | 1225 | 1238 | 1257 |
| G30D70 | 1167 | 1173 | 1182 | 1194 |
| G40D60 | 1230 | 1242 | 1263 | 1276 |
| G50D50 | 1302 | 1331 | 1368 | 1391 |


3.2. Mineral Matter Transformations of Blended Ashes
3.2.1. G100
| Samples | G100 | D100 | G10D90 | G30D70 | G50D50 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral crystals | Temperature, °C | ||||||||||||||||||||||||
| 900 | 1000 | 1100 | 1200 | 1300 | 900 | 1000 | 1100 | 1200 | 1300 | 900 | 1000 | 1100 | 1200 | 1300 | 900 | 1000 | 1100 | 1200 | 1300 | 900 | 1000 | 1100 | 1200 | 1300 | |
| Anhydrite | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| Sodiumsillicate | √ | ||||||||||||||||||||||||
| Mullite | √ | √ | √ | √ | |||||||||||||||||||||
| Sillimanite | √ | √ | √ | ||||||||||||||||||||||
| Cristobalite | √ | √ | |||||||||||||||||||||||
| Nepheline | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||
| Magnetite | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Gehlenite | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| Periclase | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||
| Anorthite | √ | √ | √ | √ | √ | ||||||||||||||||||||
| Rankinite | √ | ||||||||||||||||||||||||
| Merwinite | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||
| Quartz | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| Hematite | √ | √ | √ | √ | √ | √ | |||||||||||||||||||
| Diopside | √ | √ | √ | √ | |||||||||||||||||||||
| Hercynite | √ | √ | √ | ||||||||||||||||||||||
| “√” means the corresponding mineral crystal is formed in the sample. | |||||||||||||||||||||||||
3.2.2. D100
3.2.3. G10D90
3.2.4. G30D70
3.2.5. G50D50


3.3. Thermodynamic Calculations with FactSage
3.3.1. Ternary Phase Diagram and Liquidus Analysis

3.3.2. Eutectic Processes Simulated with FactSage
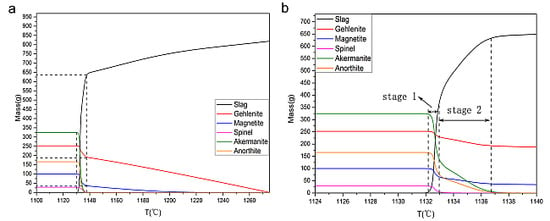
3.3.2.1. D100
| Samples | Reactants | Mass Content (%) | Mass (g) |
|---|---|---|---|
| D100 | Magnetite | 21.4% | 100.0 |
| Gehlenite | 69.4% | 343.0 | |
| Merwinite | 9.2% | 45.3 | |
| G10D90 | Magnetite | 20.9% | 100.0 |
| Gehlenite | 61.2% | 293.1 | |
| Merwinite | 17.9% | 85.5 | |
| G30D70 | Magnetite | 7.9% | 100.0 |
| Gehlenite | 54.2% | 470.1 | |
| Diopside | 37.9% | 297.0 |


3.3.2.2. G10D90

3.3.2.3. G30D70
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, P.; Massoudi, M. Slag Behavior in Gasifiers. Part I: Influence of Coal Properties and Gasification Conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef]
- Bryant, G.W.; Browning, G.J.; Emanuel, H.; Gupta, S.K.; Gupta, R.P.; Lucas, J.A.; Wall, T.F. The fusibility of blended coal ash. Energy Fuels 2000, 14, 316–325. [Google Scholar] [CrossRef]
- Sarkar, P.; Mukherjee, A.; Sahu, S.G.; Choudhury, A.; Adak, A.K.; Kumar, M.; Choudhury, N.; Biswas, S. Evaluation of combustion characteristics in thermogravimetric analyzer and drop tube furnace for Indian coal blends. Appl. Therm. Eng. 2013, 60, 145–151. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, Y.H.; Wang, C.A.; Che, D.F. Experimental study on interaction and kinetic characteristics during combustion of blended coals. J. Therm. Anal. Calorim. 2012, 107, 935–942. [Google Scholar] [CrossRef]
- Lolja, S.A.; Haxhi, H.; Dhimitri, R.; Drushku, S.; Malja, A. Correlation between ash fusion temperatures and chemical composition in Albanian coal ashes. Fuel 2002, 81, 2257–2261. [Google Scholar] [CrossRef]
- Van Dyk, J.C. Understanding the influence of acidic components (Si, Al, and Ti) on ash flow temperature of South African coal sources. Miner. Eng. 2006, 19, 280–286. [Google Scholar] [CrossRef]
- Liu, B.; He, Q.H.; Jiang, Z.H.; Xu, R.F.; Hu, B.X. Relationship between coal ash composition and ash fusion temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Rong, Y.Q.; Zhu, Z.B.; Koyama, S. Fusibility and flow properties of coal ash and slag. Fuel 2009, 88, 297–304. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhao, F.; Wang, F.C.; Guo, Q.H.; Yu, G.S. Study on Fusibility and Flow Behavior of High-Calcium Coal Ash. J. Chem. Eng. Jpn. 2014, 47, 711–716. [Google Scholar] [CrossRef]
- Gupta, S.; Dubikova, M.; French, D.; Sahajwalla, V. Characterization of the origin and distribution of the minerals and phases in metallurgical cokes. Energy Fuels 2007, 21, 303–313. [Google Scholar] [CrossRef]
- Vassileva, C.G.; Vassilev, S.V. Behaviour of inorganic matter during heating of Bulgarian coals: 2. Subbituminous and bituminous coals. Fuel Process Technol 2006, 87, 1095–1116. [Google Scholar] [CrossRef]
- Rodrigues, S.; Marques, M.; Ward, C.R.; Suarez-Ruiz, I.; Flores, D. Mineral transformations during high temperature treatment of anthracite. Int. J. Coal Geol. 2012, 94, 191–200. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Banerjee, A.; Tripathy, R.; Mandal, G.K.; Basariya, M.R.; Sharma, M. Composition, mineral matter characteristics and ash fusion behavior of some Indian coals. Fuel 2015, 150, 96–101. [Google Scholar] [CrossRef]
- Saikia, B.K.; Wang, P.P.; Saikia, A.; Song, H.J.; Liu, J.J.; Wei, J.P.; Gupta, U.N. Mineralogical and Elemental Analysis of Some High-Sulfur Indian Paleogene Coals: A Statistical Approach. Energy Fuels 2015, 29, 1407–1420. [Google Scholar] [CrossRef]
- Li, F.H.; Huang, J.J.; Fang, Y.T.; Liu, Q.R. Fusibility Characteristics of Residual Ash from Lignite Fluidized-Bed Gasification to Understand Its Formation. Energy Fuels 2012, 26, 5020–5027. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Prediction of Chinese Coal Ash Fusion Temperatures in Ar and H2 Atmospheres. Energy Fuels 2009, 23, 1990–1997. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Li, Y.; Lu, D.; Zhou, Z.J.; Wang, Z.H.; Zhou, J.H.; Cen, K.F. Improvement of the Coal Ash Slagging Tendency by Coal Washing and Additive Blending with Mullite Generation. Energy Fuels 2013, 27, 2049–2056. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Folgueras, J.R. Modification of lignite ash fusion temperatures by the addition of different types of sewage sludge. Fuel Process. Technol. 2015, 131, 348–355. [Google Scholar] [CrossRef]
- Nel, M.V.; Strydorn, C.A.; Schobert, H.H.; Beukes, J.P.; Bunt, J.R. Reducing atmosphere ash fusion temperatures of a mixture of coal-associated minerals—The effect of inorganic additives and ashing temperature. Fuel Process. Technol. 2014, 124, 78–86. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E. Ash properties and environmental impact of various biomass and coal fuels and their blends. Fuel Process. Technol. 2011, 92, 570–581. [Google Scholar] [CrossRef]
- Kocabas-Atakli, Z.O.; Okyay-Oner, F.; Yurum, Y. Combustion characteristics of Turkish hazelnut shell biomass, lignite coal, and their respective blends via thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 119, 1723–1729. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhang, Z.X.; Chen, Y.S.; Zhou, T.; Fan, J.J.; Piao, G.L.; Kobayashi, N.; Mori, S.; Itaya, Y. Main mineral melting behavior and mineral reaction mechanism at molecular level of blended coal ash under gasification condition. Fuel Process. Technol. 2010, 91, 1591–1600. [Google Scholar] [CrossRef]
- Qiu, J.R.; Li, F.; Zheng, Y.; Zheng, C.G.; Zhou, H.C. The influences of mineral behaviour on blended coal ash fusion characteristics. Fuel 1999, 78, 963–969. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Li, B.Q. Characterization of low-temperature coal ash behaviors at high temperatures under reducing atmosphere. Fuel 2008, 87, 583–591. [Google Scholar] [CrossRef]
- Wang, C.A.; Liu, Y.H.; Zhang, X.M.; Che, D.F. A Study on Coal Properties and Combustion Characteristics of Blended Coals in Northwestern China. Energy Fuels 2011, 25, 3634–3645. [Google Scholar] [CrossRef]
- Djordjevic, D.; Stojkovic, D.; Djordjevic, N.; Smelcerovic, M. Thermodynamics of Reactive Dye Adsorption from Aqueous Solution on the Ashes from City Heating Station. Ecol. Chem. Eng. S. 2011, 18, 527–536. [Google Scholar]
- Song, W.J.; Sun, Y.M.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Measurement and Simulation of Flow Properties of Coal Ash Slag in Coal Gasification. AICHE J. 2011, 57, 801–818. [Google Scholar] [CrossRef]
- Kong, L.X.; Bai, J.; Bai, Z.Q.; Guo, Z.X.; Li, W. Effects of CaCO3 on slag flow properties at high temperatures. Fuel 2013, 109, 76–85. [Google Scholar] [CrossRef]
- Zhang, G.J.; Reinmoller, M.; Klinger, M.; Meyer, B. Ash melting behavior and slag infiltration into alumina refractory simulating co-gasification of coal and biomass. Fuel 2015, 139, 457–465. [Google Scholar] [CrossRef]
- Ilyushechkin, A.Y.; Hla, S.S. Viscosity of High-Iron Slags from Australian Coals. Energy Fuels 2013, 27, 3736–3742. [Google Scholar] [CrossRef]
- Van Dyk, J.C.; Keyser, M.J. Influence of discard mineral matter on slag-liquid formation and ash melting properties of coal—A FACTSAGE (TM) simulation study. Fuel 2014, 116, 834–840. [Google Scholar] [CrossRef]
- Yuan, H.P.; Liang, Q.F.; Gong, X. Crystallization of Coal Ash Slags at High Temperatures and Effects on the Viscosity. Energy Fuels 2012, 26, 3717–3722. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Piotrowska, P.; van Eyk, P.J.; Bostrom, D.; Kwong, C.W.; Wang, D.B.; Cole, A.J.; de Nys, R.; Gentili, F.G.; Ashman, P.J. Cogasification of Australian Brown Coal with Algae in a Fluidized Bed Reactor. Energy Fuels 2015, 29, 1686–1700. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Helble, J.J.; Boni, A.A. Mineral Behavior during Coal Combustion 1. Pyrite Transformations. Prog. Energy Combust. 1990, 16, 281–292. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Qiu, K.; Zhang, L.; Huang, Z.; Wang, Z.; Liu, J. Influence of Coal Blending on Ash Fusibility in Reducing Atmosphere. Energies 2015, 8, 4735-4754. https://doi.org/10.3390/en8064735
Shen M, Qiu K, Zhang L, Huang Z, Wang Z, Liu J. Influence of Coal Blending on Ash Fusibility in Reducing Atmosphere. Energies. 2015; 8(6):4735-4754. https://doi.org/10.3390/en8064735
Chicago/Turabian StyleShen, Mingke, Kunzan Qiu, Long Zhang, Zhenyu Huang, Zhihua Wang, and Jianzhong Liu. 2015. "Influence of Coal Blending on Ash Fusibility in Reducing Atmosphere" Energies 8, no. 6: 4735-4754. https://doi.org/10.3390/en8064735