Enhancement of Oxygen Reduction and Mitigation of Ionomer Dry-Out Using Insoluble Heteropoly Acids in Intermediate Temperature Polymer-Electrolyte Membrane Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization


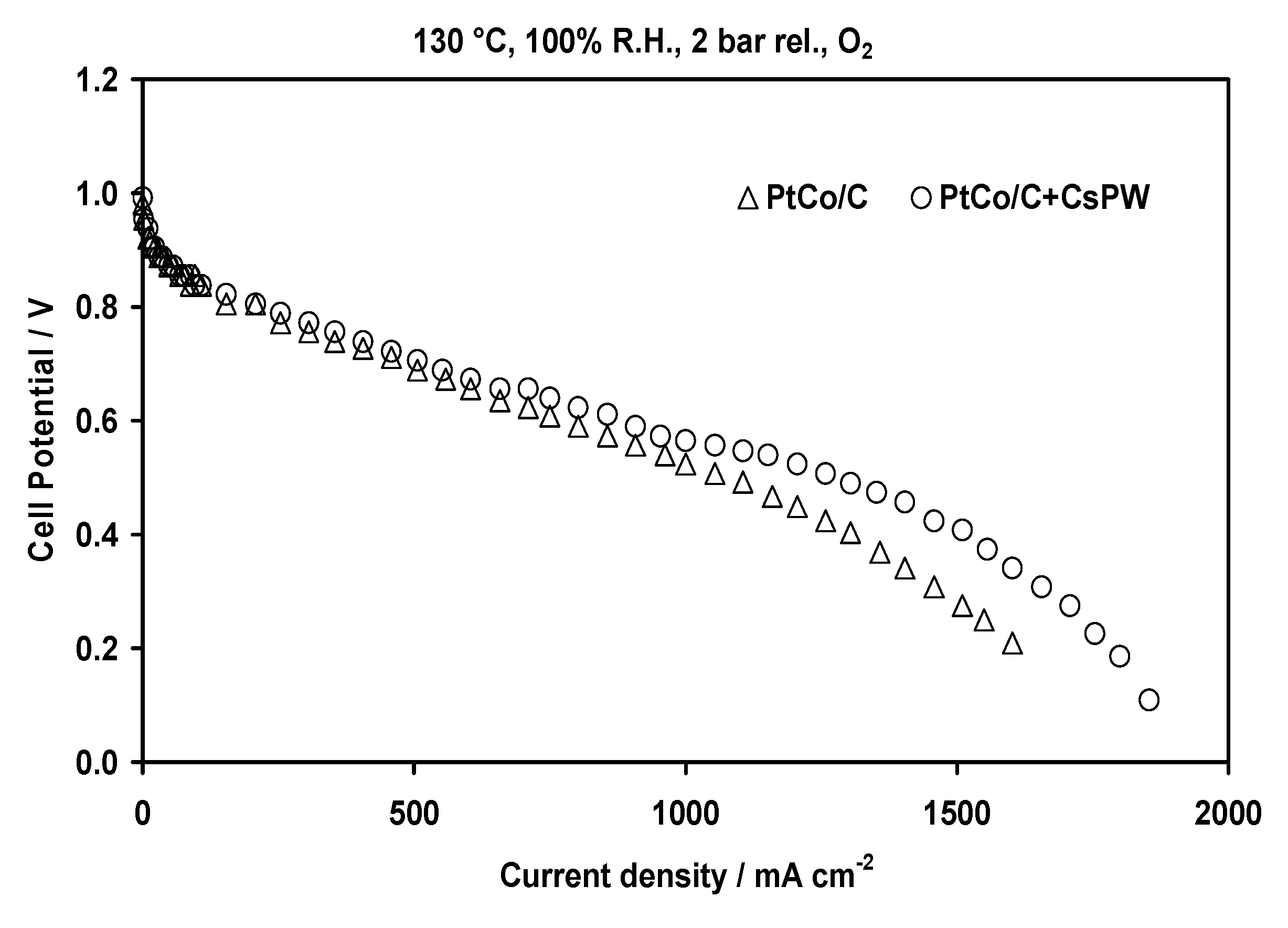
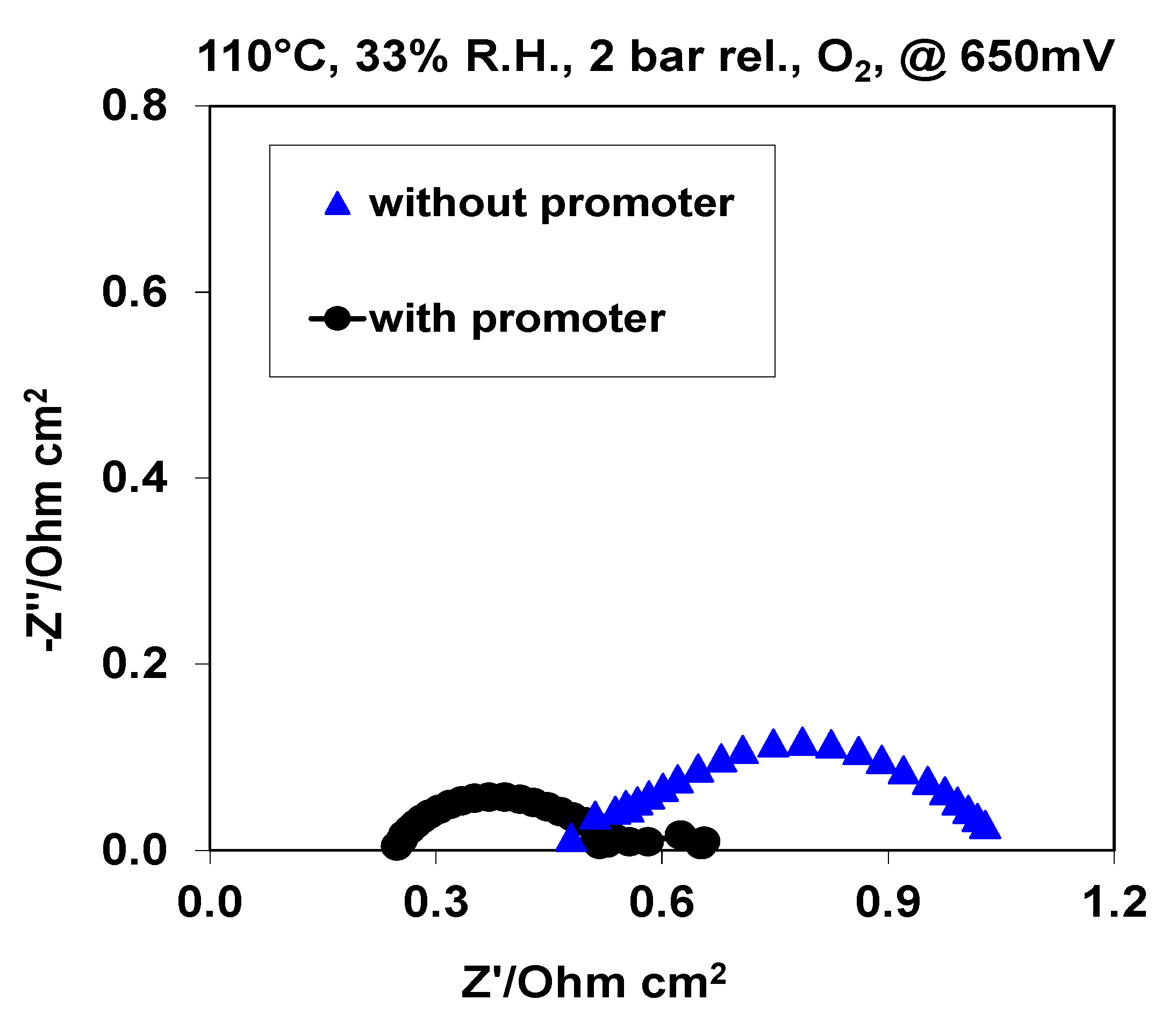
2.2. Electrochemical Characterization






3. Experimental Section
3.1. Promoter Preparation
3.2. Catalyst Preparation
3.3. Physico-Chemical Analysis
3.4. Electrochemical Studies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Jaffray, C.; Hards, G.A. Handbook of Fuel Cells—Fundamentals, Technology and Applications; Vielstich, W., Lamm, A., Gasteiger, H., Eds.; John Wiley & Sons: Chichester, UK, 2003. [Google Scholar]
- Srinivasan, S.; Dillon, R.; Krishnan, L.; Aricò, A.S.; Antonucci, V.; Bocarsly, A.B.; Lee, W.J.; Hsueh, K.L.; Lai, C.C.; Peng, A. Techno-Economic Challenges for PEMFCs and DMFCs Entering Energy Sector. In Proceedings of the 1st Fuel Cell Science Engineering and Technology, New York, NY, USA, 21–23 April 2003; pp. 529–536.
- Ji, M.; Wei, Z. A Review of Water Management in Polymer Electrolyte Membrane Fuel Cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K.; Schmoeckel, A.K.; Vernstrom, G.D.; Atanasoski, R. High voltage stability of nanostructured thin film catalysts for PEM fuel cells. J. Power Sources 2006, 161, 1002–1011. [Google Scholar] [CrossRef]
- Wood, T.E.; Tan, Z.; Schmoeckel, A.K.; O’Neill, D.; Atanasoski, R. Non-precious metal oxygen reduction catalyst for PEM fuel cells based on nitroaniline precursor. J. Power Sources 2008, 178, 510–516. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal Nanoparticles and Related Materials Supported on Carbon Nanotubes: Methods and Applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Norskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sheng, W.; Yabuuchi, N.; Ferreira, P.J.; Allard, L.F.; Yang, S.H. Origin of Oxygen Reduction Reaction Activity on “Pt3Co” Nanoparticles: Atomically Resolved Chemical Compositions and Structures. J. Phys. Chem. C. 2009, 113, 1109–1125. [Google Scholar] [CrossRef]
- Shim, J.; Lee, C.R.; Lee, H.K.; Lee, J.S.; Cairns, E.J. Electrochemical characteristics of Pt-WO3/C and Pt-TiO2/C electrocatalysts in a polymer electrolyte fuel cell. J. Power Sources 2001, 102, 172–177. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, Z.; Kaufman, A. Effect of oxygen storage materials on the performance of proton-exchange membrane fuel cells. J. Power Sources 2003, 115, 40–43. [Google Scholar] [CrossRef]
- Giordano, N.; Aricò, A.S.; Hocevar, S.; Staiti, P.; Antonucci, P.L.; Antonucci, V. Oxygen Reduction Kinetics in Phosphotungstic Acid at Low Temperature. Electrochim. Acta 1993, 38, 1733–1741. [Google Scholar] [CrossRef]
- Giordano, N.; Staiti, P.; Hocevar, S.; Aricò, A.S. High performance fuel cell based on phosphotungstic acid as proton conducting electrolyte. Electrochim. Acta 1996, 41, 397–403. [Google Scholar] [CrossRef]
- Giordano, N.; Staiti, P.; Aricò, A.S.; Passalacqua, E.; Abate, L.; Hocevar, S. Analysis of the chemical cross-over in a phosphotungstic acid electrolyte based fuel cell. Electrochim. Acta 1997, 42, 1645–1652. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Wang, X.; Mamlouk, M.; Scott, K. Composite membranes of polybenzimidazole and cesium-salts-of heteropolyacids for intermediate temperature fuel cells. J. Mater. Chem. 2011, 21, 6014–6019. [Google Scholar] [CrossRef]
- Amirinejad, M.; Madaeni, S.S.; Rafiee, E.; Amirinejad, S. Cesium hydrogen salt of heteropolyacids/Nafion nanocomposite membranes for proton exchange membrane fuel cells. J. Membr. Sci. 2011, 377, 89–98. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Wu, X.; Cao, Y.; Scott, K. A Composite Membrane of Cesium Salt of Heteropolyacids/Quaternary Diazabicyclo-Octane Polysulfone with Poly (Tetrafluoroethylene) for Intermediate Temperature Fuel Cells. Membranes 2012, 2, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kourasi, M.; Wills, R.G.A.; Shah, A.A.; Walsh, F.C. Heteropolyacids for fuel cell applications. Electrochim. Acta 2014, 127, 454–466. [Google Scholar] [CrossRef]
- Wlodarczyk, R.; Chojak, M.; Miecznikowski, K.; Kolary, A.; Kulesza, P.J.; Marassi, R. Electroreduction of oxygen at polyoxometallate-modified glassy carbon-supported Pt nanoparticles. J. Power Sources 2006, 159, 802–809. [Google Scholar] [CrossRef]
- Wlodarczyk, R.; Kolary-Zurowska, A.; Marassi, R.; Chojak, M.; Kulesza, P.J. Enhancement of oxygen reduction by incorporation of heteropolytungstate into the electrocatalytic ink of carbon supported platinum nanoparticles. Electrochim. Acta 2007, 52, 3958–3964. [Google Scholar] [CrossRef]
- Aricò, A.S.; Di Blasi, A.; Brunaccini, G.; Sergi, F.; Dispenza, G.; Andaloro, L.; Ferraro, M.; Antonucci, V.; Asher, P.; Buche, S.; et al. High Temperature Operation of a Solid Polymer Electrolyte Fuel Cell Stack Based on a New Ionomer Membrane. Fuel Cells 2010, 10, 1013–1023. [Google Scholar] [CrossRef]
- Dsoke, S.; Kolary-Zurowska, A.; Zurowski, A.; Mignini, P.; Kulesza, P.J.; Marassi, R. Rotating disk electrode study of Cs2.5H0.5PW12O40 as mesoporous support for Pt nanoparticles for PEM fuel cells electrodes. J. Power Sources 2011, 196, 10591–10600. [Google Scholar] [CrossRef]
- Zhao, J.; Ukshe, A.E.; Leonova, L.S.; Chub, A.V.; Frolova, L.A.; Dobrovolsky, Y.A. Composite catalytic system based on the platinised heteropolycompounds with addition of electronic conductors for low-temperature hydrogen-air fuel cells. Int. Sc. J. Altern. Energy Ecol. 2010, 12, 37–49. [Google Scholar]
- Aricò, A.S.; Stassi, A.; Gatto, I.; Monforte, G.; Passalacqua, E.; Antonucci, V. Surface properties of Pt and PtCo electrocatalysts and their influence on the performance and degradation of high-temperature polymer electrolyte fuel cells. J. Phys. Chem. C 2010, 114, 15823–15836. [Google Scholar] [CrossRef]
- Stassi, A.; Gatto, I.; Monforte, G.; Baglio, V.; Passalacqua, E.; Antonucci, V.; Aricò, A.S. The effect of thermal treatment on structure and surface composition of PtCo electro-catalysts for application in PEMFCs operating under automotive conditions. J. Power Sources 2012, 208, 35–45. [Google Scholar] [CrossRef]
- Aricò, A.S.; Stassi, A.; Modica, E.; Ornelas, R.; Gatto, I.; Passalacqua, E.; Antonucci, V. Performance and degradation of high temperature polymer electrolyte fuel cell catalysts. J. Power Sources 2008, 178, 525–536. [Google Scholar] [CrossRef]
- Stassi, A.; Modica, E.; Antonucci, V.; Aricò, A.S. A half cell study of performance and degradation of oxygen reduction catalysts for application in low temperature fuel cells. Fuel Cells 2009, 9, 201–208. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Di Blasi, A.; Modica, E.; Antonucci, P.L.; Antonucci, V. Analysis of the high-temperature methanol oxidation behaviour at carbon-supported Pt–Ru catalysts. J. Electroanal. Chem. 2003, 557, 167–176. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stassi, A.; Gatto, I.; Saccà, A.; Baglio, V.; Aricò, A.S. Enhancement of Oxygen Reduction and Mitigation of Ionomer Dry-Out Using Insoluble Heteropoly Acids in Intermediate Temperature Polymer-Electrolyte Membrane Fuel Cells. Energies 2015, 8, 7805-7817. https://doi.org/10.3390/en8087805
Stassi A, Gatto I, Saccà A, Baglio V, Aricò AS. Enhancement of Oxygen Reduction and Mitigation of Ionomer Dry-Out Using Insoluble Heteropoly Acids in Intermediate Temperature Polymer-Electrolyte Membrane Fuel Cells. Energies. 2015; 8(8):7805-7817. https://doi.org/10.3390/en8087805
Chicago/Turabian StyleStassi, Alessandro, Irene Gatto, Ada Saccà, Vincenzo Baglio, and Antonino S. Aricò. 2015. "Enhancement of Oxygen Reduction and Mitigation of Ionomer Dry-Out Using Insoluble Heteropoly Acids in Intermediate Temperature Polymer-Electrolyte Membrane Fuel Cells" Energies 8, no. 8: 7805-7817. https://doi.org/10.3390/en8087805
APA StyleStassi, A., Gatto, I., Saccà, A., Baglio, V., & Aricò, A. S. (2015). Enhancement of Oxygen Reduction and Mitigation of Ionomer Dry-Out Using Insoluble Heteropoly Acids in Intermediate Temperature Polymer-Electrolyte Membrane Fuel Cells. Energies, 8(8), 7805-7817. https://doi.org/10.3390/en8087805







