Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells
Abstract
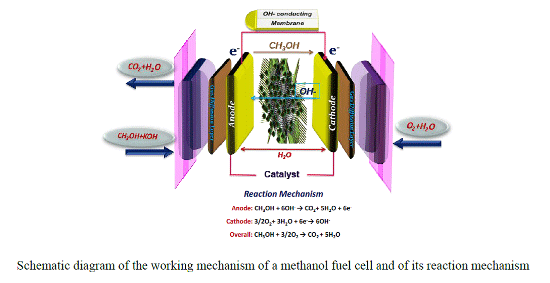
:1. Introduction
2. Results and Discussion
2.1. Morphological and Composition Analysis

2.2. Structural Analysis


| Nanocomposite Membranes | Tm (°C) | Entropy ΔH (J/g) | Crystallinity (%) |
|---|---|---|---|
| QPVA | 189.84 | 14.26 | 10.56 |
| QPVA/5% FS | 176.68 | 11.18 | 8.71 |
| QPVA/10% FS | 177.17 | 12.19 | 10.03 |
| QPVA/20% FS | 185.21 | 13.30 | 12.31 |
2.3. Thermal Analysis

2.4. Water Vapor Uptake and Diffusion Coefficients

2.5. Methanol Permeability from Aqueous Solutions

2.6. Ethanol Permeability from Aqueous Solutions

2.7. Alkaline Uptakes and Ionic Conductivity

2.8. Direct Methanol Fuel Cell Performance of Pristine QPVA Membrane


2.9. Direct Methanol Fuel Cell Performance of QPVA/FS Nanocomposites


| Electrolyte | Operating Conditions | Peak Power Density (mW/cm2) | References | |
|---|---|---|---|---|
| Temperature °C | Feed Methanol (MeOH) + Alkaline | |||
| QPVA/PECH/KOH | ambient | 4 M MeOH + 4 M KOH | 20.81 | Yang [10] |
| QPVA/Al2O3/KOH | ambient | 4 M MeOH + 4 M KOH | 36.25 | Yang et al. [22] |
| PVA/sodium alginate/KOH | 40 °C | 2 M MeOH + 2 M KOH | 29.2 | Yang et al. [14] |
| PVA/CNT/KOH | 40 °C | 2 M MeOH +6 M KOH | 30.1 | Lue et al. [11] |
| QPVA/Q-SiO2/KOH | 50 °C | 2 M MeOH + 8 M KOH | 35.13 | Yang et al. [16] |
| PVA/FS/KOH | 60 °C | 2 M MeOH + 6 M KOH | 15.3 | Lue et al. [24] |
| PVA/FS/KOH | 60 °C | 2 M MeOH + 6 M KOH | 39 | Lue et al. [34] |
| PVA/MCNTs/KOH | 60 °C | 2 M MeOH + 6 M KOH | 39 | Pan et al. [38] |
| QPVA/Q-chitosan | 60 °C | 2 M MeOH + 6 M KOH | 73 | Liao et al. [17] |
| QPVA/5%FS/KOH | 30 °C | 2 M MeOH + 6 M KOH | 37.5 | This work |
| QPVA/5%FS/KOH | 60 °C | 2 M MeOH + 6 M KOH | 88.4 | This work |
| QPVA/5%FS/KOH | 80 °C | 2 M MeOH + 6 M KOH | 96.8 | This work |
2.10. Direct Alkaline Ethanol Fuel Cell Performance

3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of QPVA
3.3. Preparation of Nanocomposite Membrane and Electrolytes
3.4. Physico-Chemical Properties of Pristine and Nanocomposites Membranes
3.5. Water Vapor Uptake and Diffusion Rate Coefficients
3.6. Methanol Permeability
3.7. Ionic Conductivity Measurements
3.8. Fuel Cell Performance Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An, L.; Zhao, T.S.; Li, Y.S. Carbon-neutral sustainable energy technology: Direct ethanol fuel cells. Renew. Sustain. Energy Rev. 2015, 50, 1462–1468. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer–nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Cho, E.-B.; Kim, H.; Kim, D. Effect of morphology and pore size of sulfonated mesoporous benzene-silicas in the preparation of poly(vinyl alcohol)-based hybrid nanocomposite membranes for direct methanol fuel cell application. J. Phys. Chem. B 2009, 113, 9770–9778. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Tseng, C.K.; Shih, C.-M.; Pai, Y.-L.; Kuo, H.-P.; Lue, S.J. Polytetrafluoroethylene (PTFE)/silane cross-linked sulfonated poly(styrene-ethylene/butylene-styrene) (sSEBS) composite membrane for direct alcohol and formic acid fuel cells. J. Memb. Sci. 2014, 464, 43–54. [Google Scholar] [CrossRef]
- Lo, C.-F.; Wu, J.-F.; Li, H.-Y.; Hung, W.-S.; Shih, C.-M.; Hu, C.-C.; Liu, Y.-L.; Lue, S.J. Novel polyvinyl alcohol nanocomposites containing carbon nano-tubes with Fe3O4 pendants for alkaline fuel cell applications. J. Memb. Sci. 2013, 444, 41–49. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, X.; Wang, L.; Liu, Y. An alkaline direct methanol fuel cell with a polymer fiber membrane and MnO2-catalyzed cathode. Electrochimica Acta 2013, 90, 421–425. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Yu, E.H.; Krewer, U.; Scott, K. Principles and materials aspects of direct alkaline alcohol fuel cells. Energies 2010, 3, 1499–1528. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 2013, 42, 5768–5787. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C. Alkaline direct methanol fuel cell based on a novel anion-exchange composite polymer membrane. J. Appl. Electrochem. 2012, 42, 305–317. [Google Scholar] [CrossRef]
- Lue, S.J.; Pan, W.-H.; Chang, C.-M.; Liu, Y.-L. High-performance direct methanol alkaline fuel cells using potassium hydroxide-impregnated polyvinyl alcohol/carbon nano-tube electrolytes. J. Power Sources 2012, 202, 1–10. [Google Scholar] [CrossRef]
- Wu, J.-F.; Lo, C.-F.; Li, L.-Y.; Li, H.-Y.; Chang, C.-M.; Liao, K.-S.; Hu, C.-C.; Liu, Y.-L.; Lue, S.J. Thermally stable polybenzimidazole/carbon nano-tube composites for alkaline direct methanol fuel cell applications. J. Power Sources 2014, 246, 39–48. [Google Scholar] [CrossRef]
- Gomes, A.D.S.; Dutra Filho, J.C. Hybrid membranes of PVA for direct ethanol fuel cells (DEFCs) applications. Int. J. Hydrog. Energy 2012, 37, 6246–6252. [Google Scholar] [CrossRef]
- Yang, J.-M.; Wang, N.-C.; Chiu, H.-C. Preparation and characterization of poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Memb. Sci. 2014, 457, 139–148. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Lin, R.; Ma, J.; Liu, J. Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-S.; Kuo, S.-C.; Liou, T.-H. Fabrication of anion-exchange composite membranes for alkaline direct methanol fuel cells. J. Power Sources 2012, 199, 37–45. [Google Scholar] [CrossRef]
- Liao, G.-M.; Yang, C.-C.; Hu, C.-C.; Pai, Y.-L.; Lue, S.J. Novel quaternized polyvinyl alcohol/quaternized chitosan nano-composite as an effective hydroxide-conducting electrolyte. J. Memb. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Zhu, A.M.; Xiong, Y.; Ren, L. Pervaporation performance of quaternized poly(vinyl alcohol) and its crosslinked membranes for the dehydration of ethanol. J. Memb. Sci. 2009, 335, 68–75. [Google Scholar] [CrossRef]
- Fang, J.; Shen, P.K. Quaternized poly(phthalazinon ether sulfone ketone) membrane for anion exchange membrane fuel cells. J. Memb. Sci. 2006, 285, 317–322. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.; Zeng, Q.H.; Liu, Q.L. Preparation and characterization of cross-linked quaternized poly(vinyl alcohol) membranes for anion exchange membrane fuel cells. J. Memb. Sci. 2008, 311, 319–325. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternized poly(vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-J.; Chien, W.-C.; Chiu, S.-S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Sources 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Efficient utilization of anion exchange composites using silica filler for low temperature alkaline membrane fuel cells. Int. J. Plast. Technol. 2013, 17, 35–50. [Google Scholar] [CrossRef]
- Lue, S.J.; Mahesh, K.P.O.; Wang, W.-T.; Chen, J.-Y.; Yang, C.-C. Permeant transport properties and cell performance of potassium hydroxide doped poly(vinyl alcohol)/fumed silica nanocomposites. J. Memb. Sci. 2011, 367, 256–264. [Google Scholar] [CrossRef]
- Khoonsap, S.; Supanchaiyamat, N.; Hunt, A.J.; Klinsrisuk, S.; Amnuaypanich, S. Improving water selectivity of poly (vinyl alcohol) (PVA)—Fumed silica (FS) nanocomposite membranes by grafting of poly (2-hydroxyethyl methacrylate) (PHEMA) on fumed silica particles. Chem. Eng. Sci. 2015, 122, 373–383. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Memb. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhu, A.M.; Huang, S.M.; Zeng, Q.H. Performance of organic-inorganic hybrid anion-exchange membranes for alkaline direct methanol fuel cells. J. Power Sources 2009, 186, 328–333. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, S.F.; Wang, L.D.; Chen, W.W.; Du, K.-M.; Wu, S.Y. Diffusion of multicomponent vapors in a poly(dimethyl siloxane) membrane. Desalination 2008, 233, 277–285. [Google Scholar] [CrossRef]
- Jessie Lue, S.; Chen, J.Y.; Ming Yang, J. Crystallinity and Stability of Poly(vinyl alcohol)-Fumed Silica Mixed Matrix Membranes. J. Macromol. Sci. Part B 2007, 47, 39–51. [Google Scholar]
- Wang, E.D.; Zhao, T.S.; Yang, W.W. Poly (vinyl alcohol)/3-(trimethylammonium) propyl-functionalized silica hybrid membranes for alkaline direct ethanol fuel cells. Int. J. Hydrog. Energy 2010, 35, 2183–2189. [Google Scholar] [CrossRef]
- Lue, S.J.; Lee, D.-T.; Chen, J.-Y.; Chiu, C.-H.; Hu, C.-C.; Jean, Y.C.; Lai, J.-Y. Diffusivity enhancement of water vapor in poly(vinyl alcohol)-fumed silica nano-composite membranes: Correlation with polymer crystallinity and free-volume properties. J. Memb. Sci. 2008, 325, 831–839. [Google Scholar] [CrossRef]
- Ye, L.; Zhai, L.; Fang, J.; Liu, J.; Li, C.; Guan, R. Synthesis and characterization of novel cross-linked quaternized poly(vinyl alcohol) membranes based on morpholine for anion exchange membranes. Solid State Ion. 2013, 240, 1–9. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Qiao, J.; Liu, L.; Jiang, G.; Zhang, J.; Liu, Y. High durable poly(vinyl alcohol)/Quaterized hydroxyethylcellulose ethoxylate anion exchange membranes for direct methanol alkaline fuel cells. J. Power Sources 2013, 227, 291–299. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, W.-T.; Mahesh, K.P.O.; Yang, C.-C. Enhanced performance of a direct methanol alkaline fuel cell (DMAFC) using a polyvinyl alcohol/fumed silica/KOH electrolyte. J. Power Sources 2010, 195, 7991–7999. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Moo Lee, Y. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Memb. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Zugic, L.D.; Perovic, M.I.; Nikolic, M.V.; Maslovara, L.S.; Marceta Kaninski, P.M. Enhanced Performance of the Solid Alkaline Fuel Cell Using PVA-KOH Membrane. Int. J. Electrochem. Sci. 2013, 8, 949–957. [Google Scholar]
- Fu, J.; Qiao, J.; Lv, H.; Ma, J.; Yuan, X.-Z.; Wang, H. Alkali doped poly(vinyl alcohol) (PVA) for anion-exchange membrane fuel cells-Ionic conductivity, chemical stability and FT-IR characterizations. ECS Trans. 2010, 25, 15–23. [Google Scholar]
- Fu, J.; Qiao, J.; Wang, X.; Ma, J.; Okada, T. Alkali doped poly(vinyl alcohol) for potential fuel cell applications. Synth. Met. 2010, 160, 193–199. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Cheng, M.-Y.; Xie, X.-L.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Sources 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Pan, W.-H.; Lue, S.J.; Chang, C.-M.; Liu, Y.-L. Alkali doped polyvinyl alcohol/multi-walled carbon nano-tube electrolyte for direct methanol alkaline fuel cell. J. Memb. Sci. 2011, 376, 225–232. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Li, Y.S. Synthesis and characterization of crosslinked poly (vinylalcohol)/layered double hydroxide composite polymer membranes for alkaline direct ethanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 18425–18432. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajesh Kumar, S.; Juan, C.-H.; Liao, G.-M.; Lin, J.-S.; Yang, C.-C.; Ma, W.-T.; You, J.-H.; Jessie Lue, S. Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells. Energies 2016, 9, 15. https://doi.org/10.3390/en9010015
Rajesh Kumar S, Juan C-H, Liao G-M, Lin J-S, Yang C-C, Ma W-T, You J-H, Jessie Lue S. Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells. Energies. 2016; 9(1):15. https://doi.org/10.3390/en9010015
Chicago/Turabian StyleRajesh Kumar, Selvaraj, Cheng-Hsin Juan, Guan-Ming Liao, Jia-Shiun Lin, Chun-Chen Yang, Wei-Ting Ma, Jiann-Hua You, and Shingjiang Jessie Lue. 2016. "Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells" Energies 9, no. 1: 15. https://doi.org/10.3390/en9010015