A Viable Electrode Material for Use in Microbial Fuel Cells for Tropical Regions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Start-up and Inoculum Selection

| Electrode/Inoculum | ROhm (Total) Ω | COD (Anode) g/L | Conductivity (Anode) mS/cm | Conductivity (Cathode) mS/cm | Electrode Distance (Total) mm |
|---|---|---|---|---|---|
| CP-FS | 34 | 13.1 ± 1.6 | 17.2 ± 2.4 | 32.0 ± 0.9 | 60 |
| AC-FS | 8 | 0 (60 mm granules) | |||
| CP-GW | 158 | 3.6 ± 0.2 | 1.4 ± 0.2 | 60 | |
| AC-GW | 34 | 0 (60 mm granules) |

2.2. Electricity Generation from Palm Kernel Shell AC
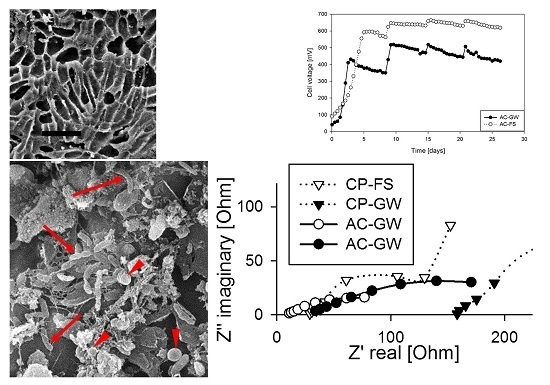
2.3. Electrochemical Interpretation of Resistance from the EIS Data

2.4. Microbial Analysis of CP and AC Biofilms
| Band | Accession Number. | Gene Bank Match | Identity (%) | Characteristics [24,25] |
|---|---|---|---|---|
| 1 | LN651003 | Thermanaerovibrio acidaminovorans | 87 | Thermophilic anaerobe fermenting amino acids. |
| 2 | LN651037 | Shigella flexneri | 99 | Facultative anaerobe. |
| 3 | LN651020 | Enterobacter cancerogenus | 99 | Glucose fermenting anaerobe. |
| 4 | LN651027 | Geobacter sulfurreducens | 99 | Metal-reducing anaerobe oxidizing short-chain fatty acids able to generate electricity. |
| 5 | LN650991 | Desulfuromonas acetexigens | 98 | Obligate anaerobic and sulphur-reducing eubacterium oxidizing acetate as carbon resource. |
| Electrode Material | Palm Kernel Shell AC | CP | ||
|---|---|---|---|---|
| Inoculum | FS | GW | FS | GW |
| DGGE Bands (S) | 10 | 7 | 16 | 14 |
| Shannon diversity index (H) | 2.24 | 1.91 | 2.70 | 2.56 |
| Margalef species richness (d) | 1.44 | 0.94 | 2.15 | 1.95 |
| Pielou species evenness | 0.972 | 0.980 | 0.972 | 0.969 |

2.5. SEM Analysis of Electrode Biofilm


2.6. Effect of Granular Volume on Power Generation
| Parameter | 40 vol. % Granules | 25 vol. % Granules | 15 vol. % Granules | |
|---|---|---|---|---|
| Mass of granules (g) | 57 | 35 | 21 | |
| Projected surface area (m2) | 0.37 | 0.23 | 0.14 | |
| Granular bed height (mm) | 36 | 27 | 17 | |

2.7. Performance of AC Relative to CP
| Electrode Material | Inoculum Used | Length of Start-up (Days) | Maximum Voltage Under Load (mV) | Power Density (mW/m3) |
|---|---|---|---|---|
| CP | FS | 6 ± 1 | 714 ± 10 | 2040 ± 55 |
| GW | 3 ± 0.5 | 540 ± 26 | 1169 ± 110 | |
| AC | FS | 6 ± 2 | 657 ± 9 | 1727 ± 47 |
| GW | 4 ± 1 | 516 ± 7 | 1066 ± 32 |
3. Experimental Section
3.1. MFC Configuration
3.2. Inoculation
3.3. Electrode Preparation
3.4. MFC Operation
3.5. Microbial Analysis
3.6. SEM Microscopy
3.7. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thygesen, A.; Thomsen, A.B.; Possemiers, S.; Verstraete, W. Integration of microbial electrolysis cells (MECs) in the biorefinery for production of ethanol, H2 and phenolics. Waste Biomass Valor. 2010, 1, 9–20. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.A.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Angelidaki, I.; Min, B.; Poulsen, F.W.; Bjerre, A.B. Electricity generation by microbial fuel cells fuelled with wheat straw hydrolysate. Biomass Bioenerg. 2011, 35, 4732–4739. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Kim, H.W.; Lim, K.H.; Shin, H.S. Effects of organic loading rates on the continuous electricity generation from fermented wastewater using a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Chang, I.S.; Kim, B.H. Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresour. Technol. 2006, 97, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Curtis, M.; Troop, E.; Scheible, K.; McGrath, J.; Hu, B.; Suib, S.; Raymond, D.; Li, B. A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. Int. J. Hydrogen Energy 2011, 36, 876–884. [Google Scholar] [CrossRef]
- Jiang, D.; Li, B. Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): A design suitable for large-scale wastewater treatment processes. Biochem. Eng. J. 2009, 47, 31–37. [Google Scholar] [CrossRef]
- Santoro, C.; Artyushkova, K.; Babanova, S.; Atanassov, P.; Ieropoulos, I.; Grattieri, M.; Cristiani, P.; Trasatti, S.; Li, B.; Schuler, A.J.; et al. Parameters characterization and optimization of activated carbon (AC) cathodes for microbial fuel cell application. Bioresour. Technol. 2014, 163, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Van Bogaert, G.; De Smet, M.; Diels, L.; Vanbroekhoven, K. Use of novel permeable membrane and air cathodes in acetate microbial fuel cells. Electrochim. Acta 2010, 55, 7710–7716. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [PubMed]
- Dong, H.; Yu, H.; Wang, X.; Zhou, Q.; Feng, J. A novel structure of scalable air-cathode without nafion and pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 2012, 46, 5777–5787. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, G.; Hu, X.; Wang, S.; Zeng, D.; Hou, H.; Schroder, U. A three dimensional ordered macroporous carbon derived from a natural plant resource as anode for microbial bioelectrochemical systems. ChemSusChem 2012, 5, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, G.; Liu, Q.; Harnisch, F.; Zhou, Y.; Chen, Y.; Hanif, M.; Wang, S.; Peng, X.; Hou, H.; et al. Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ. Sci. 2012, 5, 9769–9772. [Google Scholar] [CrossRef]
- Palm Biomass Burning Oil Palm Products. Available online: http://oilpalmproducts.com (accessed on 4 January 2016).
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Yacob, A.B.; Wahab, N.; Suhaimi, N.H.; Amat, M.K.A. Microwave induced carbon from waste palm kernel shell activated by phosphoric acid. Int. J. Eng. Technol. 2013, 5, 214–217. [Google Scholar] [CrossRef]
- Herawan, S.G.; Hadi, M.S.; Ayob, R.; Putra, A. Characterization of activated carbons from oil-palm shell by CO2 activation with no holding carbonization temperature. Sci. World J. 2013, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.D.; Head, I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Rodrigues, D.S.; Thygesen, A.; Daniel, G.; Fernando, D.; Meyer, A.S. Inocula selection in microbial fuel cells based on anodic biofilm abundance of Geobacter sulfurreducens. Chin. J. Chem. Eng. 2016, in press. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Thygesen, A.; Meyer, A.S. Acetate is a superior substrate for microbial fuel cell initiation preceding bioethanol effluent utilization. Appl. Microbiol. Biotechnol. 2015, 99, 4905–4915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer-Verlag London Ltd.: London, UK, 2010. [Google Scholar]
- Marzorati, M.; Wittebolle, L.; Boon, N.; Daffonchio, D.; Verstraete, W. How to get more out of molecular fingerprints: Practical tools for microbial ecology. Environ. Microbiol. 2008, 10, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Marzorati, M.; Boon, N.; Thomsen, A.B.; Verstraete, W. Upgrading of straw hydrolysate for production of hydrogen and phenols in a microbial electrolysis cell (MEC). Appl. Microbiol. Biotechnol. 2011, 89, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Thygesen, A.; Ale, M.T.; Mensah, M.; Poulsen, F.W.; Meyer, A.S. The significance of the initiation process parameters and reactor design for maximizing the efficiency of microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Offei, F.; Thygesen, A.; Mensah, M.; Tabbicca, K.; Fernando, D.; Petrushina, I.; Daniel, G. A Viable Electrode Material for Use in Microbial Fuel Cells for Tropical Regions. Energies 2016, 9, 35. https://doi.org/10.3390/en9010035
Offei F, Thygesen A, Mensah M, Tabbicca K, Fernando D, Petrushina I, Daniel G. A Viable Electrode Material for Use in Microbial Fuel Cells for Tropical Regions. Energies. 2016; 9(1):35. https://doi.org/10.3390/en9010035
Chicago/Turabian StyleOffei, Felix, Anders Thygesen, Moses Mensah, Kwame Tabbicca, Dinesh Fernando, Irina Petrushina, and Geoffrey Daniel. 2016. "A Viable Electrode Material for Use in Microbial Fuel Cells for Tropical Regions" Energies 9, no. 1: 35. https://doi.org/10.3390/en9010035