Surface Modification of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Nano-Al2O3 to Improve Electrochemical Performance in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
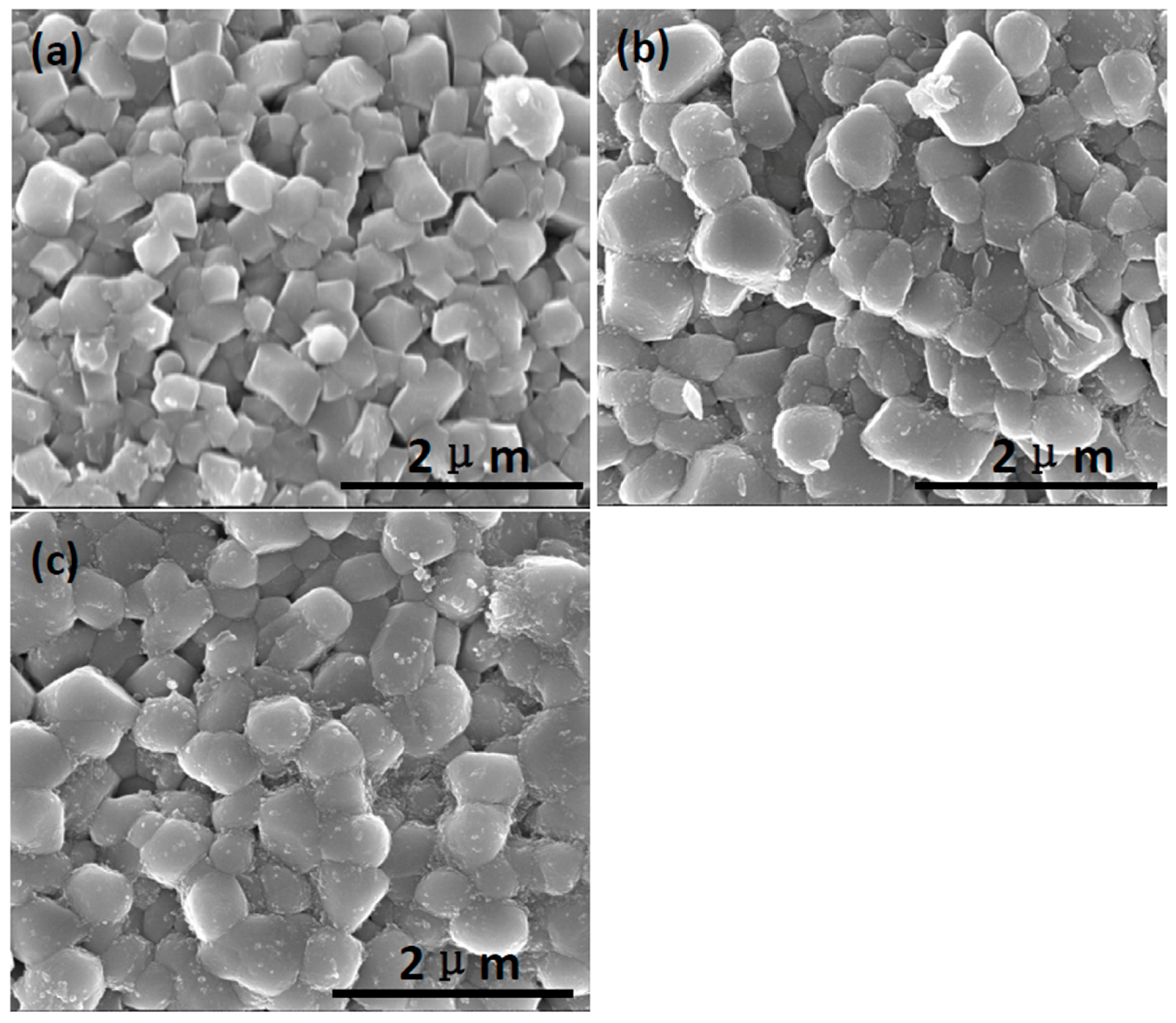
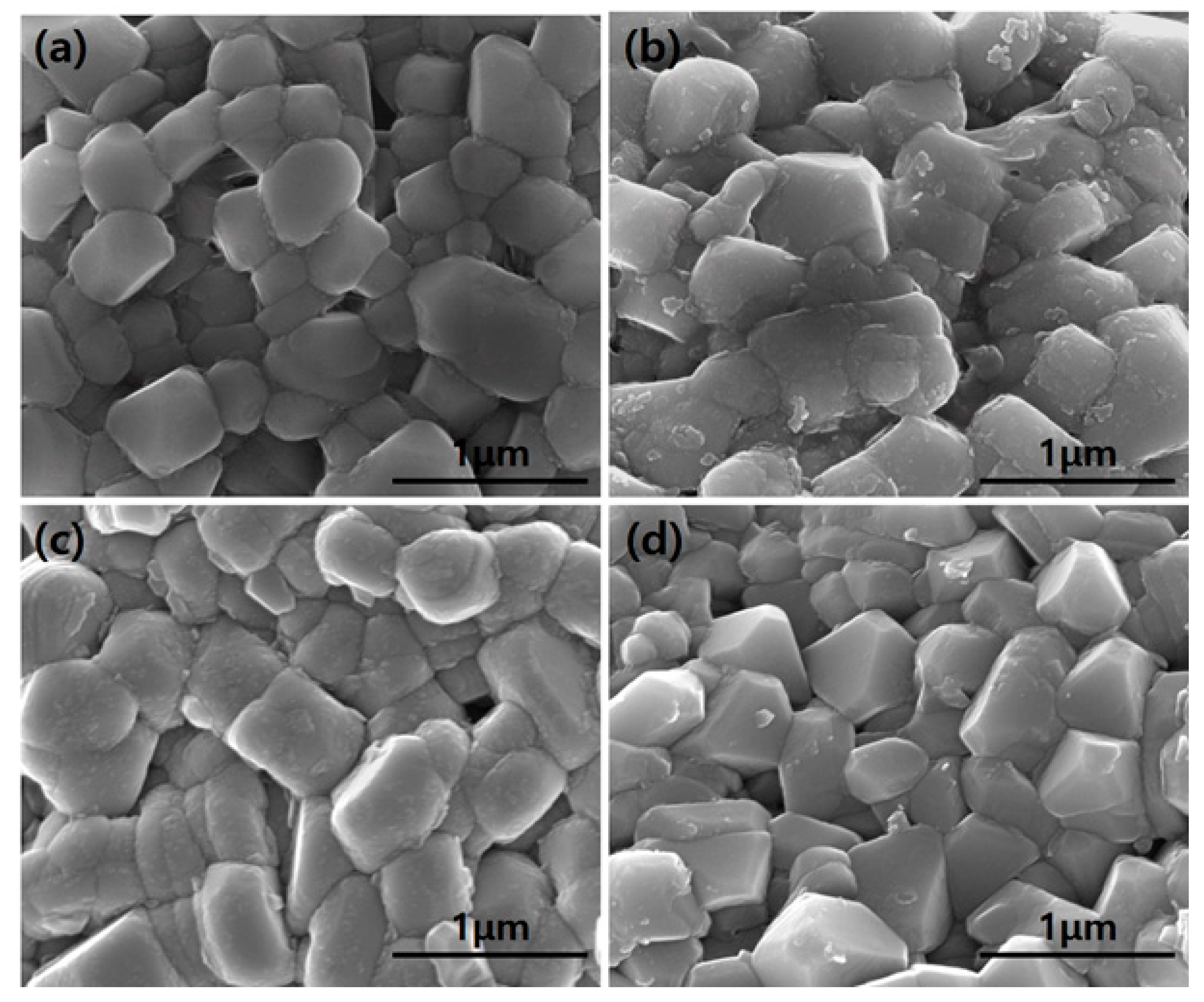
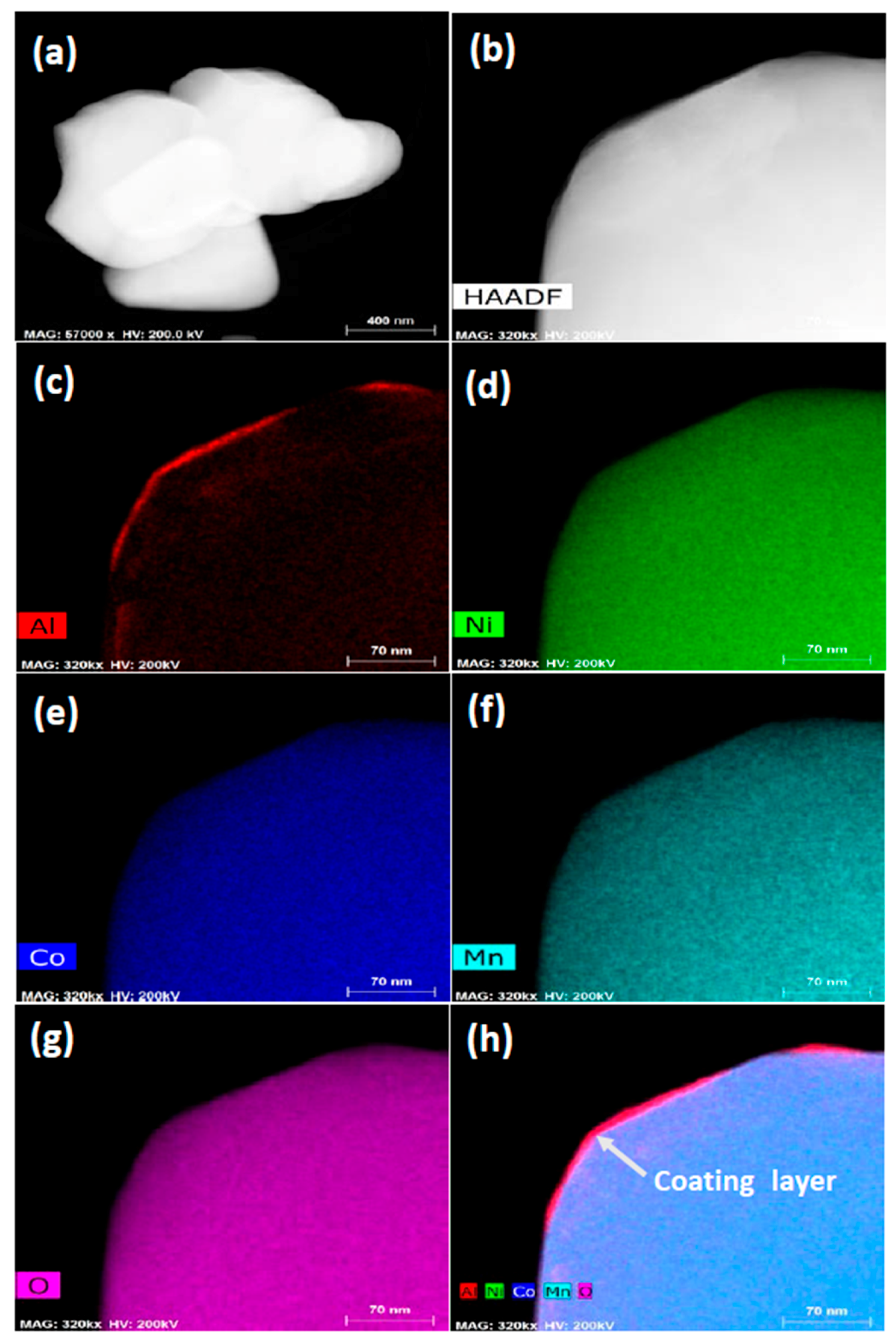
3.1. Structural Properties
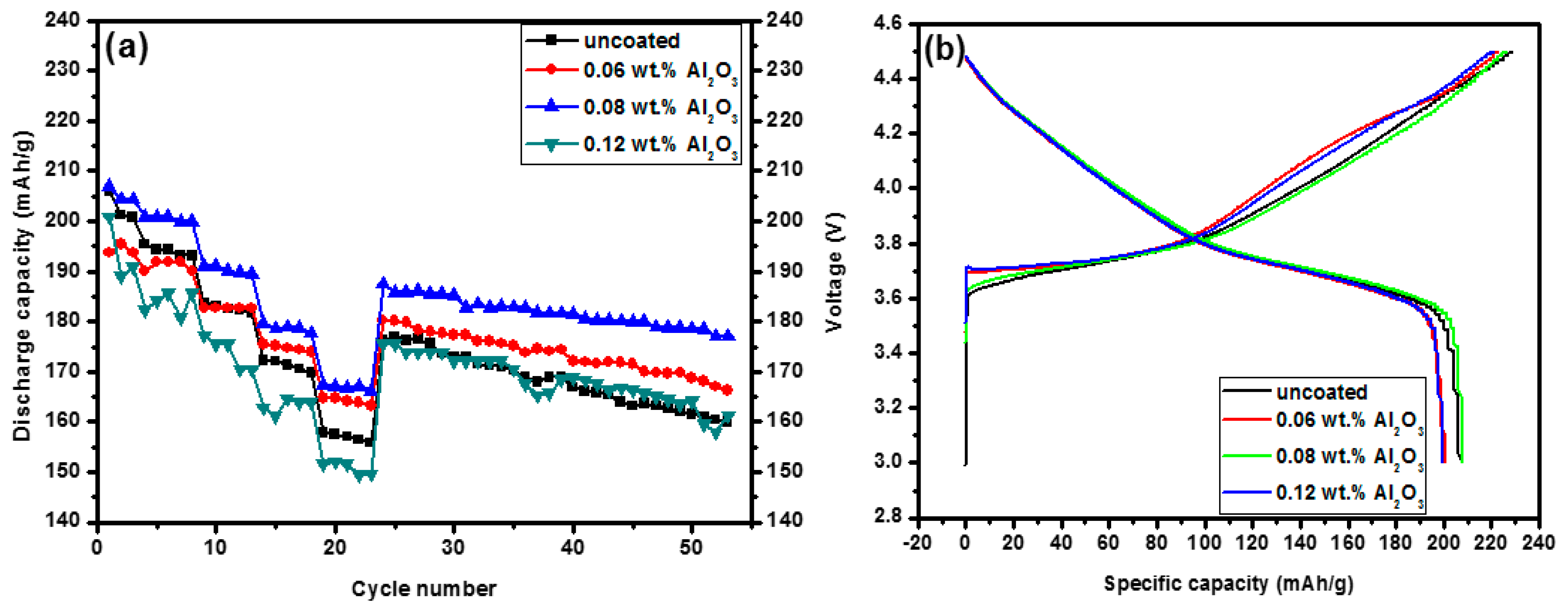
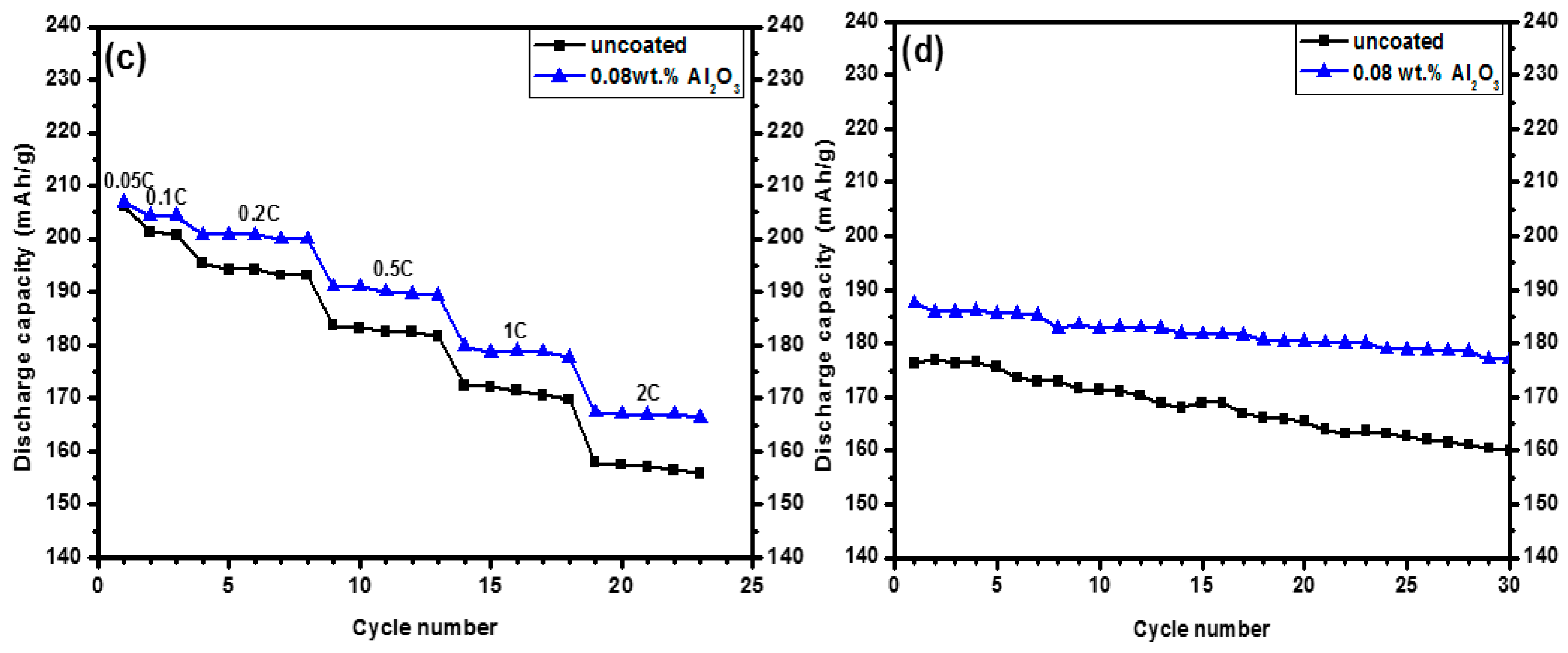
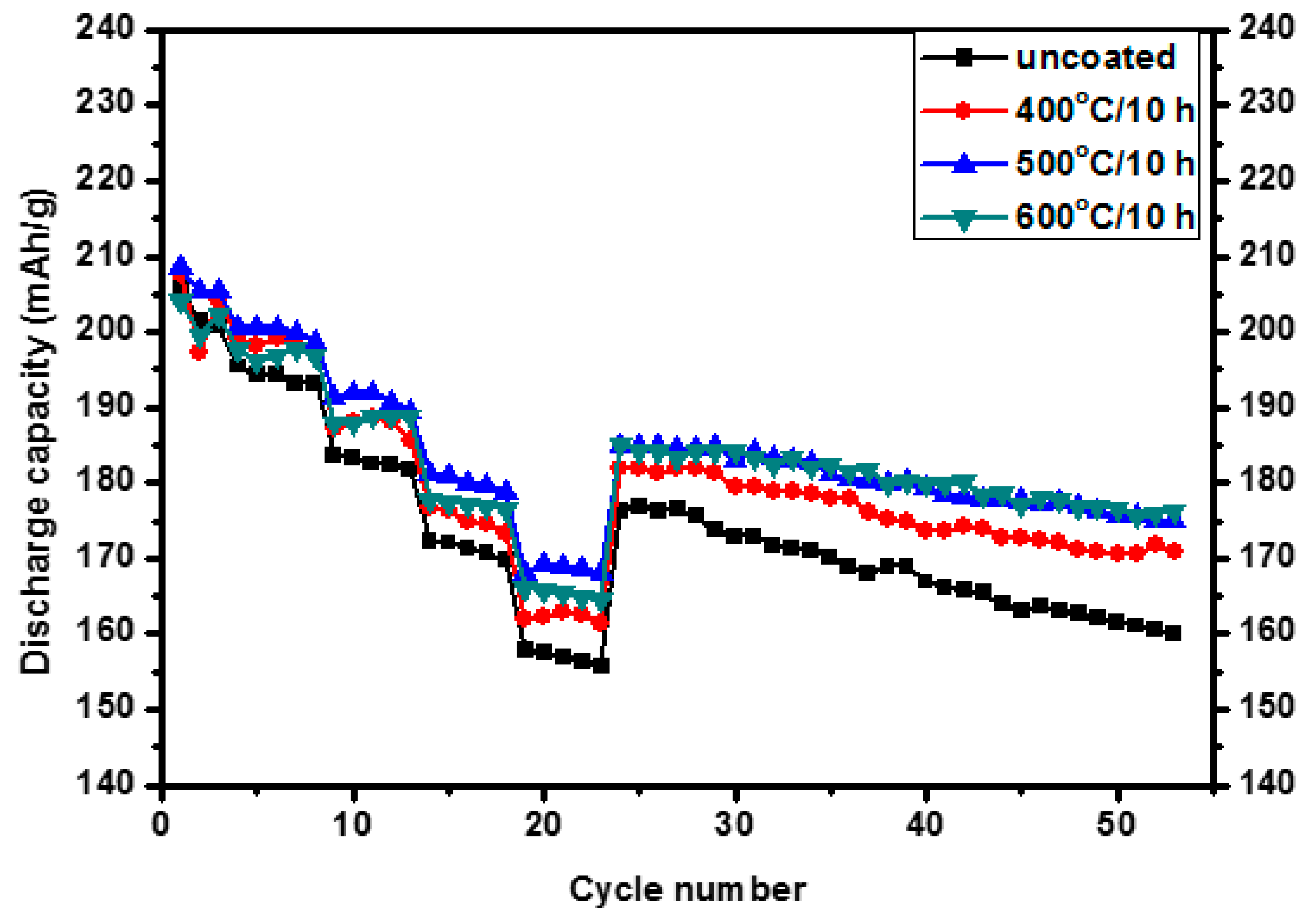
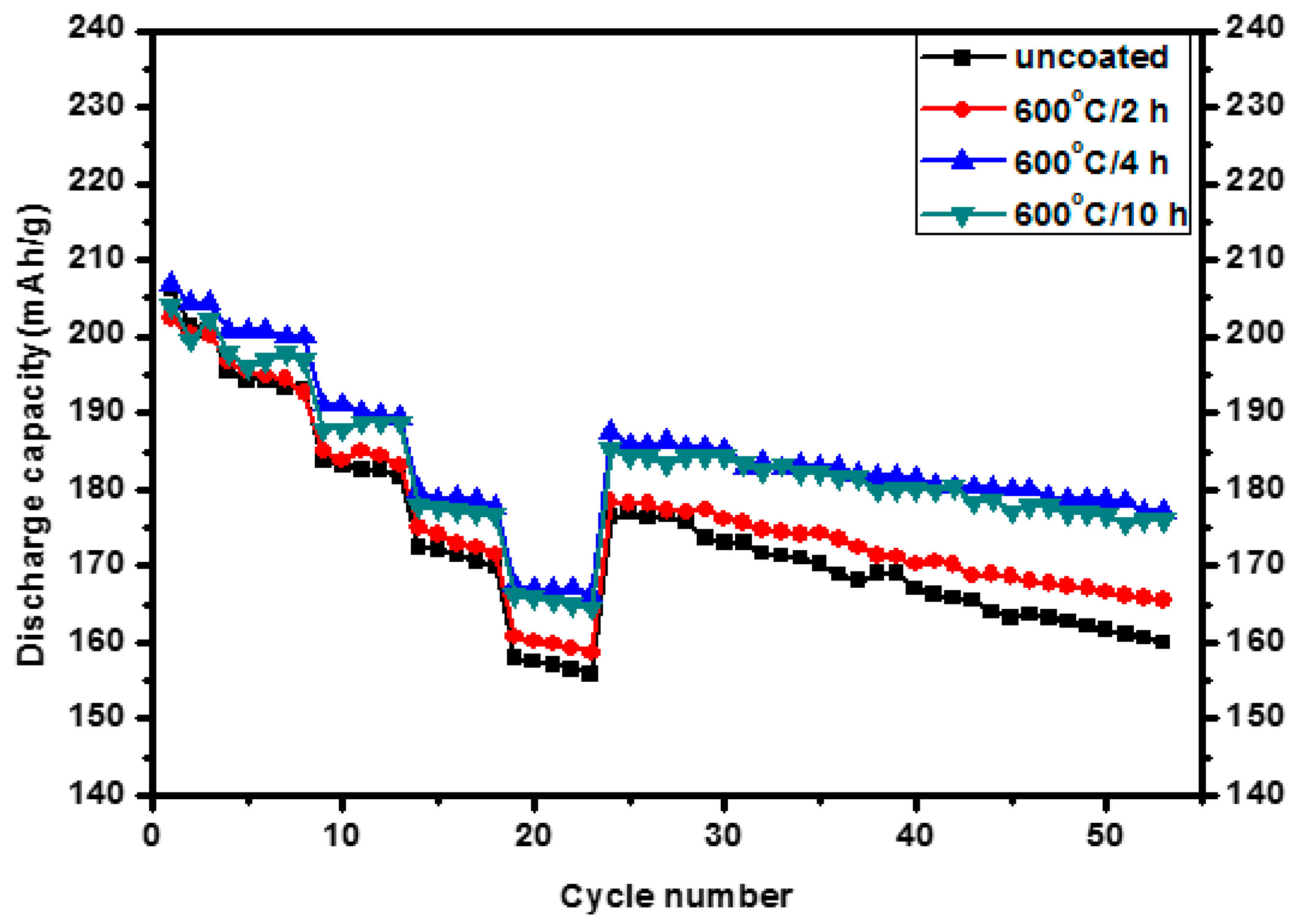
3.2. Electrochemical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J. Recent Progress in Advanced Materials for Lithium Ion Batteries. Materials 2013, 6, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Lu, Y.; Wang, Q.; Xu, J.; Wang, W.; Dai, X.; Li, J. Sputtering TiO2 on LiCoO2 Composite Electrodes as a Simple and Effective Coating to Enhance High-voltage Cathode Performance. J. Power Sources 2017, 346, 24–30. [Google Scholar] [CrossRef]
- Yoo, K.S.; Cho, N.W.; Oh, Y.-J. Structural and Electrical Characterization of Li(Mn1-δTiδ)2O4 Electrode Materials. Solid State Ion. 1998, 113, 43–49. [Google Scholar] [CrossRef]
- Bakierska, M.; Świętosławski, M.; Gajewaka, M.; Kowalczyk, A.; Piwowarska, Z.; Chmielarz, L.; Dziembaj, R.; Molenda, M. Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S. Materials 2016, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Rozier, P.; Tarascon, J.M. Review—Li-rich Layered Oxide Cathodes for Next-generation Li-ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.; Wang, Y.; Zhao, X.; Mi, Y.; Xia, B. Enhanced High-temperature Cycling Stability of Li(Ni1/3Co1/3Mn1/3)O2-coated LiMn2O4 as Cathode Material for Lithium Ion Batteries. Ionics 2015, 21, 1835–1842. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Li, X.; Xiong, X.; Wang, J.; Wu, X.; Guo, H. The Enhanced Electrochemical Performance of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Low Temperature Fluorine Substitution. Electrohcim. Acta 2013, 95, 112–118. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.-C.; Nazar, L.F. Approaching Theoretical Capacity of LiFePO4 at Room Temperature at High Rates. Electrochem. Solid-State Lett. 2001, 4, A170–A172. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Y.; Zhang, J.; Xia, B. Synthesis and Electrochemical Characteristics of Layered Li(Ni0.6Co0.2Mn0.2)O2 Cathode Material for Lithium Ion Batteries. Solid State Ion. 2005, 176, 1207–1211. [Google Scholar] [CrossRef]
- Liu, W.; Hua, W.; Zheng, Z.; Zhong, B.; Zhang, Z. Facile Synthesis of Hierarchical Porous Ni-rich Cathode Material with Superior High-rate Capability. Ionics 2016. [Google Scholar] [CrossRef]
- Cheng, K.-L.; Mu, D.-B.; Wu, B.-R.; Wang, L.; Jiang, Y.; Wang, R. Electrochemical Performance of a Nickel-rich Li(Ni0.6Co0.2Mn0.2)O2 Cathode Material for Lithium-ion Batteries under Different Cut-off Voltages. Int. J. Miner. Metall. Mater. 2017, 24, 342–351. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, Z.; Liu, Y.; Zhang, Q.; Zheng, Y.; Li, G. Synthesis and Electrochemical Properties of Mg-doped Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials for Li-ion Battery. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 211–215. [Google Scholar] [CrossRef]
- Erickson, E.M.; Schipper, F.; Penki, T.R.; Shin, J.-Y.; Erk, C.; Chesneau, F.-F.; Markovsky, B.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes II. Lithium-rich xLi2MnO3·(1-x)Li(NiaCobMnc)O2. J. Electochem. Soc. 2017, 164, A6341–A6348. [Google Scholar] [CrossRef]
- Wu, F.; Tian, J.; Su, Y.; Wang, J.; Zhang, C.; Bao, L.; He, T.; Li, J.; Chen, S. Effect of Ni2+ Content on Lithium/nickel disorder for Ni-rich Cathode Materials. ACS Appl. Mater. Interfaces 2015, 7, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, S.; Huang, J.; Yan, C. Acacia Gum-assisted Co-precipitating Synthesis of Li(Ni0.5Co0.2Mn0.3)O2 Cathode Material for Lithium Ion Batteries. Ionics 2015. [Google Scholar] [CrossRef]
- Luo, W.B.; Li, X.H.; Dahn, J.R. Synthesis, Characterization and Thermal Stability of Li[Ni1/3Mn1/3Co1/3-z (MnMg)z/2]O2. Chem. Mater. 2010, 22, 5065–5073. [Google Scholar] [CrossRef]
- Santhanam, R.; Rambabu, B. High Rate Cycling Performance of Li1.05Ni1/3Co1/3Mn1/3O2 Materials Prepared by Sol-gel and Co-precipitation methods for Lithium-ion Batteries. J. Power Sources 2010, 195, 4313–4317. [Google Scholar] [CrossRef]
- Yano, A.; Shikano, M.; Ueda, A.; Sakaebe, H.; Ogumi, Z. LiCoO2 Degradation Behavior in the High-voltage Phase Transition Region and Improved Reversibility with Surface Coating. J. Electrochem. Soc. 2017, 164, A6116–A6122. [Google Scholar] [CrossRef]
- Shim, J.-H.; Cho, N.-H.; Lee, S. Synthesis and Characterization of Mg2TiO4-coated LiCoO2 as a Cathode Material for Lithium Ion Batteries. Electrochim. Acta 2017, 243, 162–169. [Google Scholar] [CrossRef]
- Shen, B.; Zuo, P.; Fan, P.; Yang, J.; Yin, G.; Ma, Y.; Cheng, X.; Du, C.; Gao, Y. Improved Electrochemical Performance of NaAlO2-coated LiCoO2 for Lithium-ion Batteries. J. Solid State Electrochem. 2017, 21, 1195–1201. [Google Scholar] [CrossRef]
- Wu, H.M.; Belharouak, I.; Abouimrane, A.; Sun, Y.-K.; Amine, K. Surface Modification of LiNi0.5Mn1.5O4 by ZrP2O7 and ZrO2 for Lithium-ion Batteries. J. Power Sources 2010, 195, 2909–2913. [Google Scholar] [CrossRef]
- Shi, J.Y.; Yi, C.-W.; Kim, K. Improved electrochemical performance of AlPO4-coated LiMn1.5Ni0.5O4 electrode for lithium-ion batteries. J. Power Sources 2010, 195, 6860–6866. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An Approach to Application for Li(Ni0.6Co0.2Mn0.2)O2 Cathode Material as High Cutoff Voltage by TiO2 Coating. J. Power Sources 2014, 256, 20–27. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, F.; Wang, Z.; Zhang, Q. Improve the Structure and Electrochemical Performance of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Material by Nano-Al2O3 Ultrasonic Coating. J. Alloys Compd. 2014, 611, 135–141. [Google Scholar] [CrossRef]
- Sun, S.; Du, C.; Qu, D.; Zhang, X.; Tang, Z. Li2ZrO3-coatedLi(Ni0.6Co0.2Mn0.2)O2 for High-performance Cathode Material in Lithium-ion Battery. Ionics 2015, 21, 2091–2100. [Google Scholar] [CrossRef]
- Xu, X.; Lee, S.; Jeong, S.; Kim, Y.; Cho, J. Recent Progress on Nanostructured 4 V Cathode Materials for Li-ion Batteries for Mobile Electronics. Mater. Today 2013, 16, 487–495. [Google Scholar] [CrossRef] [Green Version]
- JCPDS-ICDD #87-1564, version 2.1; The International Centre for Diffraction Data: Newtown Square, PA, USA, 2000.
- Manikandan, P.; Periasamy, P.; Jagannathan, R. Microstructure—Twinning and Hexad Multiplet(s) in Lithium-rich Layered Cathode Materials for Lithium-ion Batteries. RSC Adv. 2014, 4, 40359–40367. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, J.; Zheng, Z.; Liu, W.; Peng, X.; Guo, X.-D.; Zhong, B.; Wang, Y.-J.; Wang, X. Na-doped Ni-rich LiNi0.5Co0.2Mn0.3O2 Cathode Material with Both High Rate Capability and High Tap Density for Lithium Ion Batteries. Dalton Trans. 2014, 43, 14824–14832. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G.; Park, Y.J. LiLaPO4-coated Li(Ni0.5Co0.2Mn0.3)O2 and AlF3-coated Li(Ni0.5Co0.2Mn0.3)O2 Blend Composites for Lithium Ion Batteries. Mater. Res. Bull. 2012, 47, 2843–2846. [Google Scholar] [CrossRef]
- Shi, S.J.; Tu, J.P.; Mai, Y.J.; Zhang, Y.Q.; Tang, Y.Y.; Wang, X.L. Structure and Electrochemical Performance of CaF2 coated Li Mn1/3Ni1/3Co1/3O2 Cathode Material for Li-ion Batteries. Electrochim. Acta 2012, 83, 105–112. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, K.S.; Kang, Y.H.; Im, K.R.; Kim, C.-S. Surface Modification of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Nano-Al2O3 to Improve Electrochemical Performance in Lithium-Ion Batteries. Materials 2017, 10, 1273. https://doi.org/10.3390/ma10111273
Yoo KS, Kang YH, Im KR, Kim C-S. Surface Modification of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Nano-Al2O3 to Improve Electrochemical Performance in Lithium-Ion Batteries. Materials. 2017; 10(11):1273. https://doi.org/10.3390/ma10111273
Chicago/Turabian StyleYoo, Kwang Soo, Yeon Hui Kang, Kyoung Ran Im, and Chang-Sam Kim. 2017. "Surface Modification of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Nano-Al2O3 to Improve Electrochemical Performance in Lithium-Ion Batteries" Materials 10, no. 11: 1273. https://doi.org/10.3390/ma10111273



