Li-Decorated β12-Borophene as Potential Candidates for Hydrogen Storage: A First-Principle Study
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. H2 Adsorption on β12-Borophene
3.2. H2 Adsorption on Li-β12-Borophene
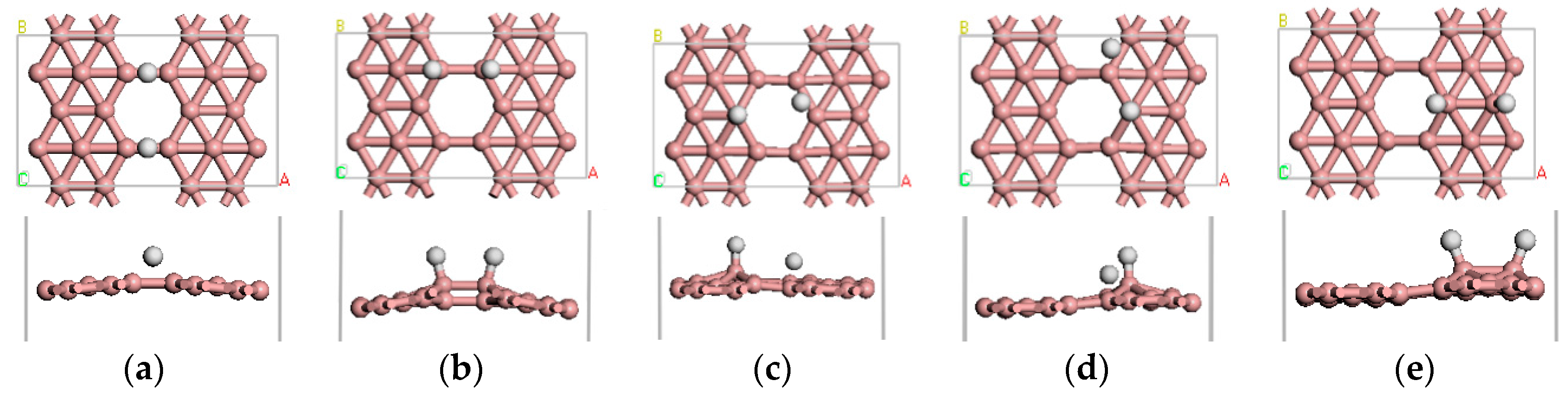
3.2.1. The Adsorption Structure of Li-β12-Borophene
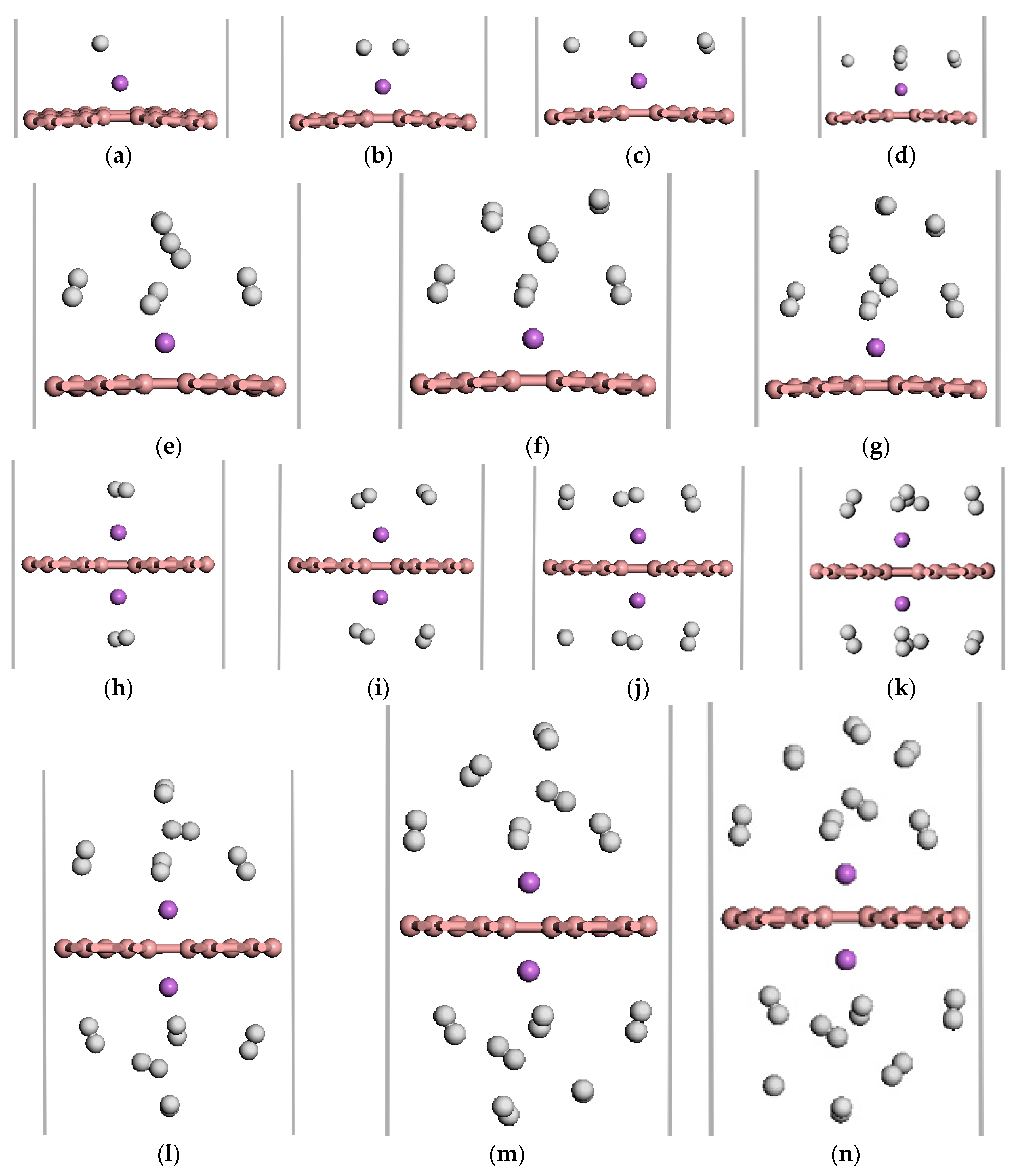
3.2.2. Adsorption of H2 Molecules on Li-β12-Borophene
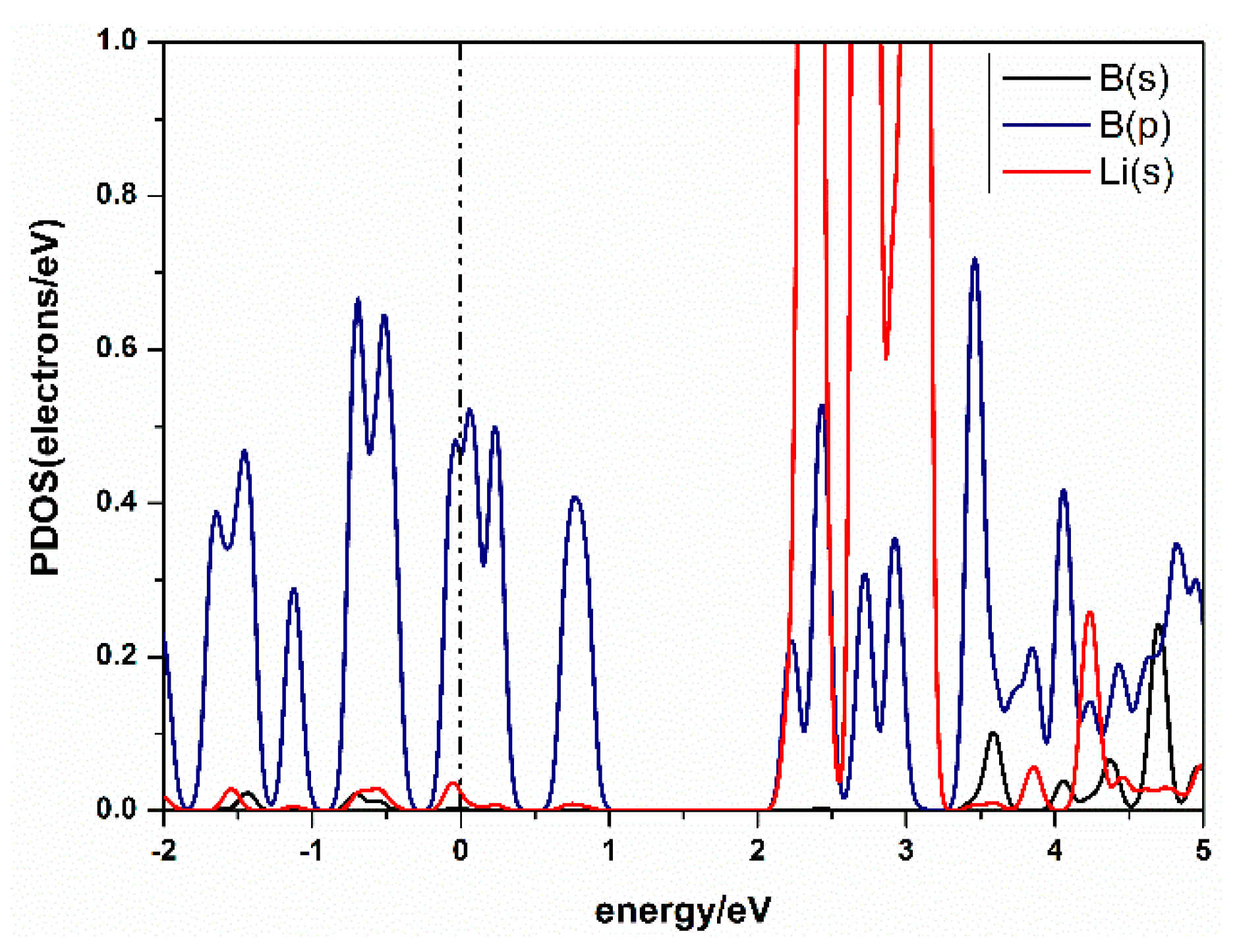
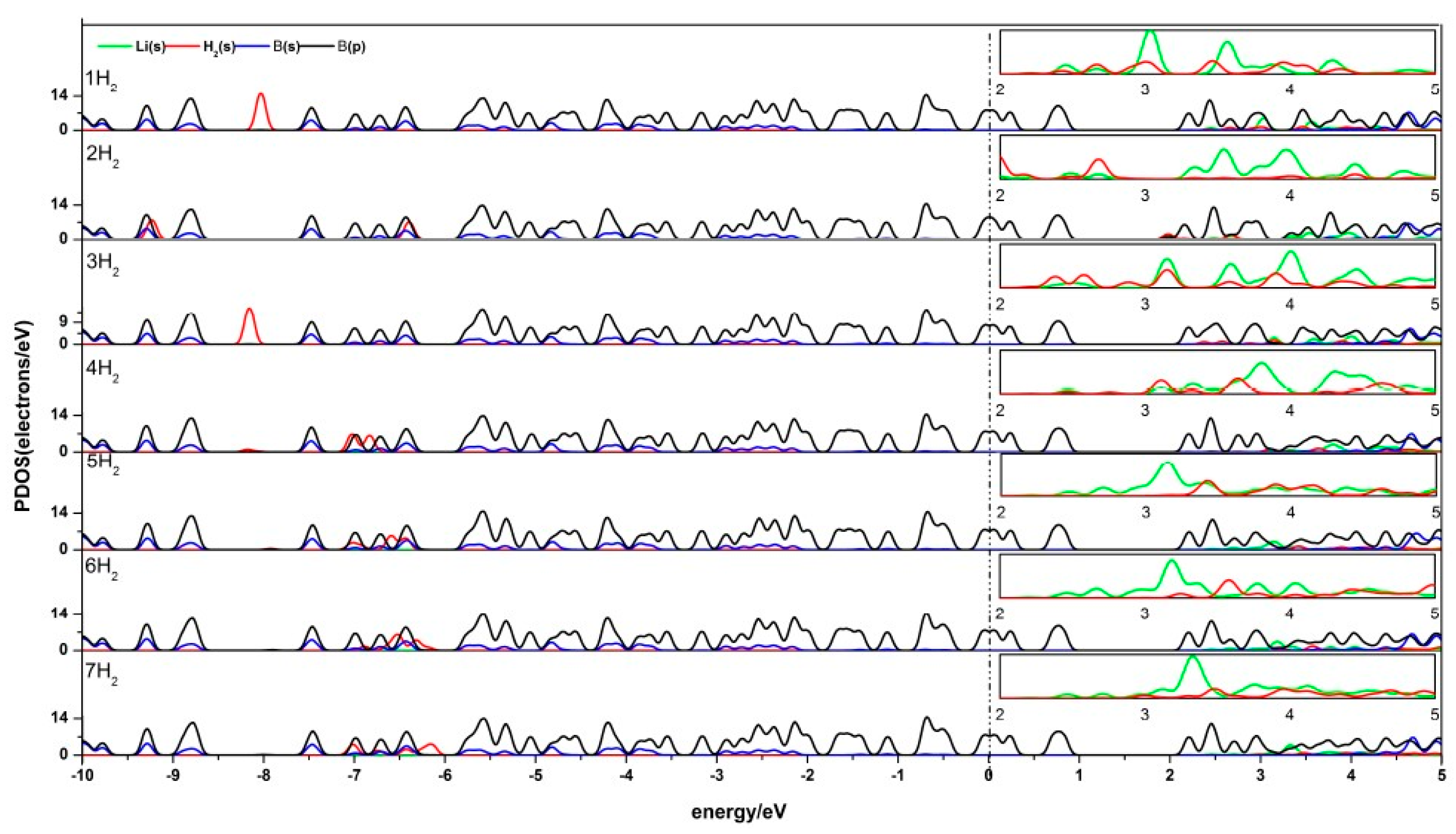
3.2.3. Electronic Properties of Li-β12-Borophene/H2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, Y.; Guo, Z.X.; Yang, R. Influence of selected alloying elements on the stability of magnesium dihydride storage applications: A first-principles investigation. Phys. Rev. B 2004, 69, 094205. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Goddard, W.A. Lighium-doped metal-organic frameworks for reversible H2 storage at ambient temperature. J. Am. Chem. Soc. 2007, 129, 8422–8423. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Hydrogen, Fuel Cells Program: FY Annual Progress Report; U.S. Department of Energy: Washington, DC, USA, 2014.
- Seenithurai, S.; Pandyan, R.K.; Kumar, S.V.; Saranya, C.; Mahendran, M. Al-decorated carbon nanotube as the molecular hydrogen storage medium. Int. J. Hydrog. Energy 2014, 39, 11990–11998. [Google Scholar] [CrossRef]
- Wu, M.H.; Gao, Y.; Zhang, Z.Y.; Zeng, X.C. Edge-decorated graphene nanoribbons by scandium as hydrogen storage media. Nanoscale 2012, 4, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, Two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Yang, Y.; Gao, G.Y.; Yakobson, B.I. Two-Dimensional Boron Monolayers Mediated by metal Substrates. Angew. Chem. Int. Ed. Engl. 2015, 127, 13022–13026. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.J.; Zhang, J.; Zhong, Q.; Li, W.B.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K.H. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.J.; Zhang, J.; Liu, R.Y.; Iimori, T.; Lian, C.; Li, H.; Chen, L.; Wu, K.H.; Meng, S.; Komori, F.; et al. Direct evidence of metallic bands in a monolayer boron sheet. Phys. Rev. B 2016, 94, 041408. [Google Scholar] [CrossRef]
- Padilha, J.E.; Miwa, R.H.; Fazzio, A. Directional dependence of the electronic and transport properties of 2D borophene and borophane. Phys. Chem. Chem. Phys. 2016, 18, 25491–25496. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.; Rahaman, O.; Dianat, A.; Rabczuk, T. Mechanical responses of borophene sheets: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 27405. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Li, Q.F.; Gao, Y.; Miao, F.; Zhou, X.F.; Wan, X.G. Strain effects on borophene: Ideal strength, negative Possion’s ration and phonon instability. New J. Phys. 2016, 18, 073016. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.J.; Tang, Z.; Wang, X.F.; Wang, L.; Hou, T.G.; Lin, H.P.; Li, Y.Y. Stable and metallic borophene nanoribbons from first-principles calculations. J. Mater. Chem. C 2016, 4, 6380–6385. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Wang, L.; Zhang, W.T.; Zhang, J.L.; Yuan, Y.Q. Ca-decorated borophene as potential candidates for hydrogen storage: A first-principle study. Int. J. Hydrog. Energy 2017, 42, 20036–20045. [Google Scholar] [CrossRef]
- Wang, Y.H.; Meng, Z.S.; Liu, Y.Z.; You, D.; Wu, K.; Lv, J.; Wang, X.Z.; Deng, K.M.; Rao, D.; Lu, R.F. Lithium decoration of three dimensional boron-doped graphene frameworks for high-capacity storage. Appl. Phys. Lett. 2015, 106, 2721. [Google Scholar] [CrossRef]
- Reunchan, P.; Jhi, S.H. Metal-dispersed porous graphene for hydrogen storage. Appl. Phys. Lett. 2011, 98, 93103. [Google Scholar] [CrossRef]
- Ao, Z.M.; Jiang, Q.; Zhang, R.Q.; Tan, T.T.; Li, S. Al doped graphene: A promising material for hydrogen storage at room temperature. J. Appl. Phys. 2009, 105, 074307. [Google Scholar] [CrossRef]
- Faye, O.; Eduok, U.; Szpunar, J.; Szpunar, B.; Samoura, A.; Beye, A. Hydrogen Storage on bare Cu atom and Cu-functionalized boron-doped graphene: A first principles study. Int. J. Hydrog. Energy 2017, 42, 4233–4243. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.A.; Szpunar, B.; Beye, A.C. Hydrogen adsorption and storage on Palladium-functionalized graphene with NH-dopant: A first principles calculation. Appl. Surf. Sci. 2017, 392, 362–374. [Google Scholar] [CrossRef]
- Yuan, L.H.; Chen, Y.H.; Kang, L.; Zhang, C.R.; Wang, D.B.; Wang, C.N.; Zhang, M.L.; Wu, X.J. First-principles investigation of hydrogen storage capacity of Y-decorated porous graphene. Appl. Surf. Sci. 2017, 399, 463–468. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, Y.H.; Dillion, A.C.; Heben, M.J.; Zhang, S.B. Hydrogen storage in novel organometallic buckyballs. Phys. Rev. Lett. 2005, 94, 155504. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Yang, S.Y.; Hicke, C.; Wang, E.; Geohegan, D.; Zhang, Z.Y. Calcium as the superior coating metal in functionalization of carbon fullerenes for high-capacity hydrogen storage. Phys. Rev. Lett. 2008, 100, 206806. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Z.G.; Liu, S.Q.; Zhao, X.H.; Huang, K.L. High-capacity hydrogen storage medium: Ti doped fullerene. Appl. Phys. Lett. 2011, 98, 023107. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.; Refson, K.R.; Payne, M.C. First Principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, T.; Gulans, A.; Krasheninnikov, A.V.; Nieminen, R.M. Van der Waals Bonding in Layered Compounds from Advanced Density-Functional First-Principles Calculations. Phys. Rev. Lett. 2012, 108, 235502. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Hu, W.; Xia, N.; Wu, X.; Li, Z.; Yang, J. Silicene as a highly sensitive molecule sensor for NH3, NO and NO2. Phys. Chem. Chem. Phys. 2014, 16, 6957–6962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, J.; Yuan, L.H.; Zhang, M.L.; Zhang, C.R. Sc-Decorated Porous Graphene for High-Capacity Hydrogen Storage: First-Principles Calculations. Materials 2017, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dai, J.; Zhuo, Z.; Yang, J.; Zeng, X. Two-Dimensional Boron Monolayer Sheets. ACS Nano 2012, 6, 7443–7453. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, H.; Shao, H.; Ning, Z.; Xu, Y.; Ni, G.; Lu, H.; Zhang, D.; Zhu, H. Stability and strength of atomically thin borophene from first principles calculations. Mater. Res. Lett. 2017, 5, 399–407. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Shao, H.Z.; Xu, Y.F.; Zhang, R.J.; Zhua, H.Y. Electronic, Optical, and thermodynamic properties of borophene from first-principle calculations. J. Mater. Chem. C 2016, 4, 3592–3598. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, Y.H.; Wu, N.; Zhang, M.L.; Yuan, L.H.; Zhang, C.R. A First Principles Study of H2 Adsorption on LaNiO3(001) Surfaces. Materials 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ataca, C.; Akturk, E.; Ciraci, S.; Ustunel, H. High-capacity hydrogen storage by metallized graphene. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wang, Q.; Jena, P.; Kawazoe, Y. Clustering of Ti on a C60 surface and its effect on hydrogen storage. J. Am. Chem. Soc. 2005, 127, 14582–14583. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Harrison, N.M.; Saunders, V.R. A density functional study of lithium bulk and surfaces. J. Phys.-Condens. Matter 1999, 11, 5007–5019. [Google Scholar] [CrossRef]
- Mulliken, R.S. Molecular Compounds and Their Spectra. V. Orientation in Molecular Complexes. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- An, H.; Liu, C.S.; Zeng, Z.; Fan, C.; Ju, X. Li-doped B2C graphene as potential hydrogen storage medium. Appl. Phys. Lett. 2011, 98, 173101–173103. [Google Scholar] [CrossRef]

| Li-β12-borophene | Number of H2 | 1 H2 | 2 H2 | 3 H2 | 4 H2 | 5 H2 | 6 H2 | 7 H2 |
| /eV | −0.247 | −0.281 | −0.154 | −0.179 | −0.139 | −0.169 | −0.134 | |
| (DFT-D)/eV | −0.385 | −0.388 | −0.251 | −0.147 | −0.160 | −0.167 | −0.142 | |
| /eV/H2 | −0.247 | −0.213 | −0.194 | −0.190 | −0.181 | −0.178 | −0.173 | |
| (DFT-D)/eV/H2 | −0.385 | −0.387 | −0.286 | −0.251 | −0.233 | −0.222 | −0.210 | |
| rH-H/Å | 0.756 | 0.757 | 0.753 | 0.755 | 0.753 | 0.753 | 0.753 | |
| rH-Li/Å | 2.164 | 2.169 | 3.813 | 3.810 | 4.661 | 5.667 | 6.368 | |
| 2Li-β12-borophene | Number of H2 | 2 H2 | 4 H2 | 6 H2 | 8 H2 | 10 H2 | 12 H2 | 14 H2 |
| (DFT-D)/eV/H2 | −0.381 | −0.298 | −0.274 | −0.262 | −0.230 | −0.226 | −0.220 |
| Atom | Mulliken | |||||
|---|---|---|---|---|---|---|
| Before Adsorption/e | After Adsorption/e | |||||
| s | p | Charge | s | p | Charge | |
| H (1) | 1.0 | 1.06 | −0.06 | |||
| H (2) | 1.0 | 1.05 | −0.05 | |||
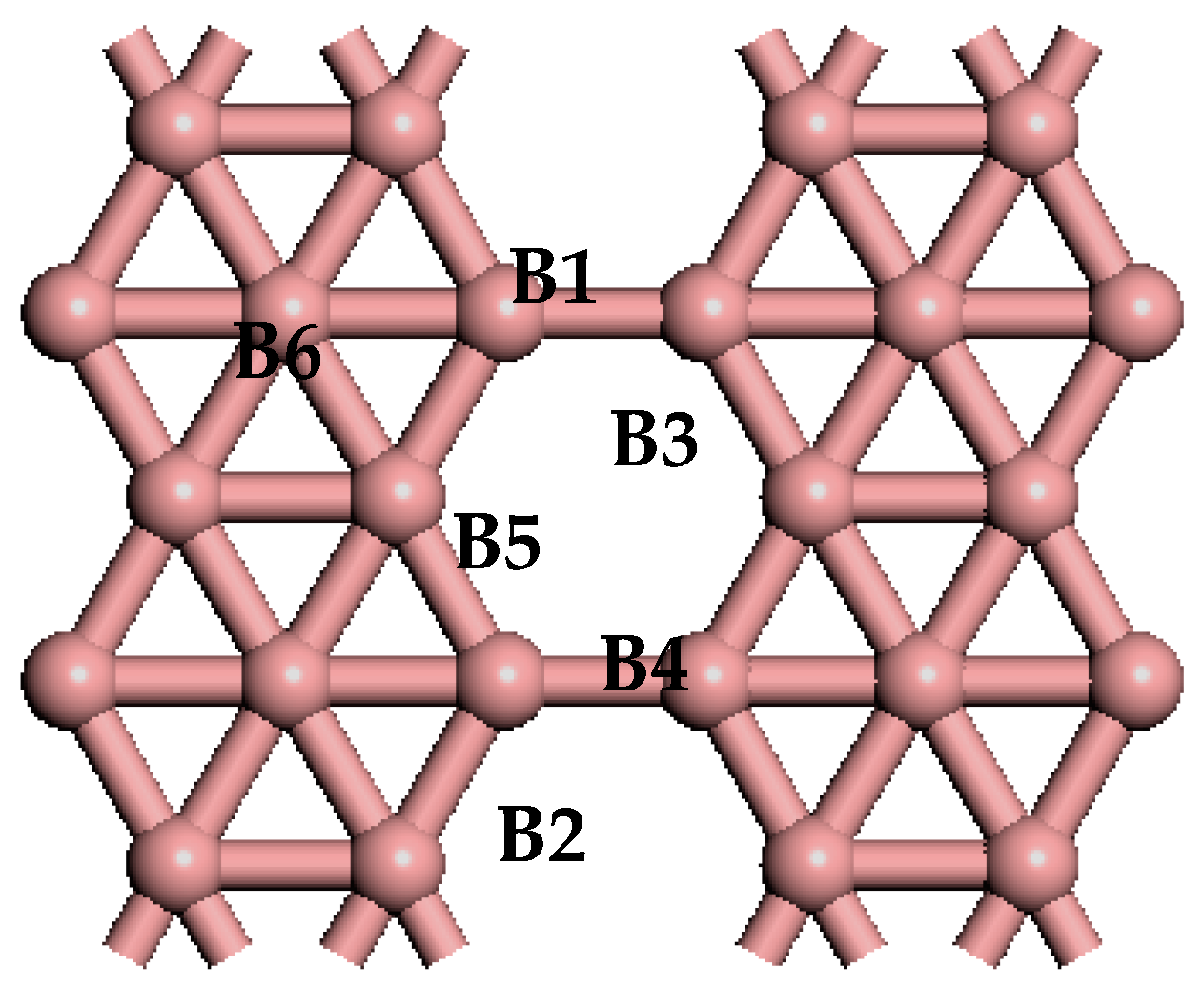
| B1 | 0.82 | 2.18 | 0 | 0.83 | 2.36 | −0.19 |
| B5 | 0.74 | 2.23 | 0.03 | 0.75 | 2.40 | −0.15 |
| B6 | 0.65 | 2.40 | −0.05 | 0.65 | 2.40 | −0.05 |
| Li | 3 | 0 | 0 | 1.60 | 1.40 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Chen, Y.; Wang, H.; Zhang, M.; Yuan, L.; Zhang, C. Li-Decorated β12-Borophene as Potential Candidates for Hydrogen Storage: A First-Principle Study. Materials 2017, 10, 1399. https://doi.org/10.3390/ma10121399
Liu T, Chen Y, Wang H, Zhang M, Yuan L, Zhang C. Li-Decorated β12-Borophene as Potential Candidates for Hydrogen Storage: A First-Principle Study. Materials. 2017; 10(12):1399. https://doi.org/10.3390/ma10121399
Chicago/Turabian StyleLiu, Tingting, Yuhong Chen, Haifeng Wang, Meiling Zhang, Lihua Yuan, and Cairong Zhang. 2017. "Li-Decorated β12-Borophene as Potential Candidates for Hydrogen Storage: A First-Principle Study" Materials 10, no. 12: 1399. https://doi.org/10.3390/ma10121399





