Optimization of a Binary Concrete Crack Self-Healing System Containing Bacteria and Oxygen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Spore Preparation
2.3. Preparation of Oxygen-Releasing Tablets
2.4. Calcium Precipitation Determination
2.5. Optimization of Spore Production Process
2.6. Optimization of the Bacterial Calcium Precipitation Process
3. Results
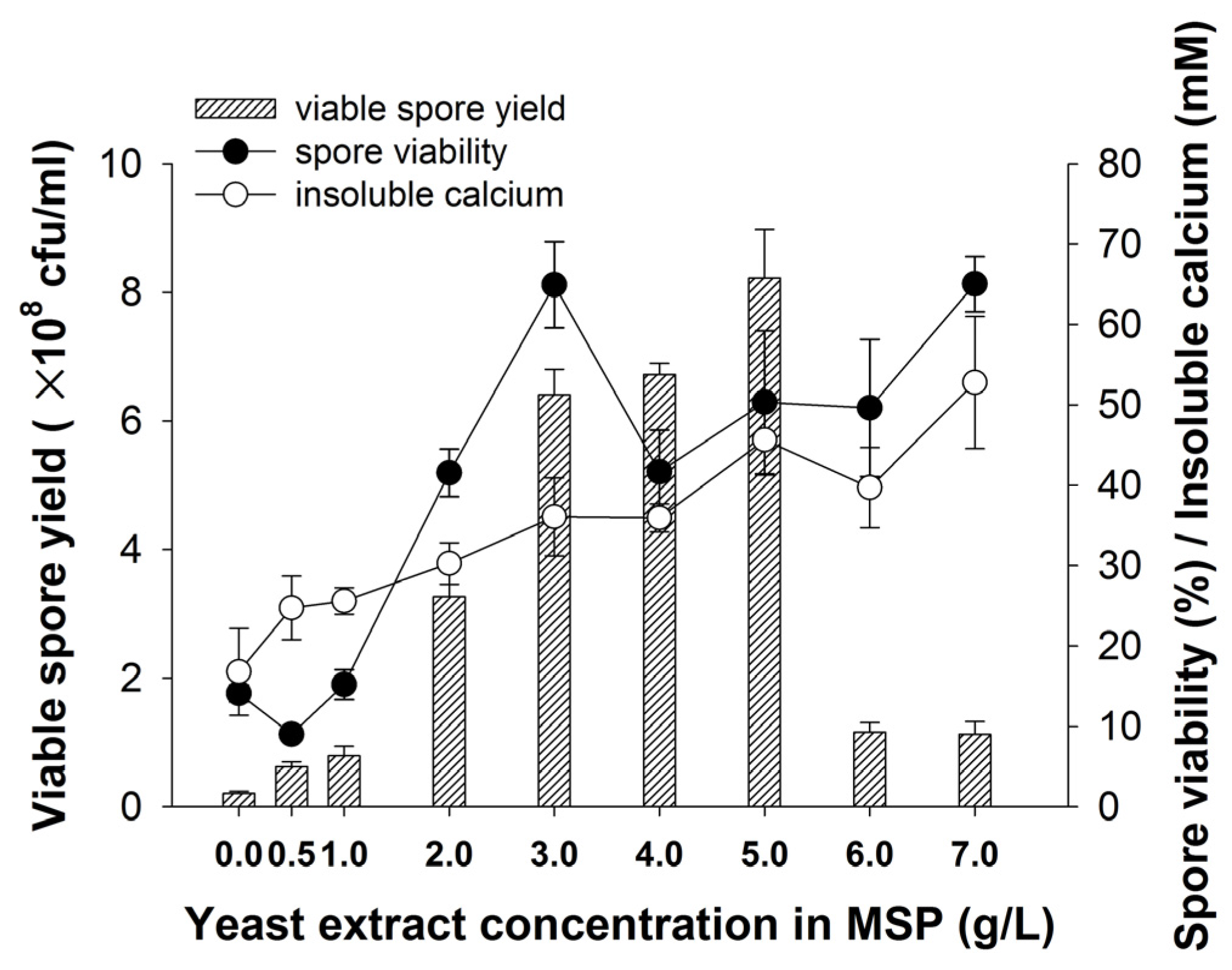
3.1. Effect of Yeast Extract on Spore Production and Calcium Precipitation
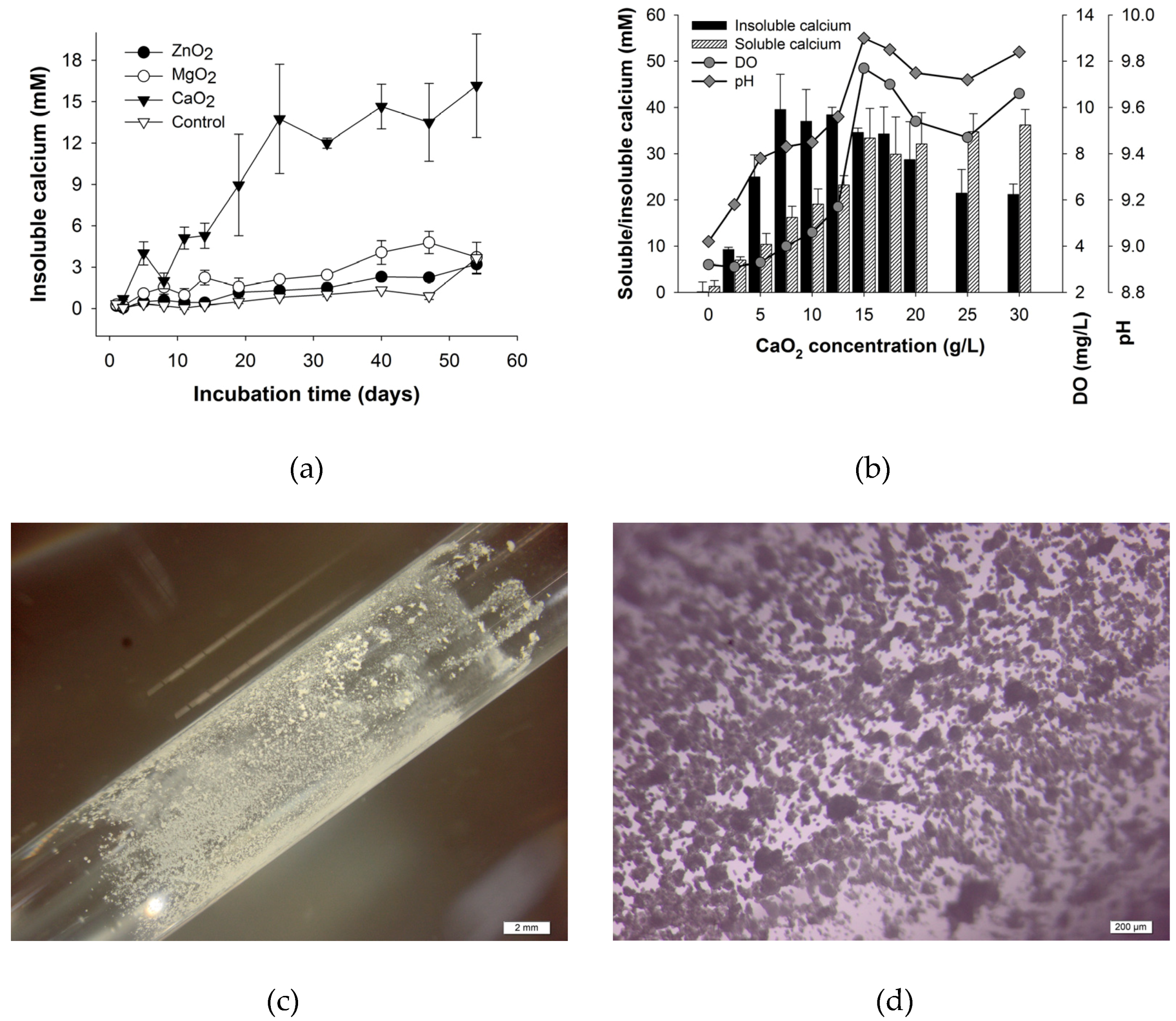
3.2. Effect of Different Peroxides and CaO2 Dosage on Calcium Precipitation
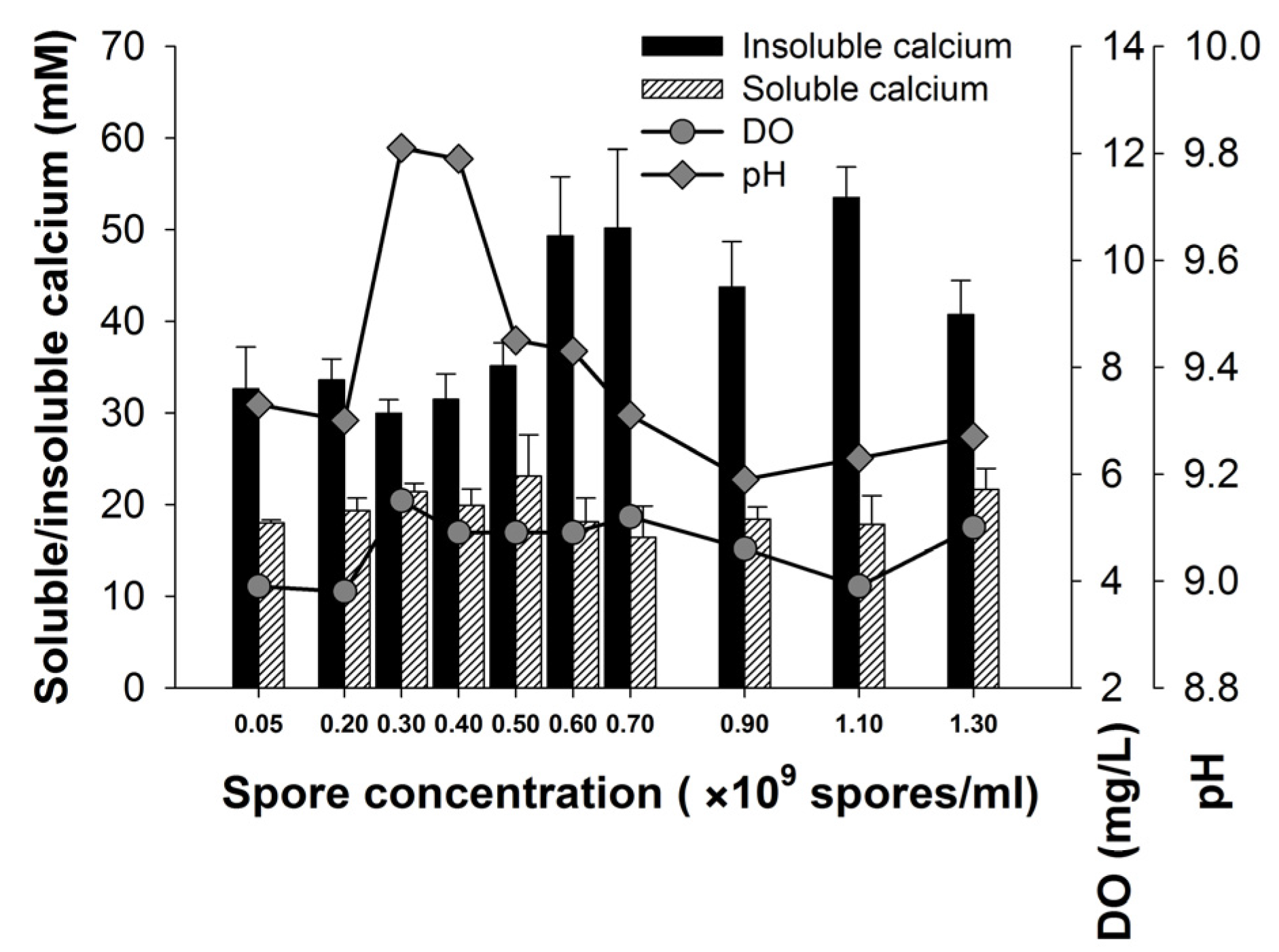
3.3. Effect of Spore Concentration on the Calcium Precipitation
3.4. Effect of Carbon and Nitrogen Sources on the Calcium Precipitation
4. Discussion
4.1. The Effect of Yeast Extract on Spore Production and Calcium Precipitation
4.2. Effect of Different Peroxides and CaO2 Dosage on Calcium Precipitation
4.3. The Effect of Carbon and Nitrogen Sources on Calcium Precipitation
5. Conclusions
- The optimal yeast extract concentration for both spore production and calcium precipitation is 5 g/L.
- CaO2 is the best oxygen-releasing compound that can improve the bacterial calcium precipitation and the most suitable CaO2 dosage is 7.5 g/L.
- The suitable spore concentration for H4 to achieve a high level of calcium precipitation is 6×108 spores/mL when the spore viability is approximately 50%.
- Lactate is the best carbon source and nitrate is the best nitrogen source for H4 in the self-healing process of concrete crack with/without oxygen supply
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.; Ersan, Y.C.; Boon, N.; De Belie, N. Application of microorganisms in concrete: a promising sustainable strategy to improve concrete durability. Appl. Microbiol. Biotechnol. 2016, 100, 2993–3007. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wu, R.S.; Li, Y.M.; Zhong, J.Y.; Deng, X.; Liu, B.; Han, N.X.; Xing, F. Screening of bacteria for self-healing of concrete cracks and optimization of the microbial calcium precipitation process. Appl. Microbiol. Biotechnol. 2016, 100, 6661–6670. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M. Microbial Concrete: Way to enhance the durability of building structures. J. Mater. Civ. Eng. 2010, 23, 730–734. [Google Scholar] [CrossRef]
- Ganendra, G.; De Muynck, W.; Ho, A.; Arvaniti, E.C.; Hosseinkhani, B.; Ramos, J.A.; Rahier, H.; Boon, N. Formate oxidation-driven calcium carbonate precipitation by Methylocystis parvus OBBP. Appl. Environ. Microbiol. 2014, 80, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wang, C.G.; Wang, Q.L.; Feng, J.L.; Pan, W.; Zheng, X.C.; Liu, B.; Han, N.X.; Xing, F.; Deng, X. A binary concrete crack self-healing system containing oxygen-releasing tablet and bacteria and its Ca2+-precipitation performance. Appl. Microbiol. Biotechnol. 2016, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Dick, J.; de Windt, W.; de Graef, B.; Saveyn, H.; van der Meeren, P.; de Belie, N.; Verstraete, W. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.M.; Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016, 122, 118–125. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Sierra-Beltran, M. G.; Jonkers, H.M.; Schlangen, E. Performance of shcc with bacteria for concrete patch repair. In Proceedings of the structural faults and repair conference, London, UK, 8–10 July 2014; The Electrochemical Society: Pennington, NJ, USA, 2014. [Google Scholar]
- Wang, J.Y.; Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2011, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; van Tittelboom, K.; de Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Wang, J.Y.; Snoeck, D.; van Vlierberghe, S.; Verstraete, W.; de Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Scotland, M.; Setlow, P. Levels of germination proteins in dormant and superdormant spores of bacillus subtilis. J. Bacteriol. 2012, 194, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Setlow, P. Isolation and characterization of superdormant spores of bacillus species. J. Bacteriol. 2009, 191, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.S.; Zhou, P.P.; Yu, L.J. Influence of enzyme concentration on bio-sequestration of CO2 in carbonate form using bacterial carbonic anhydrase. Chem. Eng. J. 2013, 232, 149–156. [Google Scholar] [CrossRef]
- Guizelini, B.P.; Vandenberghe, L.P.S.; Sella, S.R.B.R.; Soccol, C.R. Study of the influence of sporulation conditions on heat resistance of Geobacillus stearothermophilus used in the development of biological indicators for steam sterilization. Arch. Microbiol. 2012, 194, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Tsay, K.J.; Wu, W.S.; Tzeng, Y.M. Medium optimization of carbon and nitrogen sources for the production of spores from Bacillus amyloliquefaciens B128 using response surface methodology. Process Biochem. 2007, 42, 535–541. [Google Scholar] [CrossRef]
- Penna, T.C.V.; Machoshvili, I.A.; Taqueda, M.E.S.; Ishii, M. The effect of media composition on the thermal resistance of Bacillus stearothermophilus. PDA J. Pharm. Sci. Technol. 2000, 54, 398–412. [Google Scholar] [PubMed]
- Xu, J.; Yao, W.; Jiang, Z. Non-ureolytic bacterial carbonate precipitation as a surface treatment strategy on cementitious materials. J. Mater. Civ. Eng. 2013, 26, 983–991. [Google Scholar] [CrossRef]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.G.; Pedahzur, R.; Lev, O. Zinc dioxide nanoparticulates: A hydrogen peroxide source at moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef] [PubMed]
- Karagöl, F.; Demirboğa, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polat, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; de Belie, N.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Mai, B.; Cai, T.; Luo, J.; Wu, W.; Liu, B.; Han, N.; Xing, F.; Deng, X. Optimization of a Binary Concrete Crack Self-Healing System Containing Bacteria and Oxygen. Materials 2017, 10, 116. https://doi.org/10.3390/ma10020116
Zhang J, Mai B, Cai T, Luo J, Wu W, Liu B, Han N, Xing F, Deng X. Optimization of a Binary Concrete Crack Self-Healing System Containing Bacteria and Oxygen. Materials. 2017; 10(2):116. https://doi.org/10.3390/ma10020116
Chicago/Turabian StyleZhang, Jinlong, Bixia Mai, Tingwei Cai, Jiayi Luo, Wanhan Wu, Bing Liu, Ningxu Han, Feng Xing, and Xu Deng. 2017. "Optimization of a Binary Concrete Crack Self-Healing System Containing Bacteria and Oxygen" Materials 10, no. 2: 116. https://doi.org/10.3390/ma10020116



