Compressive Properties of Open-Cell Al Hybrid Foams at Different Temperatures
Abstract
:1. Introduction
2. Experimental Section
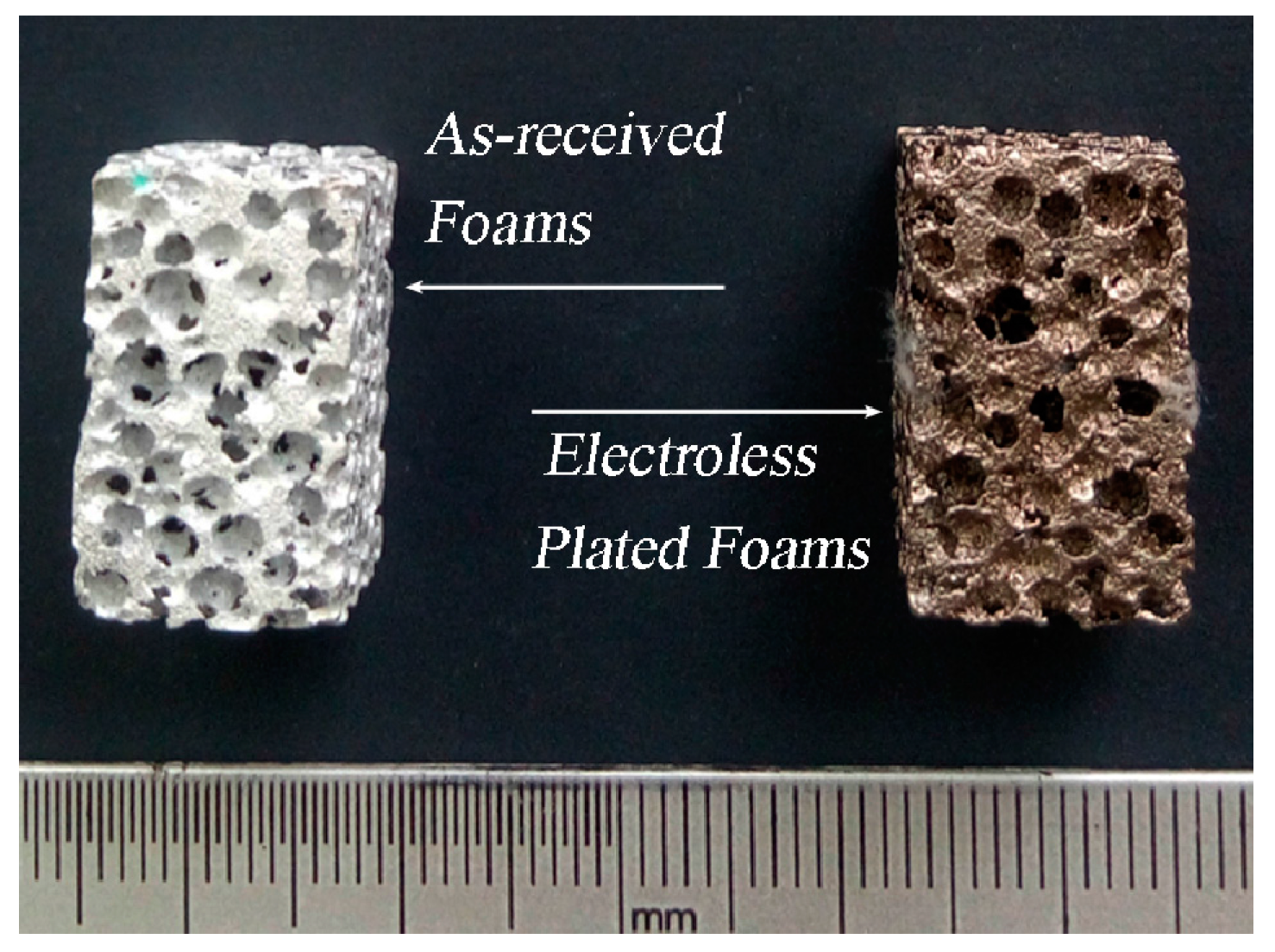
2.1. Preparation
2.2. Compression Test
2.3. Material Characterization
3. Results and Discussion
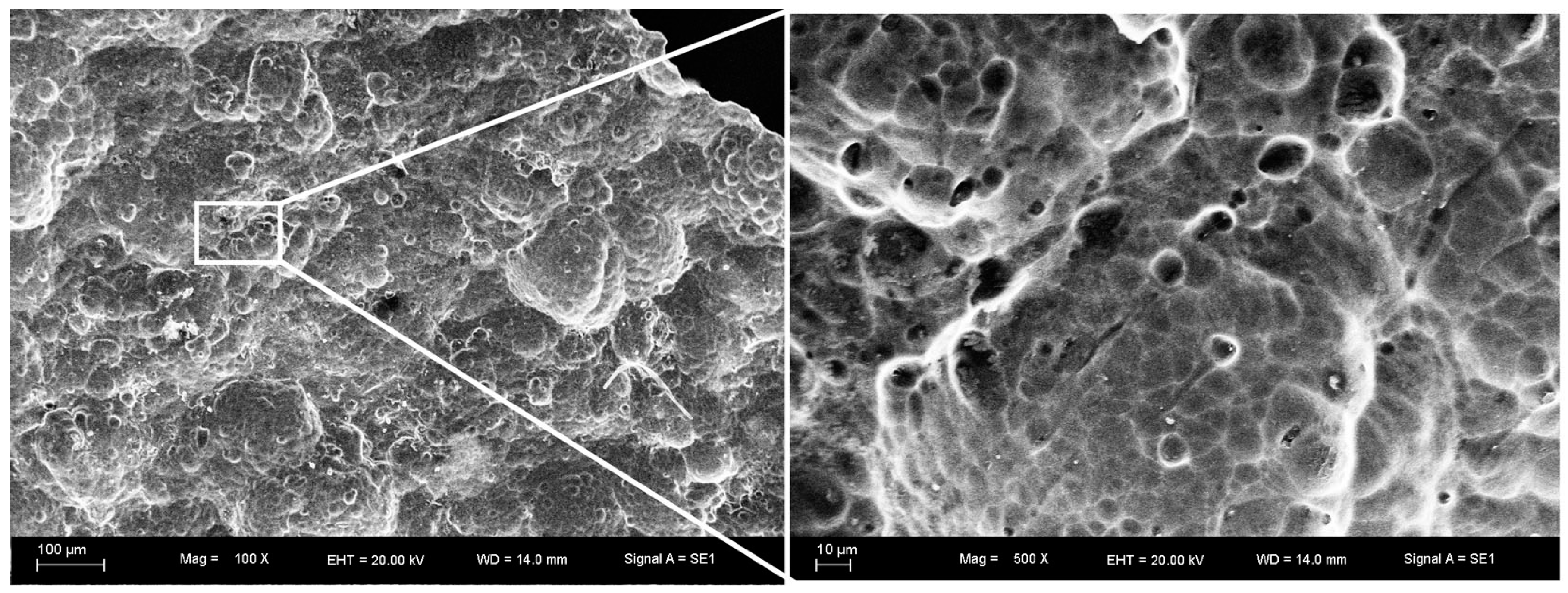
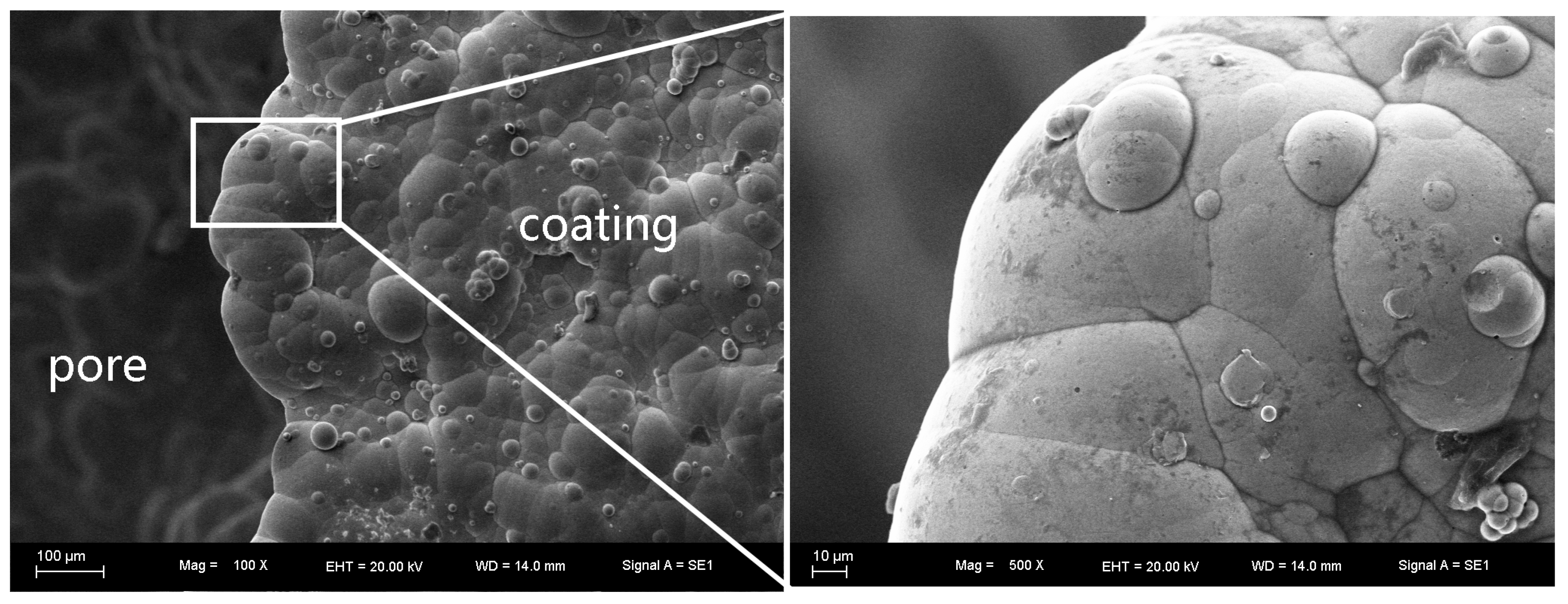
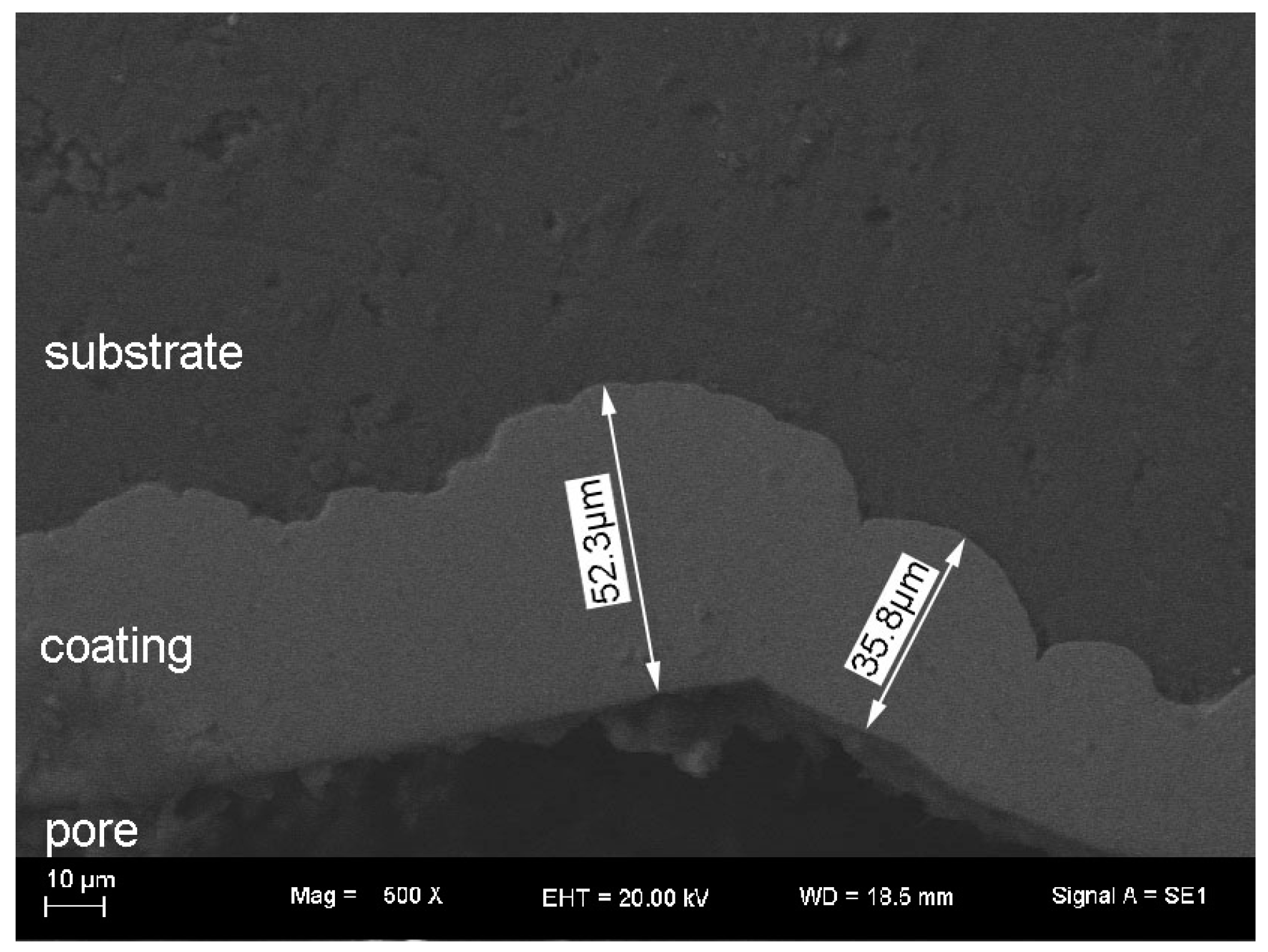
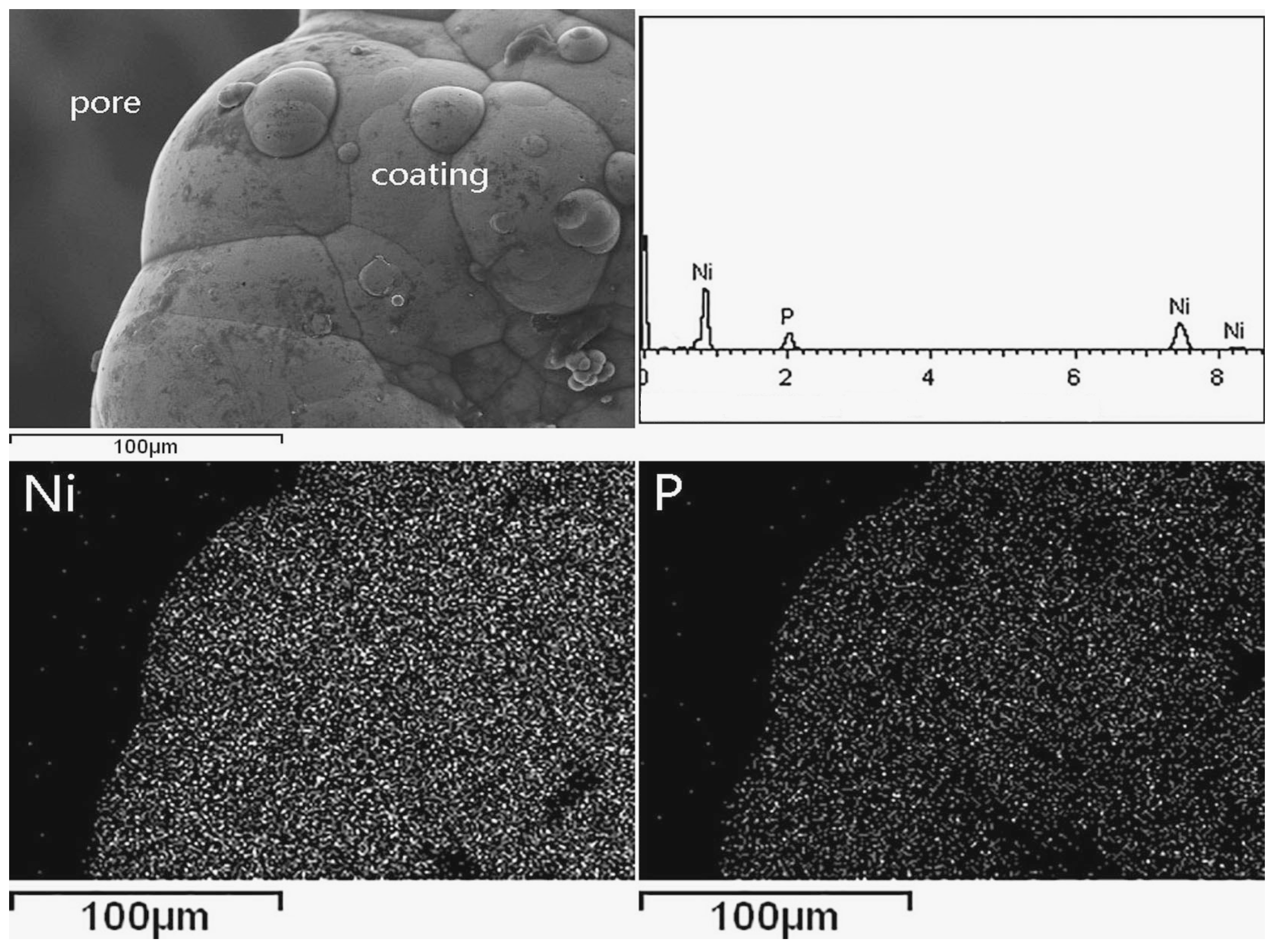
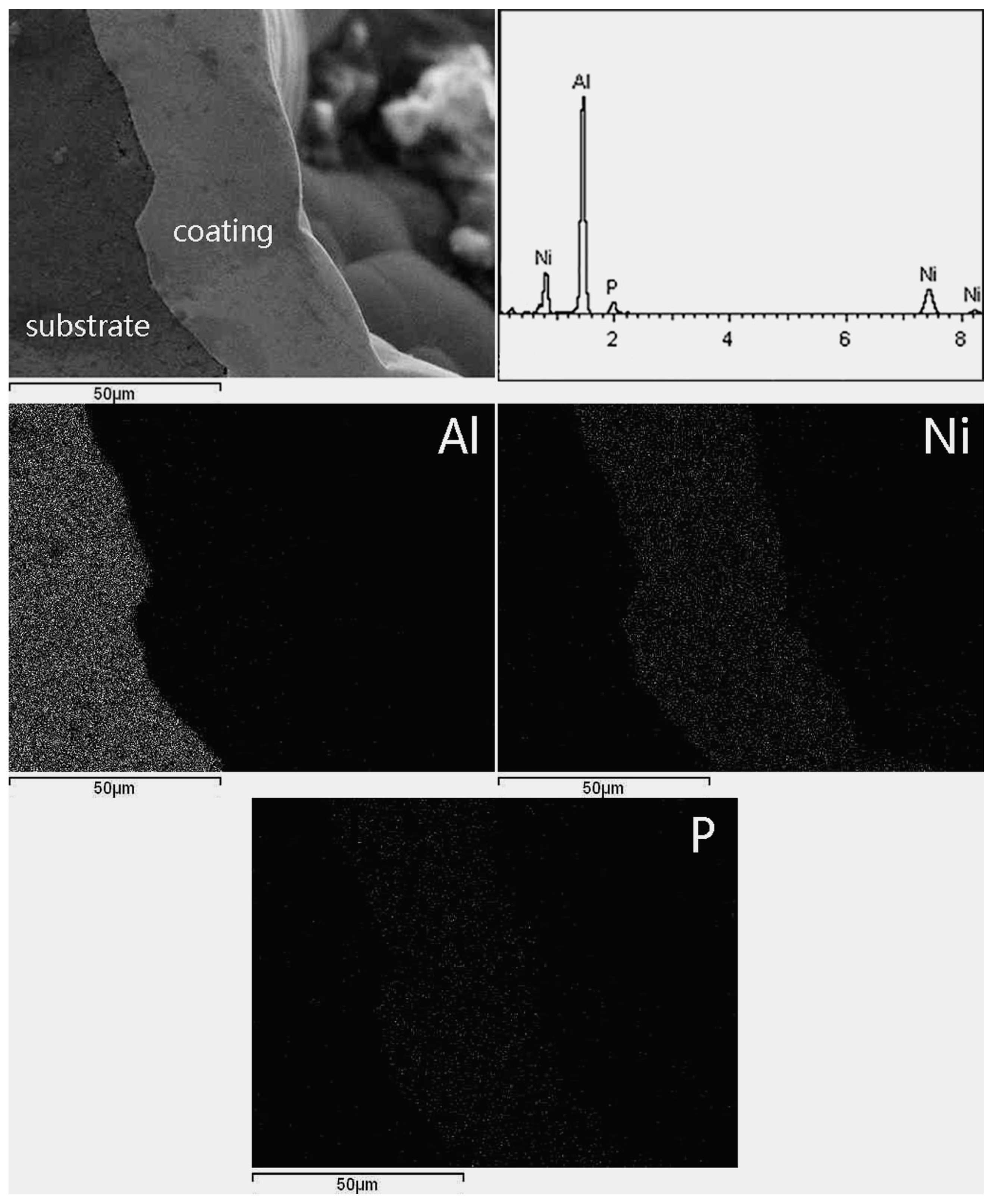
3.1. Surface Morphology and Thickness of the Coatings
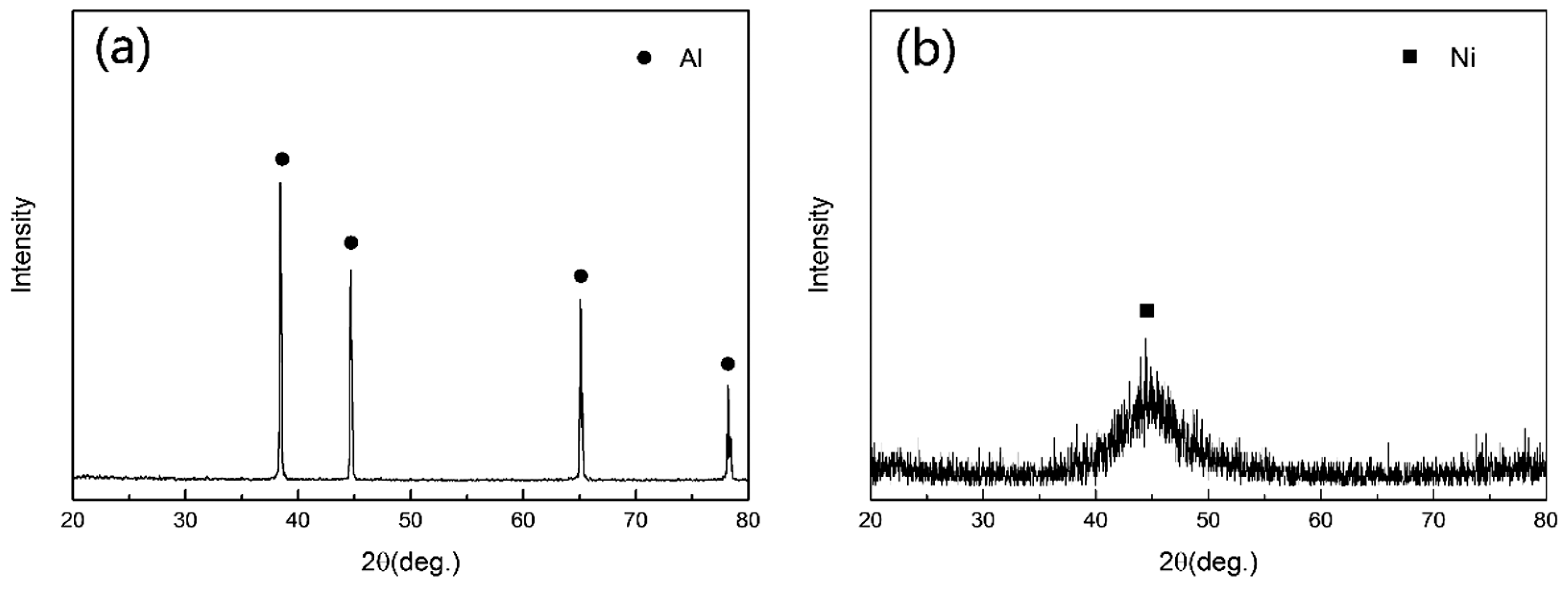
3.2. Chemical Composition and Phase Composition of Coatings
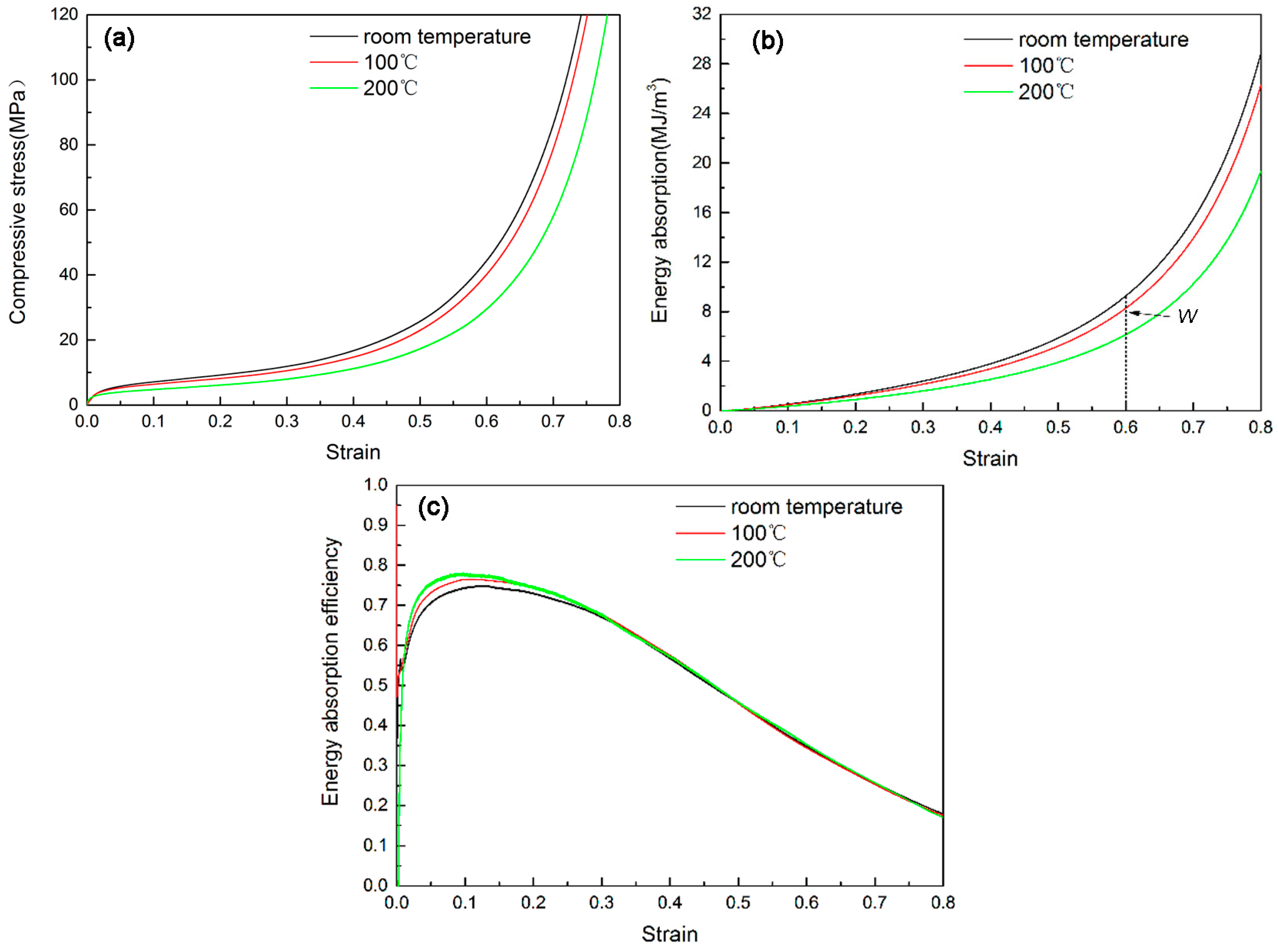
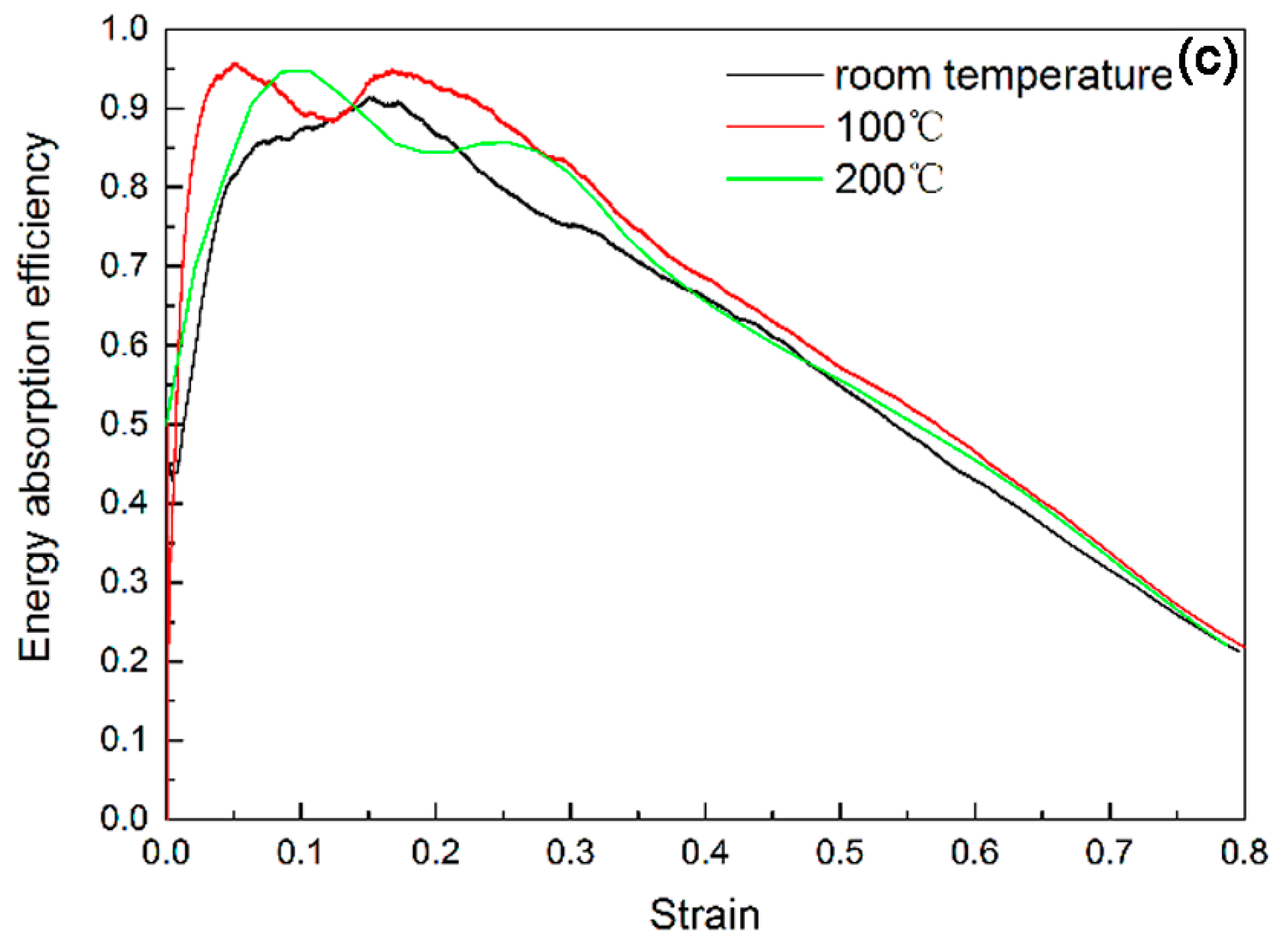
3.3. Compressive Properties of the Foam at Different Temperatures
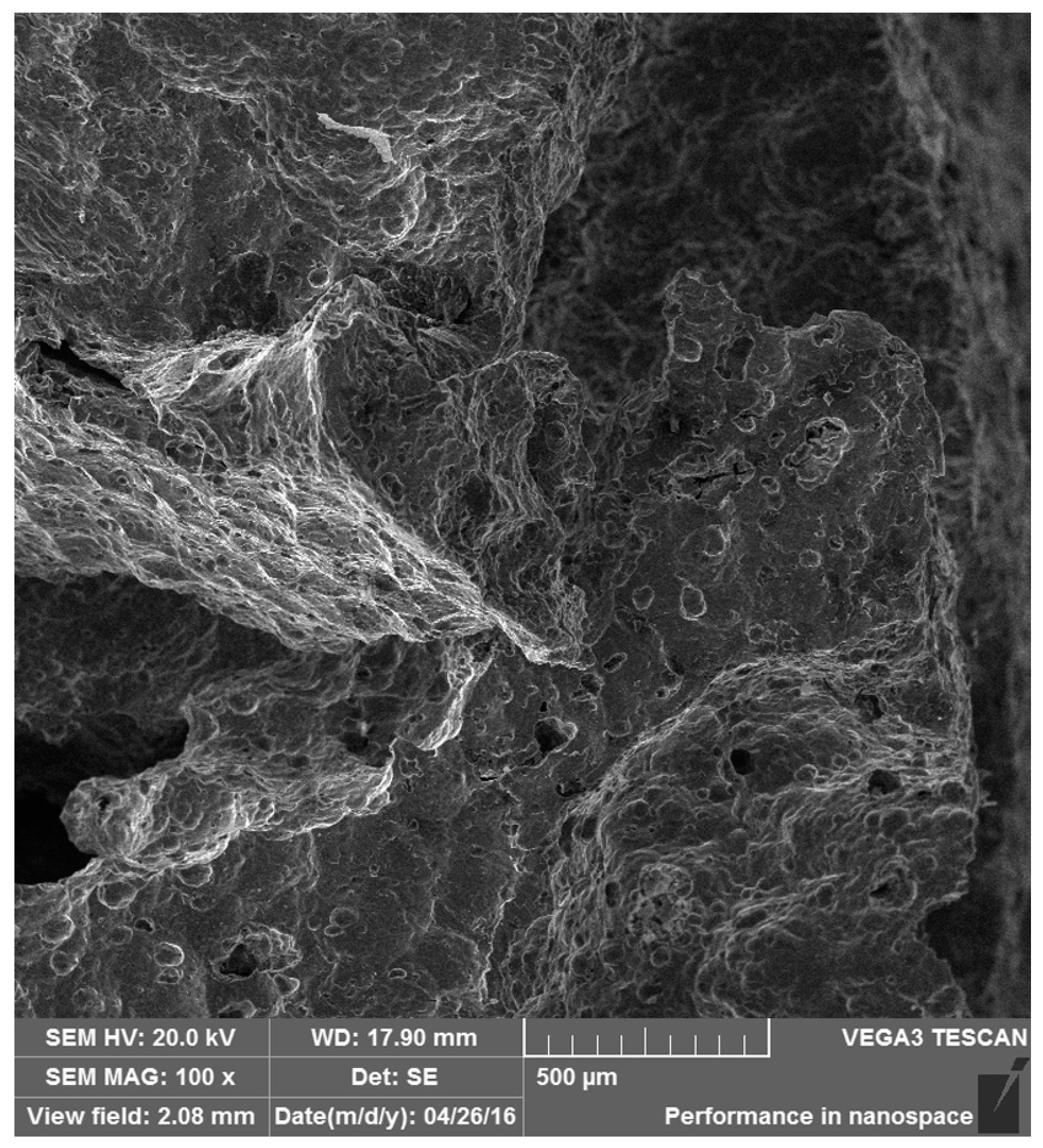
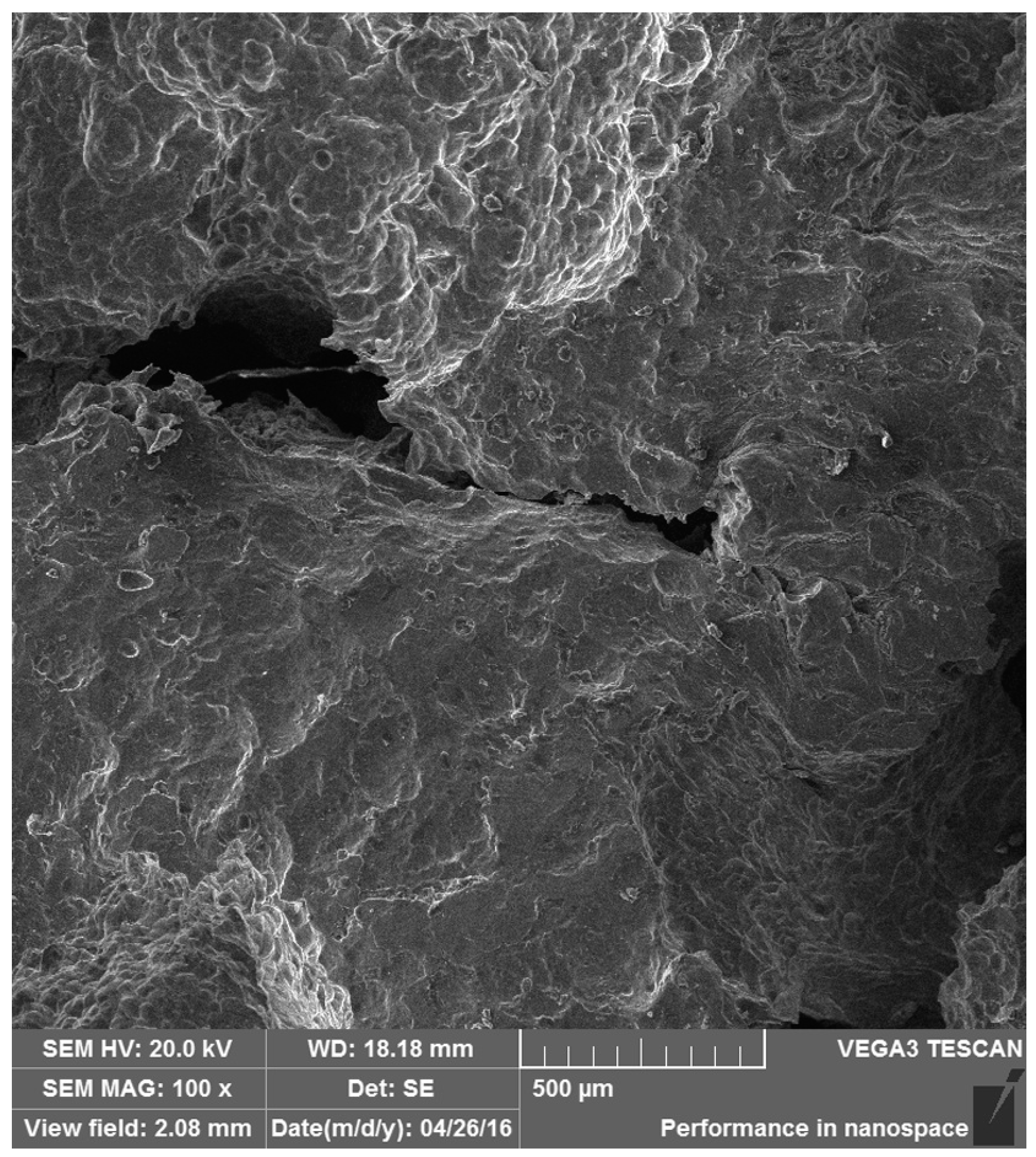
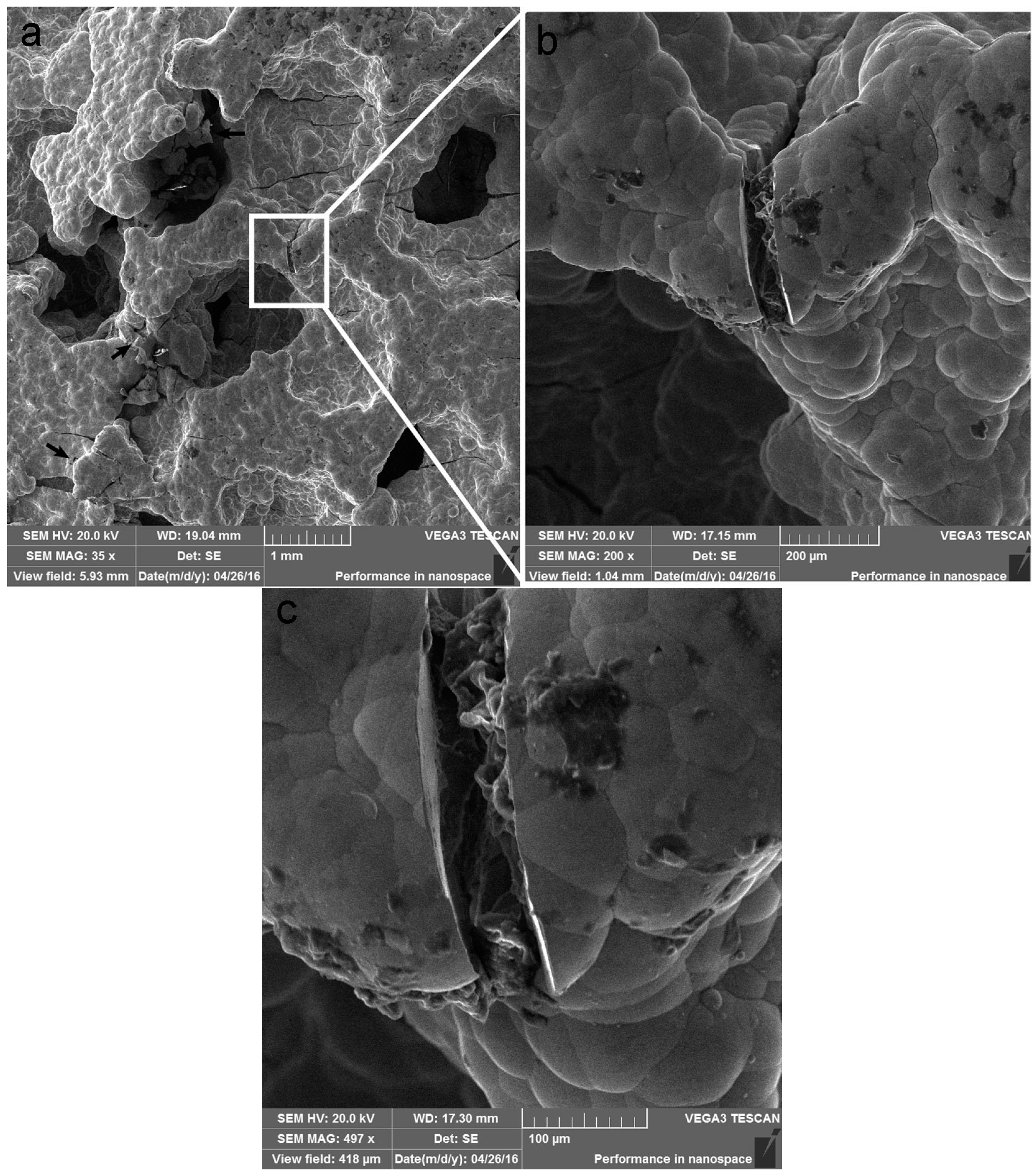
3.4. Failure Mechanism Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Oxford, UK, 1997. [Google Scholar]
- Goodall, R. Porous metals: Foams and sponges. In Advances in Powder Metallurgy: Properties, Processing and Applications, 1st ed.; Chang, I.T., Zhao, Y.Y., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; Chapter 10; pp. 273–307. [Google Scholar]
- Degischer, H.P. Handbook of Cellular Metals, 1st ed.; Wiley-VCH Gerlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Bin, J.; Zejun, W.; Naiqin, Z. Effect of pore size and relative density on the mechanical properties of open cell aluminum foams. Scr. Mater. 2007, 56, 169–172. [Google Scholar]
- Simone, A.E.; Gibson, L.J. Aluminum foams produced by liquid-state processes. Acta Mater. 1998, 46, 3109–3123. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Luo, Y. Compressive behavior and damping property of ZA22/SiCp composite foams. Mater. Sci. Eng. A 2007, 457, 325–328. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, T.W. The effect of cell wall microstructure on the deformation and fracture of aluminium-based foams. Acta Mater. 2001, 49, 1677–1686. [Google Scholar] [CrossRef]
- Lehmhus, D.; Banhart, J. Properties of heat-treated aluminium foams. Mater. Sci. Eng. A 2003, 349, 98–110. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Zhu, X.; Luo, Y.; Wei, M. Effects of heat treatment on compressive behavior and energy absorption characteristic of ZA22 foams. Adv. Eng. Mater. 2007, 9, 679–683. [Google Scholar] [CrossRef]
- Aly, M.S. Behavior of closed cell aluminium foams upon compressive testing at elevated temperatures: Experimental results. Mater. Lett. 2007, 61, 3138–3141. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Li, Z.; Yang, J.; Zheng, H.; Hu, S. Temperature effects on the mechanical behavior of aluminum foam under dynamic loading. Mater. Sci. Eng. A 2014, 599, 174–179. [Google Scholar] [CrossRef]
- Liu, J.; Qu, Q.; Liu, Y.; Li, R.; Liu, B. Compressive properties of Al-Si-SiC composite foams at elevated temperatures. J. Alloys Compd. 2016, 676, 239–244. [Google Scholar] [CrossRef]
- Abdulla, T.; Yerokhin, A.; Goodall, R. Enhancement in specific strength of open cell aluminium foams through plasma electrolytic oxidation treatment. Scr. Mater. 2014, 75, 38–41. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Curran, J.A.; Clyne, T.W. Plasma electrolytic oxidation of aluminium networks to form a metal-cored ceramic composite hybrid material. Compos. Sci. Technol. 2011, 71, 908–915. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Huang, Z.; Yu, S.; Yang, X. Characterization and property of microarc oxidation coatings on open-cell aluminum foams. J. Coat. Technol. Res. 2012, 9, 357–363. [Google Scholar] [CrossRef]
- Boonyongmaneerat, Y.; Schuh, C.; Dunand, D. Mechanical properties of reticulated aluminum foams with electrodeposited Ni–W coatings. Scr. Mater. 2008, 59, 336–339. [Google Scholar] [CrossRef]
- Bouwhuis, B.; McCrea, J.; Palumbo, G.; Hibbard, G. Mechanical properties of hybrid nanocrystalline metal foams. Acta Mater. 2009, 57, 4046–4053. [Google Scholar] [CrossRef]
- Jung, A.; Natter, H.; Diebels, S.; Lach, E.; Hempelmann, R. Nanonickel coated aluminium foam for enhanced impact energy absorption. Adv. Eng. Mater. 2011, 13, 23–28. [Google Scholar] [CrossRef]
- Sun, Y.; Burgueño, R.; Wang, W.; Lee, I. Effect of annealing on the mechanical properties of nano-copper reinforced open-cell aluminum foams. Mater. Sci. Eng. A 2014, 613, 340–351. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Sudagar, J.; Gao, F.; Feng, P. Preparation and corrosion resistance of electroless Ni–P coating on open-cell aluminum foams. Int. J. Electrochem. Sci. 2012, 7, 5951–5961. [Google Scholar]
- Liu, J.; Gao, F.; Rao, Y.Q.; Liu, Y. Compressive properties of aluminum foams produced by replication route using spheroidal calcium chloride as space holder. Mater. Trans. 2014, 12, 1906–1908. [Google Scholar] [CrossRef]
- Si, F. Study on Strengthening and Properties of Open-Cell Aluminum Foams. Master’s Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]
- Crobu, M.; Scorciapino, A.; Elsener, B.; Rossi, A. The corrosion resistance of electroless deposited nano-crystalline Ni–P alloys. Electrochim. Acta 2008, 53, 3364–3370. [Google Scholar] [CrossRef]
- Bi, H.; Kou, K.C.; Ostrikov, K. Microstructure and electromagnetic characteristics of Ni nanoparticle film coated carbon microcoils. J. Alloys Compd. 2009, 478, 796–800. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Vijayaraghavan, L.; Madhavan, S.; Almeida, A. Study on the mechanical properties of heat-treated electroless NiP coatings reinforced with Al2O3 nano particles. Metall. Mater. Trans. A 2016, 47, 2223–2231. [Google Scholar] [CrossRef]
- Yongjun, H.; Tianxu, W.; Jilong, M.; Qianyang, R. Structure and phase transformation behaviour of electroless Ni–W–P on aluminium alloy. Surf. Coat. Technol. 2006, 201, 988–992. [Google Scholar]


| Electrolyte | Concentration (g/L) | Electrolyte | Concentration (g/L) |
|---|---|---|---|
| NiSO4·6H2O | 25 | C3H6O3 | 30 |
| NaH2PO2·H2O | 30 | C3H6O2 | 2.2 |
| CH3COONa | 20 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Si, F.; Zhu, X.; Liu, Y.; Zhang, J.; Liu, Y.; Zhang, C. Compressive Properties of Open-Cell Al Hybrid Foams at Different Temperatures. Materials 2017, 10, 98. https://doi.org/10.3390/ma10020098
Liu J, Si F, Zhu X, Liu Y, Zhang J, Liu Y, Zhang C. Compressive Properties of Open-Cell Al Hybrid Foams at Different Temperatures. Materials. 2017; 10(2):98. https://doi.org/10.3390/ma10020098
Chicago/Turabian StyleLiu, Jiaan, Fujian Si, Xianyong Zhu, Yaohui Liu, Jiawei Zhang, Yan Liu, and Chengchun Zhang. 2017. "Compressive Properties of Open-Cell Al Hybrid Foams at Different Temperatures" Materials 10, no. 2: 98. https://doi.org/10.3390/ma10020098






