1. Introduction
In our previous work [
1] we developed the specialized technique of vacuum-induced surface freezing [
2] to produce aligned porous alumina monoliths that have parallel channels running through the solid from top to bottom. This is an alternate technique to the freeze-casting methods used before which require a specialized cold-finger apparatus [
3,
4,
5,
6,
7,
8,
9]. A vacuum of approximately 250 mTorr produces undercooling of the dispersion surface, which freezes to form a continuous, frozen surface layer. Subsequent sublimation keeps this surface cold and directional freezing occurs from top to bottom through the dispersion [
9]. Since this is done in a glass vial standing on the shelf of a lyophilizer, it does not need a complex cold-finger freezing apparatus. The aligned porous morphology of the lyophilized ceramic is highly similar to the structures produced by conventional freeze casting. A more-or-less dense surface layer and cellular sub-surface region border a wide region of aligned channels that stretches through most of the depth of the membrane [
1].
Aligned porous materials can be used as scaffolds for tissue engineering, e.g., synthetic bone generation on hydroxyapatite, because of their high compressive strengths [
3,
10]. There is, however, a further potential application as porous membranes for use in diffusion and separation processes. One particular application is the provision of highly-tortuous membranes for use as a surrogate for human stratum corneum. This is the outermost layer of the skin and of relevance for testing transdermal patches that deliver drugs through the skin into the systemic circulation [
11]. No synthetic membrane has a tortuosity sufficiently high to simulate that of the stratum corneum [
12]. This application is governed by the particular orientation of the linearly-aligned channels to the direction of solute or solvent movement through the membrane. In the direction parallel to the channels the membrane’s tortuosity,
will be very close to unity [
13], i.e.,
≈ 1.0, where
is the mean path (pore) length and L is the membrane thickness measured parallel to the aligned pores [
14]. This is because the aligned channels run linearly straight through the thickness of the membrane from top to bottom. In the direction perpendicular to the channels, however, the tortuosity should be larger than 1.0. The reason is that to pass sideways through the membrane, e.g., from left to right, the movement of solute or solvent must occur on a convoluted pathway that circumvents the solid lamellae walls separating the channels. This requires the existence of cross-linking pores through the lamellae that connect adjacent parallel channels which have, however, been identified in alumina monoliths made by vacuum-induced surface directional freezing [
1].
We describe, in this paper, our efforts to measure the tortuosity of alumina membranes produced by vacuum-induced surface directional freezing. The wide region of aligned channels formed by freezing, lyophilization, and sintering was isolated and used as a membrane. The steady-state diffusion of a model solute, methylene blue, in the direction perpendicular to ice crystal growth was measured to identify if a continuous channel pathway exists. From the measured diffusion rate it is readily possible to calculate the effective tortuosity of the membrane [
15], (
α/
τ)
−1, from:
where (
α/
τ)
−1 is seen to be the factor by which the solute flux, Δ
m(
t)/Δ
t, out of a donor solution of concentration
c0 through the tortuous membrane, is reduced compared with that through an isotropic membrane of the same area,
A, and thickness,
L, with solute diffusivity,
D. (
α/
τ)
−1 is, therefore, the reciprocal value of the quotient of the reduced available area for diffusion within the membrane,
=
A*/
A, where
A* is the available area and
A is the cross-sectional area of the membrane, to the increased mean diffusional path length, i.e., tortuosity,
. It was, therefore, the object of this study to determine the effective tortuosity of these aligned porous membranes.
2. Results and Discussion
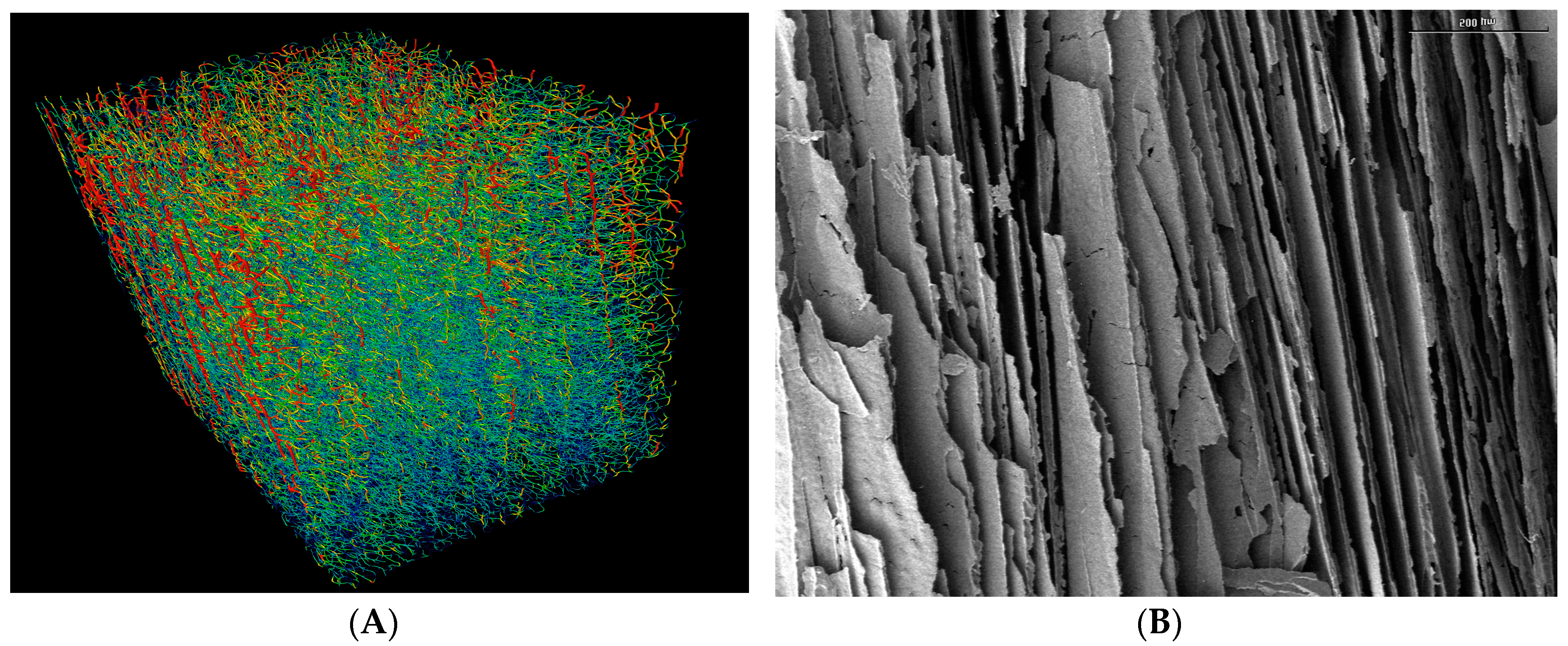
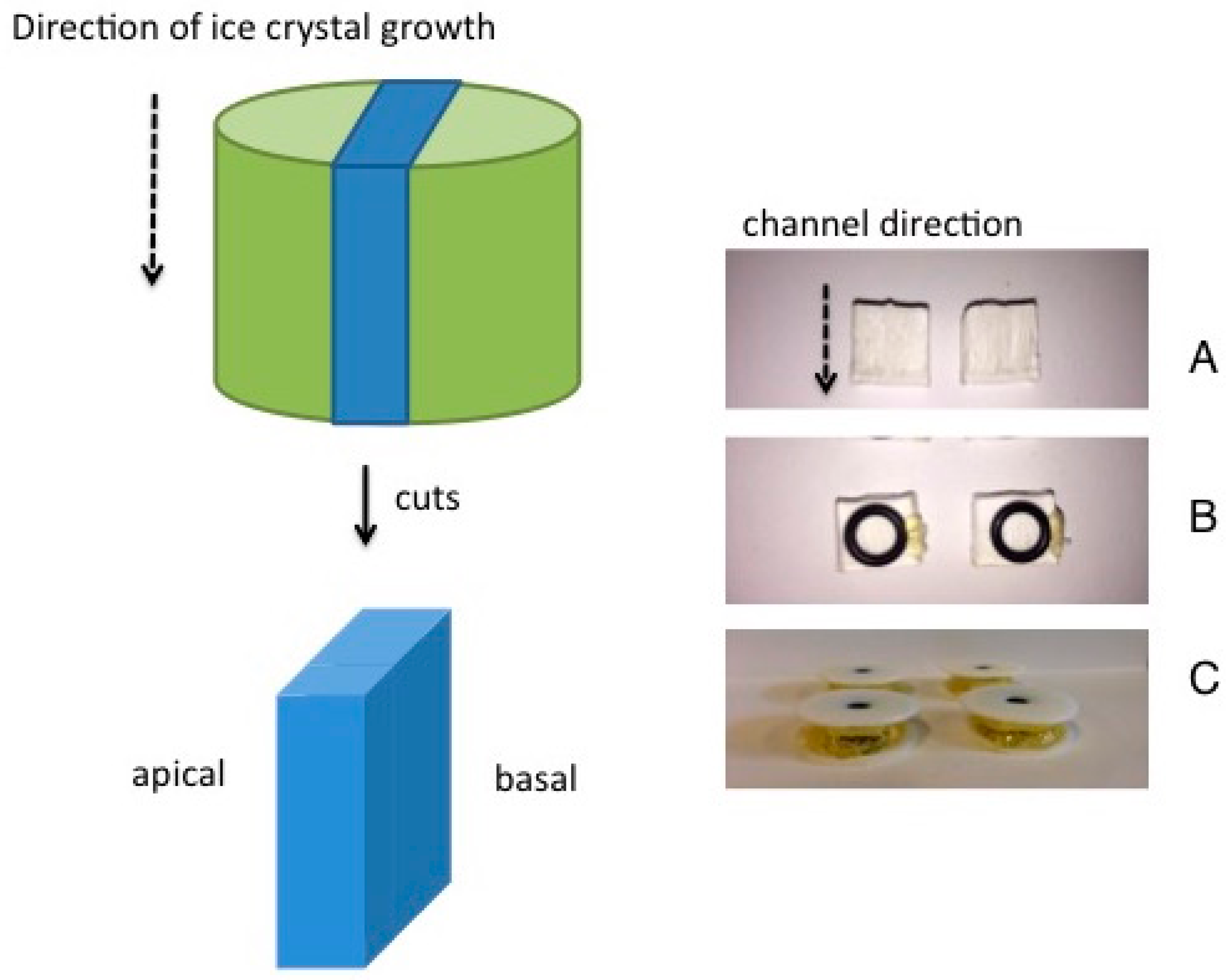
Figure 1A shows the pore channels and their distributions in the sintered membrane obtained from the example of the alumina dispersion having a volume fraction of dispersed phase of φ
v = 0.128. The pore network is represented with a red color to indicate maximum pore diameter and a blue color for minimum pore diameter. For ease of visualization the true channel diameter is reduced by a factor of 0.6 to enable an improved insight view. The channels are predominantly aligned in parallel and run from top to bottom of the sample. As far as can be judged by eye, the channels appear to be continuous between the top and bottom of the membrane. The tortuosity of this membrane perpendicular to the direction of freezing (top to bottom) should be >1.0. The SEM picture in
Figure 1B is a longitudinal section through the region of aligned channels within the same membrane (φ
v = 0.128). Additionally, in this image the parallel arrangement of aligned solid walls and channels runs through the depth of the membrane and is the same geometry at all three φ
v [
7]. The diffusion of methylene blue took place perpendicular to the direction of the aligned channels, i.e., in this image from left to right. Note the presence of pores and cracks in the walls in
Figure 1B. These become increasingly evident as φ
v is reduced from 0.128 (compare
Figure 1B) through 0.064 to 0.032 (SEMs not shown) [
1].
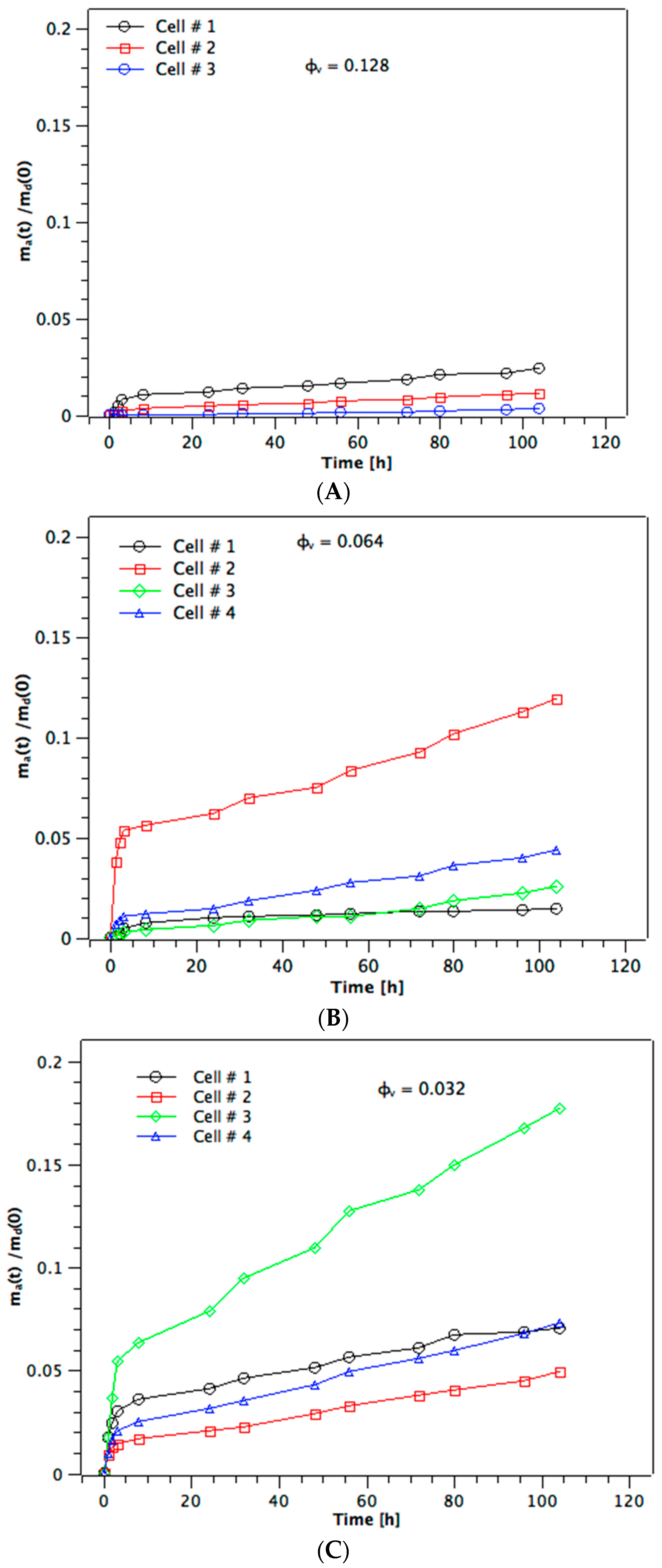
Figure 2A–C show the diffusion profiles for methylene blue obtained from a 0.20 mg/5 mL donor solution, i.e., m
d(0) = 0.2 mg methylene blue, for membranes made from the three different φ
v. We give the individual profiles (
n = 3 for
Figure 2A—one cell failed—and
n = 4 for
Figure 2B,C) rather than mean ± standard deviation since this helps understand better the variation seen at earlier times. The diffusion profiles at both the higher and lower m
d(0), i.e., of 0.1 mg and 0.3 mg, respectively, show the same pattern (not given here for brevity). It is immediately evident that the diffusion profiles do not show the expected shape for membrane permeation with a lag time followed by a linear steady-state phase [
16]. The amount of dye appearing in the acceptor solution shows a rapid initial step-increase during the first 5 h, after which it levels off and shows an approximately linear further increase up to the end of the experiment at 104 h. This behavior is the same at all φ
v, the only difference being that the magnitudes of both the initial step-increase in m
a(
t) and the subsequent linear slopes are larger at lower values of φ
v. Furthermore, although the magnitude of the initial step varies substantially within the 3–4 membranes tested at any single φ
v, the linear slope is more consistent at given volume fraction of dispersed phase. At the lowest φ
v of 0.032 (
Figure 2C) the value of m
a(
t)/m
d(0) reaches, at most, 0.18 = 18% of the total mass of dye in the initial donor solution, with three out of four profiles only reaching 0.07. At both the higher φ
v the maximum values reached for m
a(
t)/m
d(0) are 0.12 and 0.025. The near–linear second phase of the diffusion profiles is, therefore, a steady-state [
17].
The rapid initial step-increase cannot come from a diffusion-controlled transport process through the sintered membranes. Even if the channels were oriented horizontally straight through the membrane from the donor to the acceptor, i.e.,
τ = 1, the diffusion lag time,
tlag =
2/6D
meth, is calculated to be 1.8 h. Indeed, detailed micro-computer tomography has shown that the channels in these aligned porous membranes run in domains that are perpendicular to the direction of diffusion [
1], and the tortuosity is, therefore, expected to be larger than 1.0. This is also the impression given by the image in
Figure 1A. If we assume
τ = 5, for example, the calculated value of
tlag rises to 46 h. It is, therefore, highly unlikely that the initial step-increase in m
a(
t) comes only from diffusion through the channels. Up to 1% of the total mass of dye is already detected in the acceptor at the first sampling point of
t = 1 h.
It is plausible that the initial step-increase is a result of capillarity through the aligned porous membrane. The Laplace capillary pressure will draw the donor solution through non-wetted channels and into the acceptor as soon as the donor chamber of the diffusion cells is filled with dye solution. This behavior can be approximated by the Washburn equation [
17]. The effects of atmospheric and hydrostatic pressure can be neglected. In this case the rate of liquid movement through a cylindrical channel of radius,
r, is [
18]:
where
l(
t) is the distance penetrated into the channel after time,
t,
is the liquid/air interfacial tension,
θls is the liquid/solid contact angle and
η is the liquid dynamic viscosity. Integration of Equation (2) and solving for
t gives:
where
is the time it takes for the liquid’s meniscus to travel through the channel of length
. The channel radius is taken as 40 µm which was determined from micro computer tomography of cross sections through the aligned region of the membrane [
1]. Note that the validity of Washburn in such fine channels (down to 3 µm radius) has been demonstrated with water [
19]. The contact angle of water on sintered alumina compacts,
θls, is 37° [
20]. For an assumed tortuosity of
τ = 1 the calculated value for
using Equation (2) is 50 ms. For an assumed
τ = 5, the
lengthens to 0.86 s and for assumed
τ = 10 to 4.5 s. It is, therefore, clear that capillarity through the very fine aligned channels in the membrane is rapid. This was confirmed by observing the almost instantaneous uptake of a droplet into the surface of a membrane (result not shown). We suggest that this is the cause of the step-increase in m
a(
t) observed in the first time-samples. The capillary pressure falls to zero once the donor liquid passing through the channel reaches the acceptor solution and further dye transport can then only occur by diffusion. The reason for the non-wetting of some channels is unclear but could be caused by the complex interconnected geometry of the channel system as observed by SEM and µ
Ct [
1].
Once the near-linear steady-state phase has been reached at
t ≈ 10 h, the slopes are fairly uniform for the membranes from a given φ
v (cf.
Figure 2A–C). The values of the slope give the flux of the steady-state diffusion through the channels of the membrane, Δm
a(
t)/
AΔ
t.
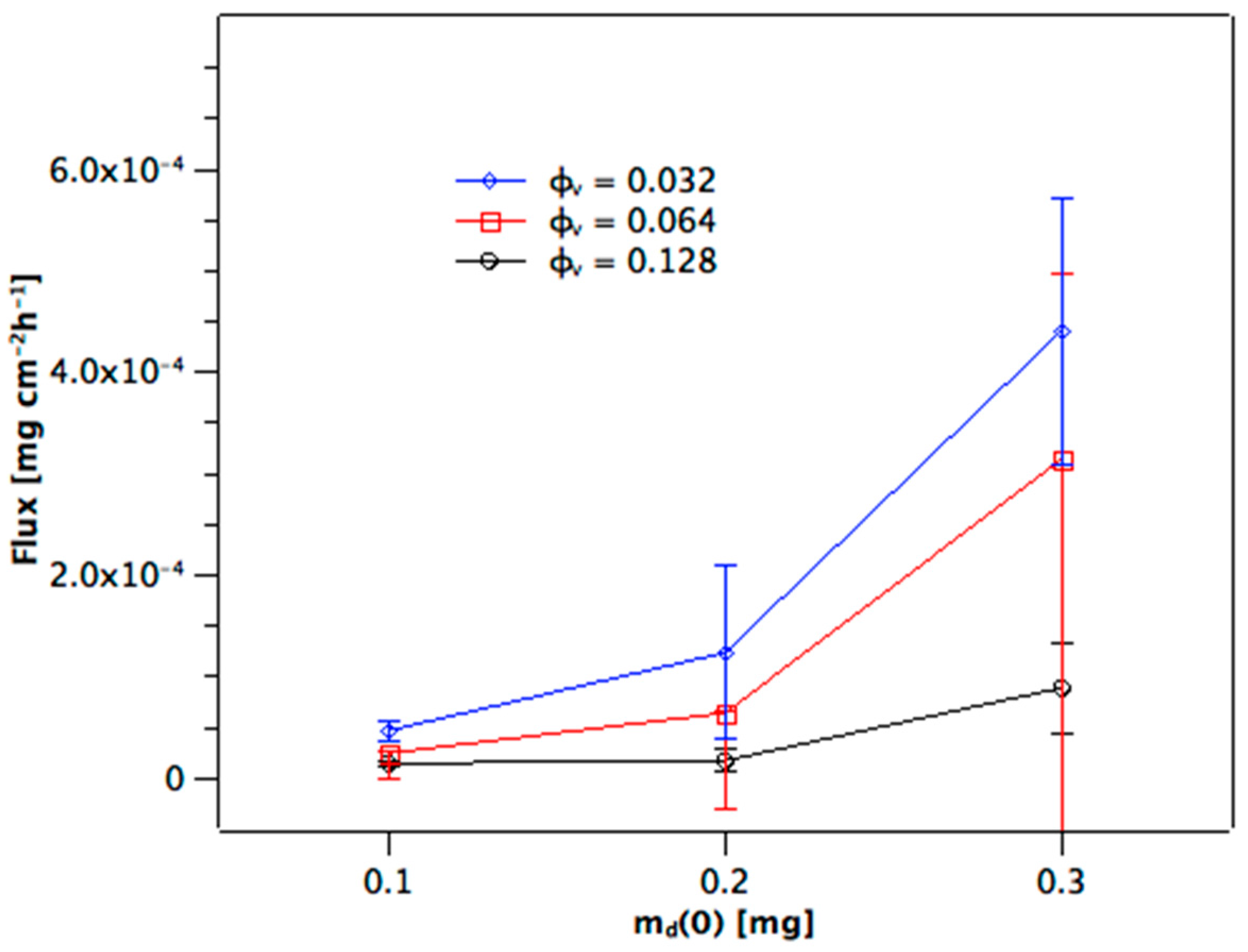
Figure 3 shows that flux increases over-proportionally at higher m
d(0), whereas this is expected to be linear from Equation (1). Additionally, the flux increases as the volume fraction of dispersed phase of the alumina colloid used to prepare the membrane is reduced. This is because by decreasing φ
v the porosity of the sintered membrane becomes larger. This must increase the effective area available for diffusion within the membrane. How it likely changes the path length, which will be discussed below.
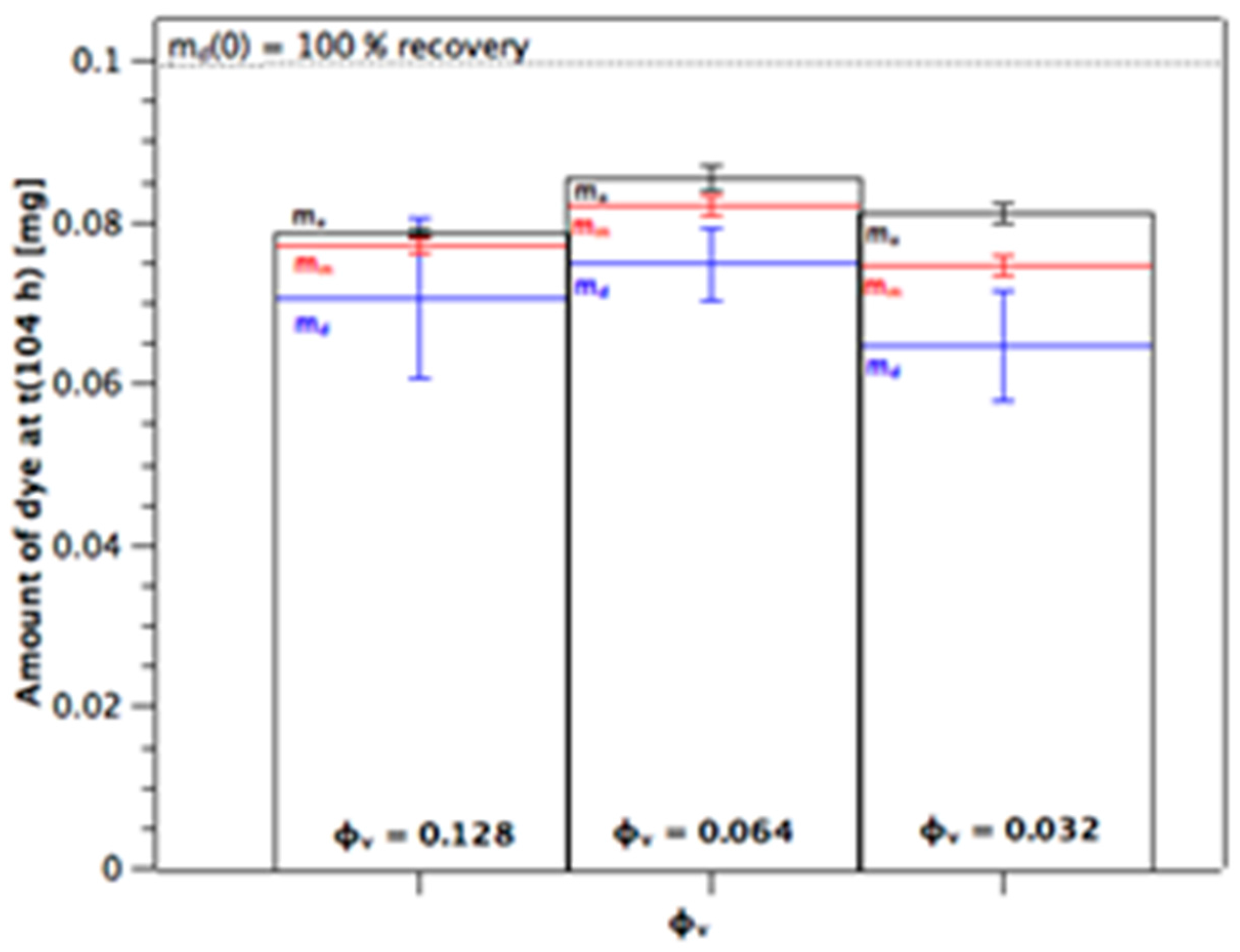
Figure 4 shows the distribution of the dye between donor (blue), membrane (red), and acceptor (black) as measured at the end of the experiment for the m
d(0) = 0.1 mg series. The amount detected at the acceptor, m
a(104 h), is larger as the volume fraction decreases because of the higher flux (cf.
Figure 3). There is also a slight trend to higher amounts of dye in the membrane as the volume fraction decreases which would be the case for higher porosity. Note that the total recoveries lie below 100%. We suggest that the reason for this is the very high relative donor concentration; a small analytical error in m
d(104 h) represents a high amount of dye compared to the amounts present in membrane and acceptor. The recoveries with both the m
d(0) = 0.2 mg and 0.3 mg series were 90%–105% (not shown), with the distributions being very similar to that shown in
Figure 4 for the m
d(0) = 0.1 mg series.
Note that the position of the membrane held between the two halves of the diffusion cell will result in radial diffusion of the dye out to the edge regions of the membrane that lie outside of the rubber sealant ring and have no direct contact to donor or receiver solutions (cf.
Figure 5B). Although this effect will increase the diffusional lag-time,
tlag, especially for a thick membrane, such as that used here (
L = 0.5 cm), its effects on steady-state flux will be marginal [
21]. Equation (1) is now used to calculate the effective tortuosity
τ* = (
α/
τ)
−1 from the measured values of Δm
a(
t)/Δ
t. Recall that
τ* is the factor by which the solute flux through a tortuous membrane is reduced compared with that through an isotropic membrane [
15], i.e.,
The value for D
meth of methylene blue in water was taken from the literature.
Table 1 gives the calculated values for
τ* that were each measured at three donor loadings, m
d(0), and at the different φ
v.
The results for
τ* illustrate a couple of points. First, the values for
τ* are substantially higher than unity. It follows that either the tortuosity in the membrane,
τ, is >1.0, or the surface area factor,
α, <1.0, or a combination of both factors exists. Scanning electron microscopy has shown a parallel alignment of channels and solid walls through the membrane from top to bottom [
1]. When cut and placed within the diffusion cell, the direction of solute diffusion is perpendicular to the run of the channels. Since the sintered alumina walls may be assumed to be impermeable, it follows that the diffusional path-length through the membrane must be longer than the membrane’s outer thickness. The presence of these walls between the channels must also reduce the area available for diffusion within the membrane. The structural information available from SEM and µCT suggests, therefore, that the values for
τ* are a combination of both path length and area factors.
Secondly, for the membranes prepared from a higher volume fraction of dispersed phase, φ
v, the values for
τ* are greater. The µCT data for these membranes showed that higher φ
v produced thicker walls between the channels [
1]. This effect as also been observed for alumina slurries that were freeze cast between two cold-fingers [
22] rather than vacuum-induced surface frozen, as in the current work. If the walls between the channels become thicker, volume constraints mean that the diffusion path length through the channels cannot increase. It follows that
cannot be larger at higher φ
v; if anything, it should become smaller. It seems plausible that higher φ
v decreases primarily the value of
=
A*/
A, with the result that
τ* becomes larger. Indeed, the SEM data showed that higher φ
v produced denser walls between the channels, which have fewer perforations and cracks than at lower alumina concentrations [
7].