Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Analysis Coupled with FTIR
2.2. Cone Calorimeter
2.3. Elemental Analysis
2.4. Materials
2.4.1. Flame Retardants
2.4.2. Resin Systems
3. Results and Discussion
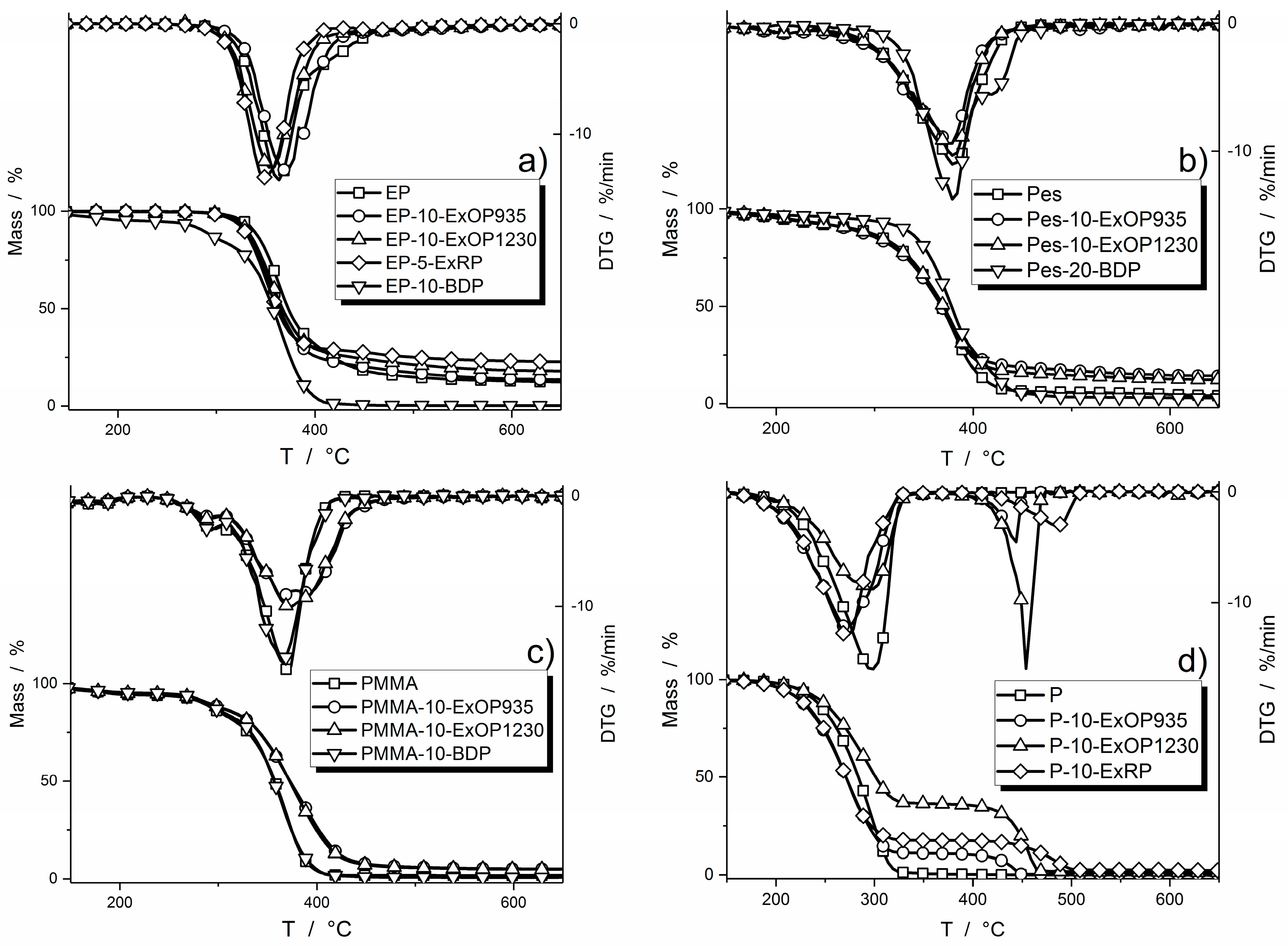
3.1. Thermogravimetric Analysis Results
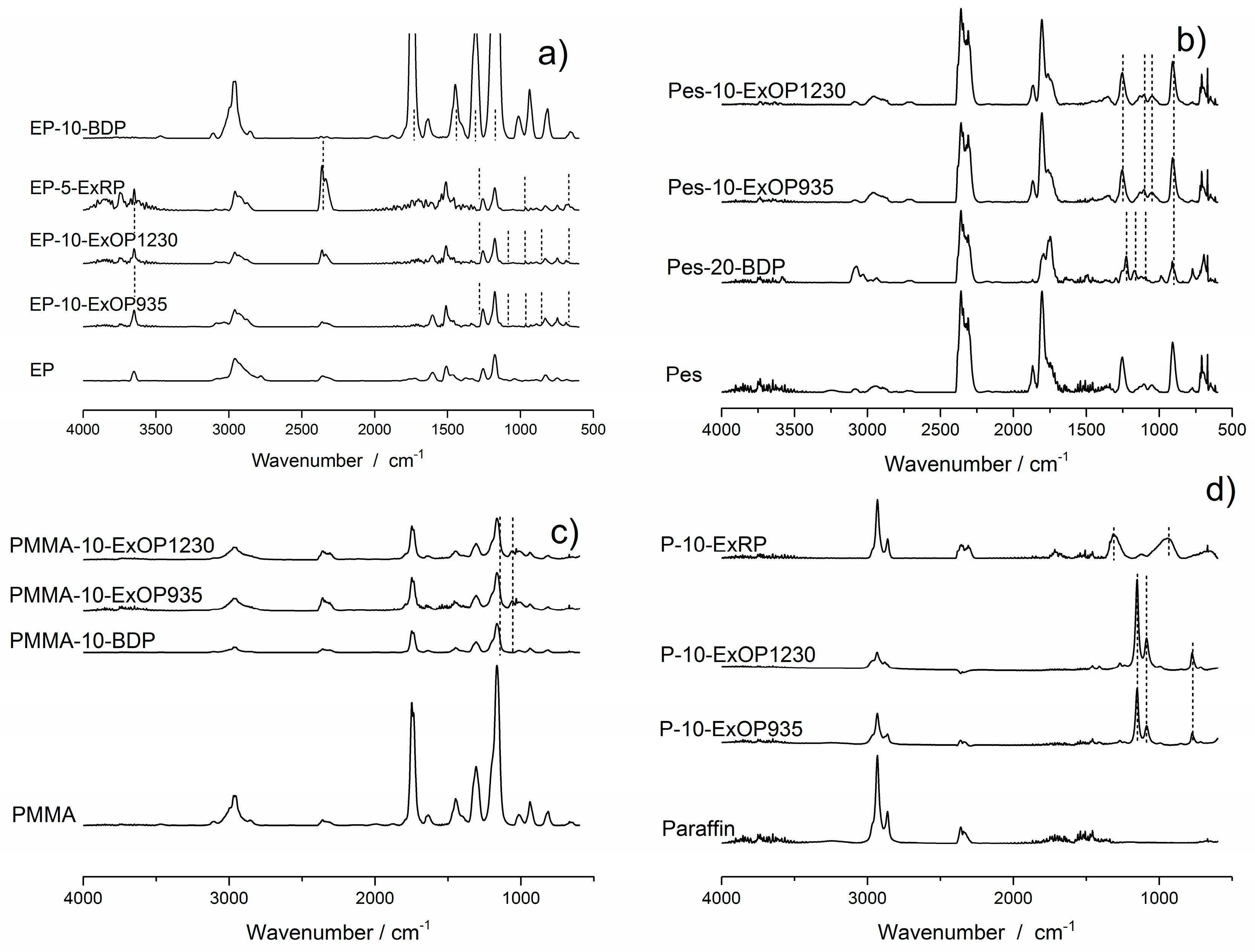
3.2. FTIR Spectra of the Gaseous Phase
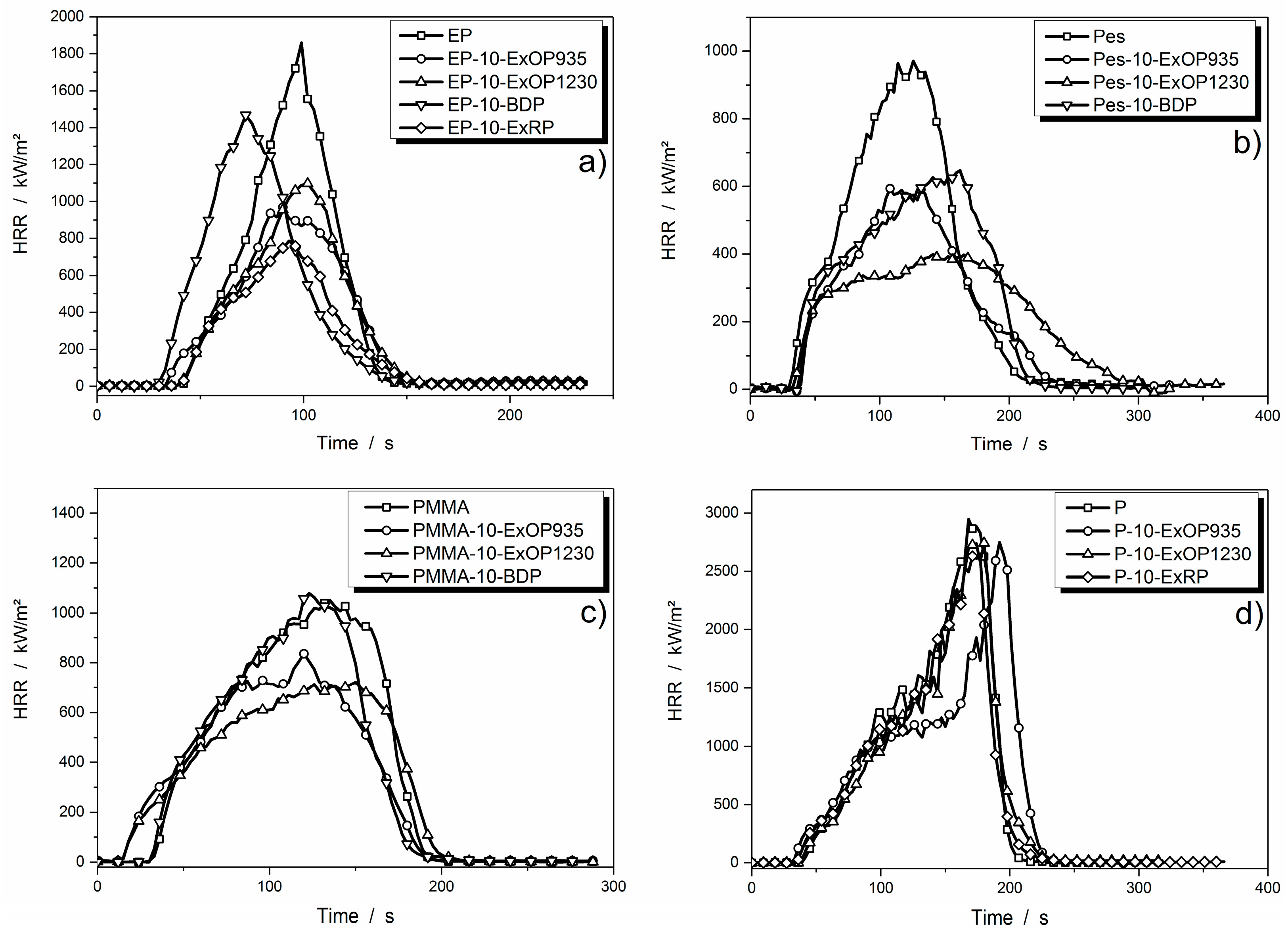
3.3. Forced Flaming Fire Behavior: Cone Calorimeter
3.3.1. Comparison of Heat Release Rates
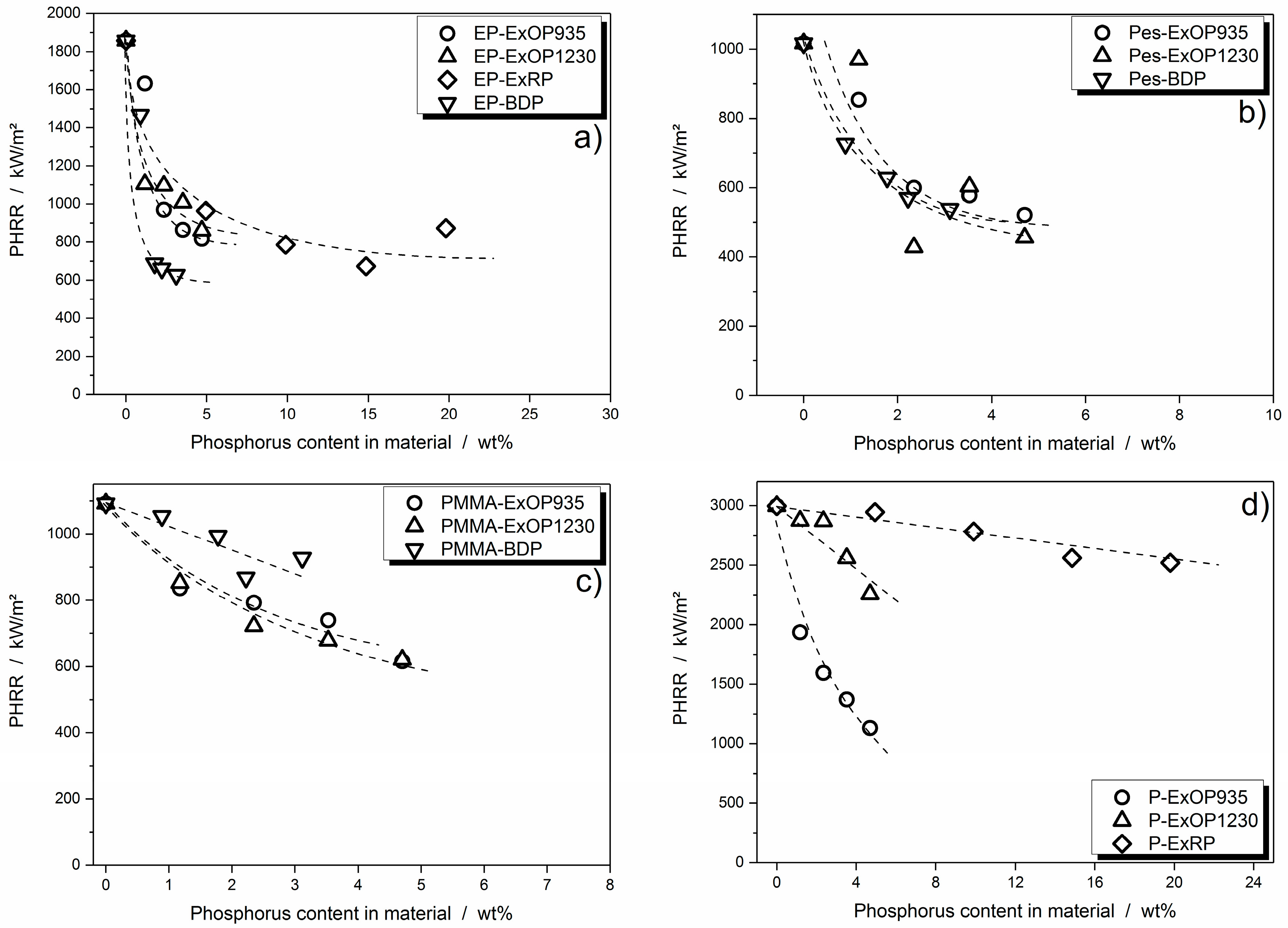
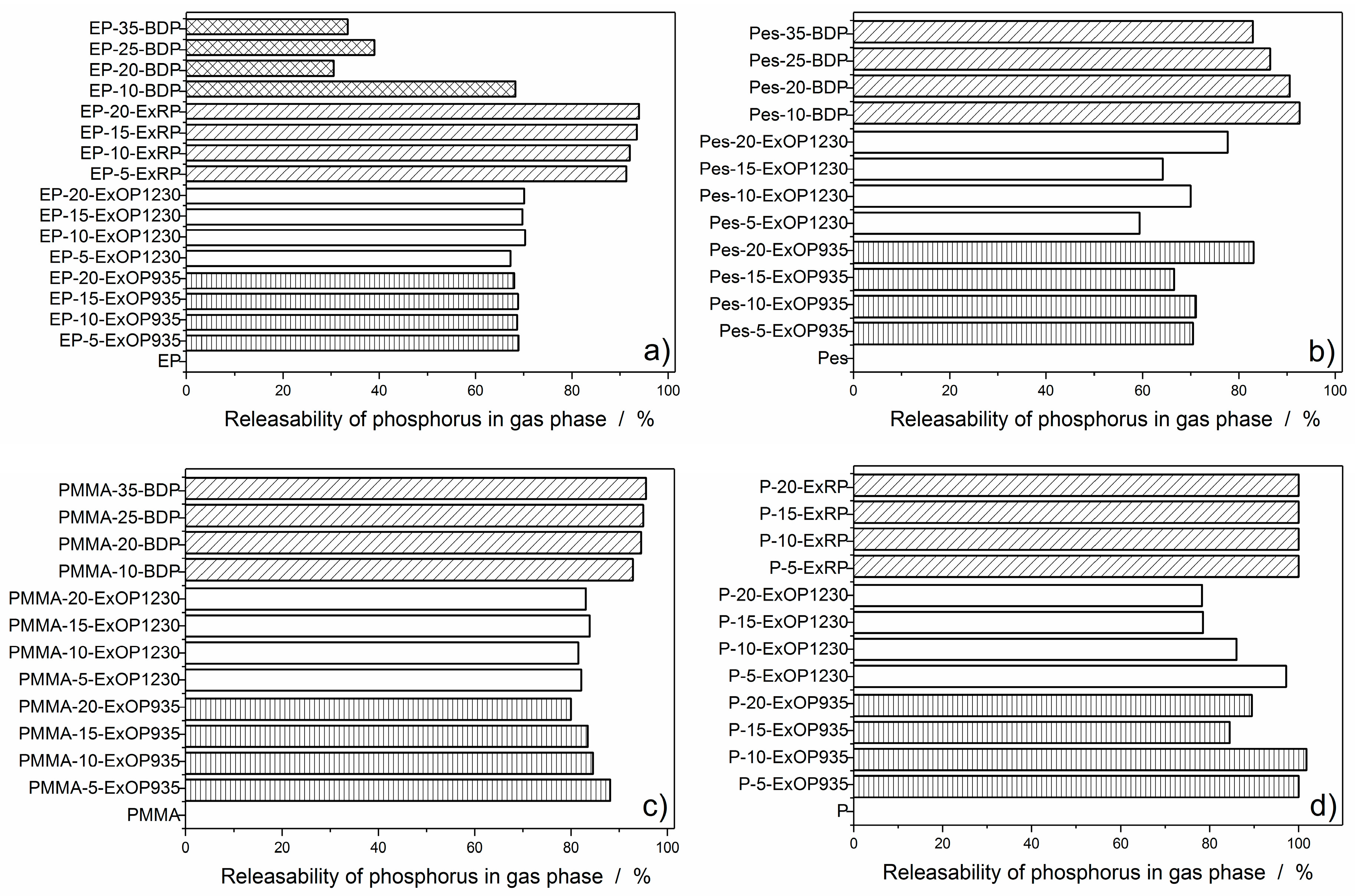
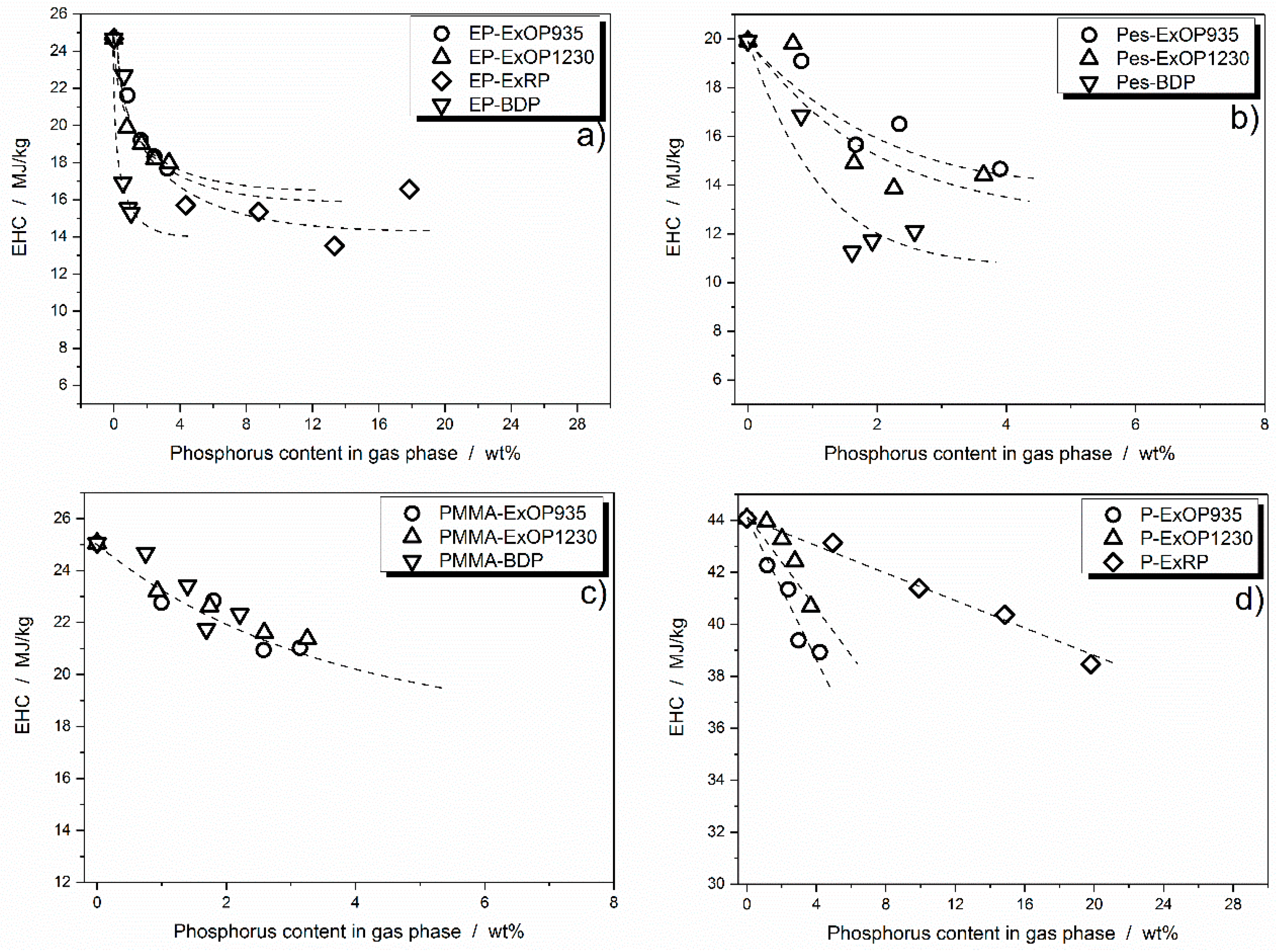
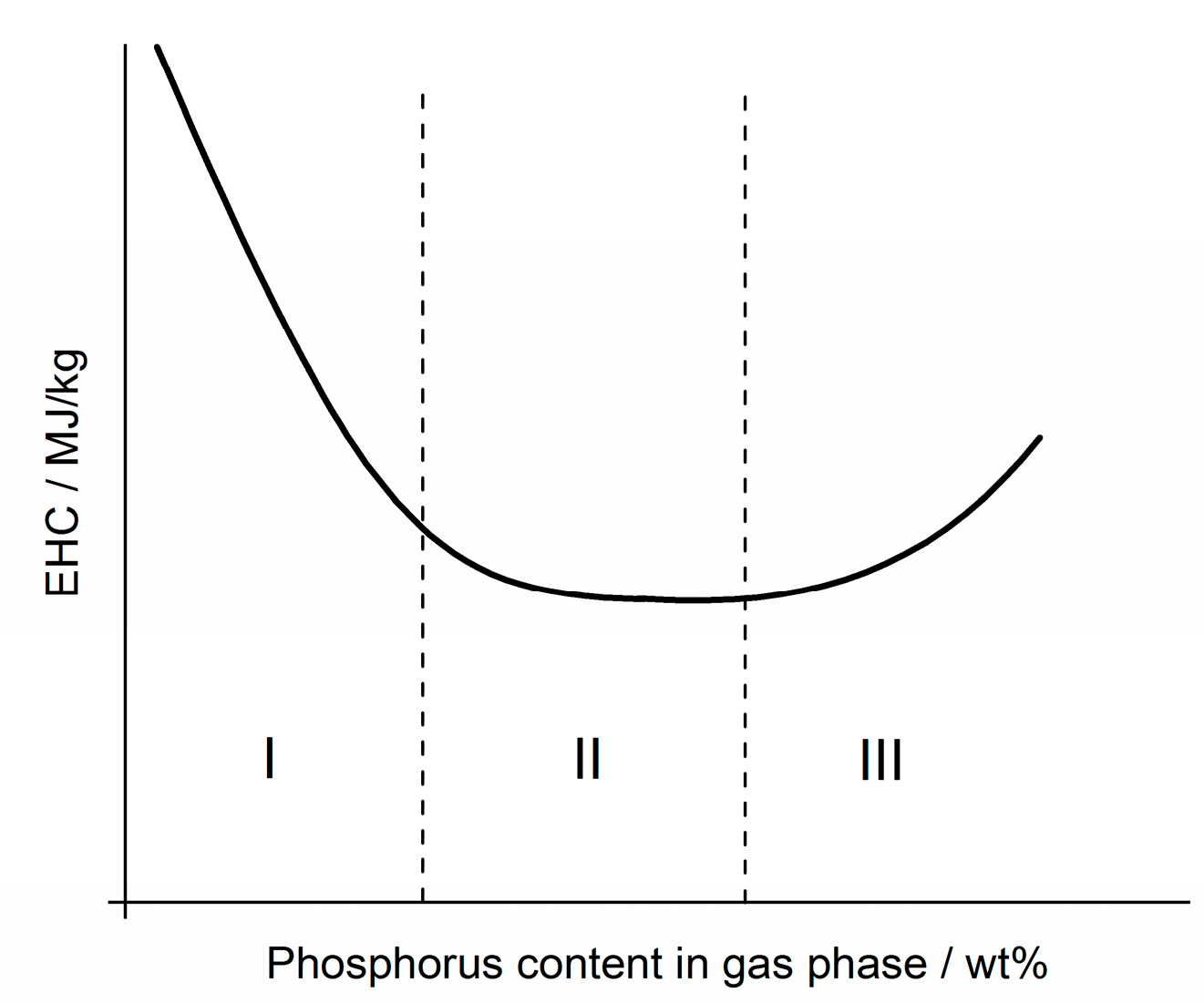
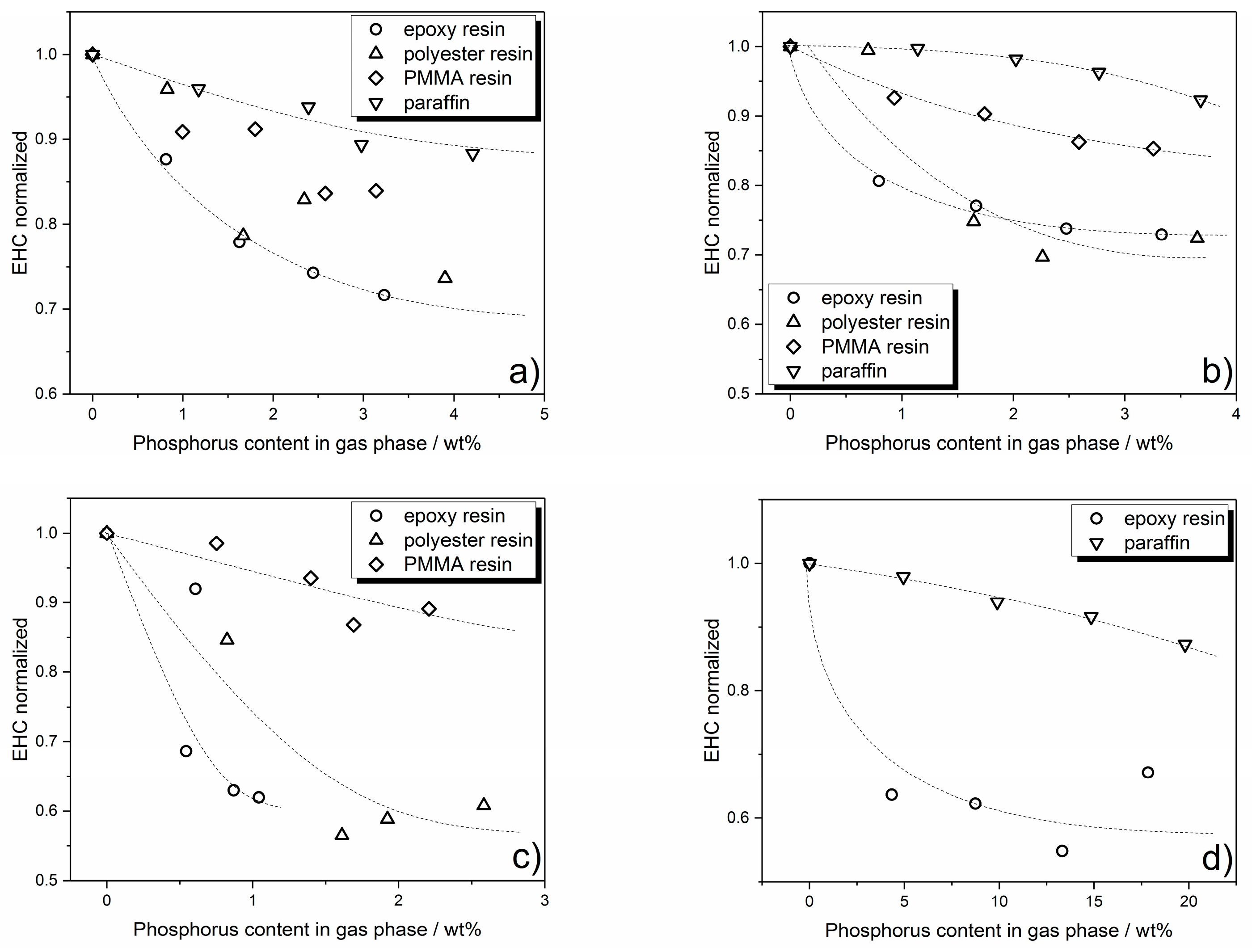
3.3.2. Flame Inhibition Dependency on Phosphorus Content Released into Gas Phase
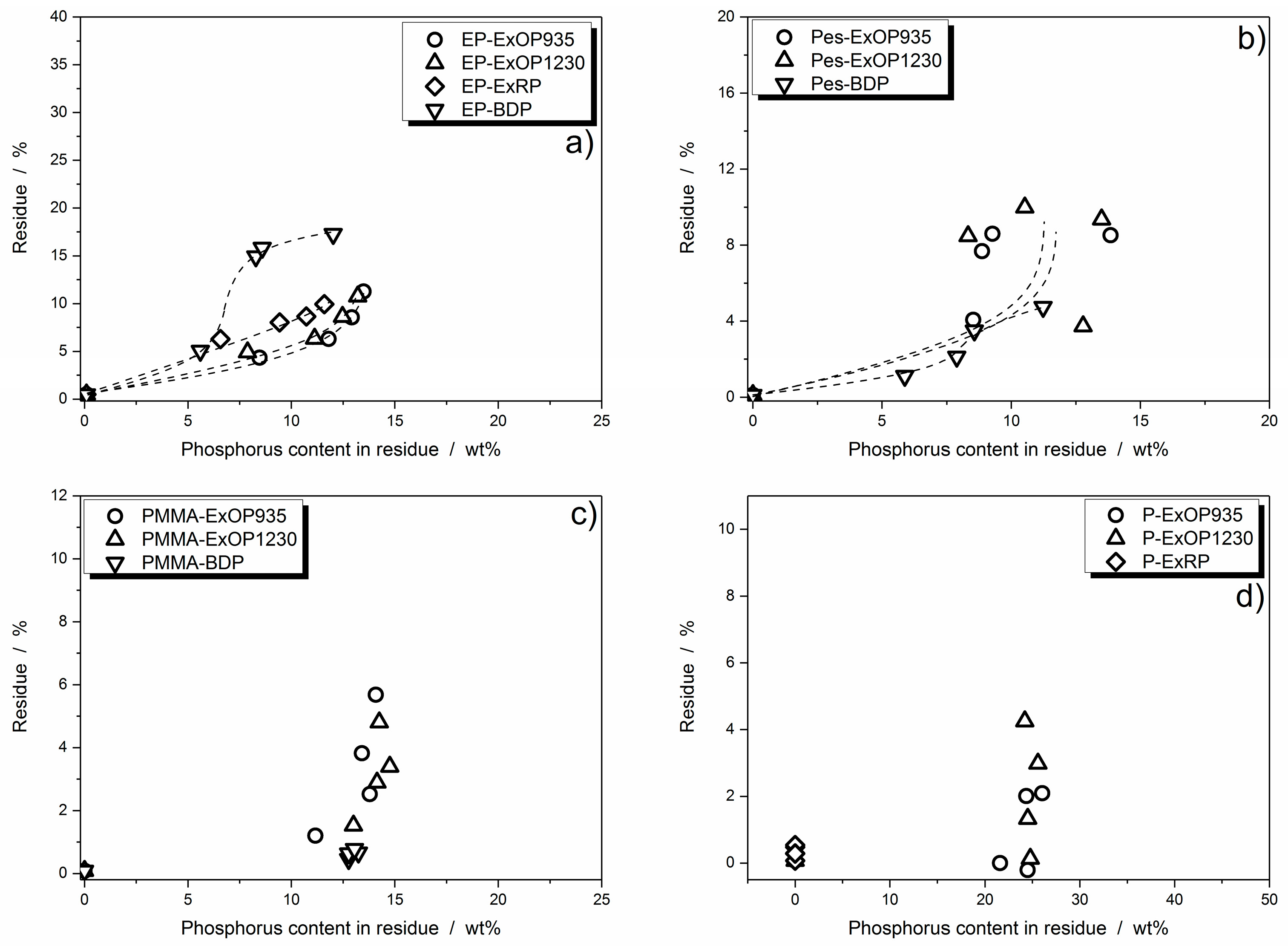
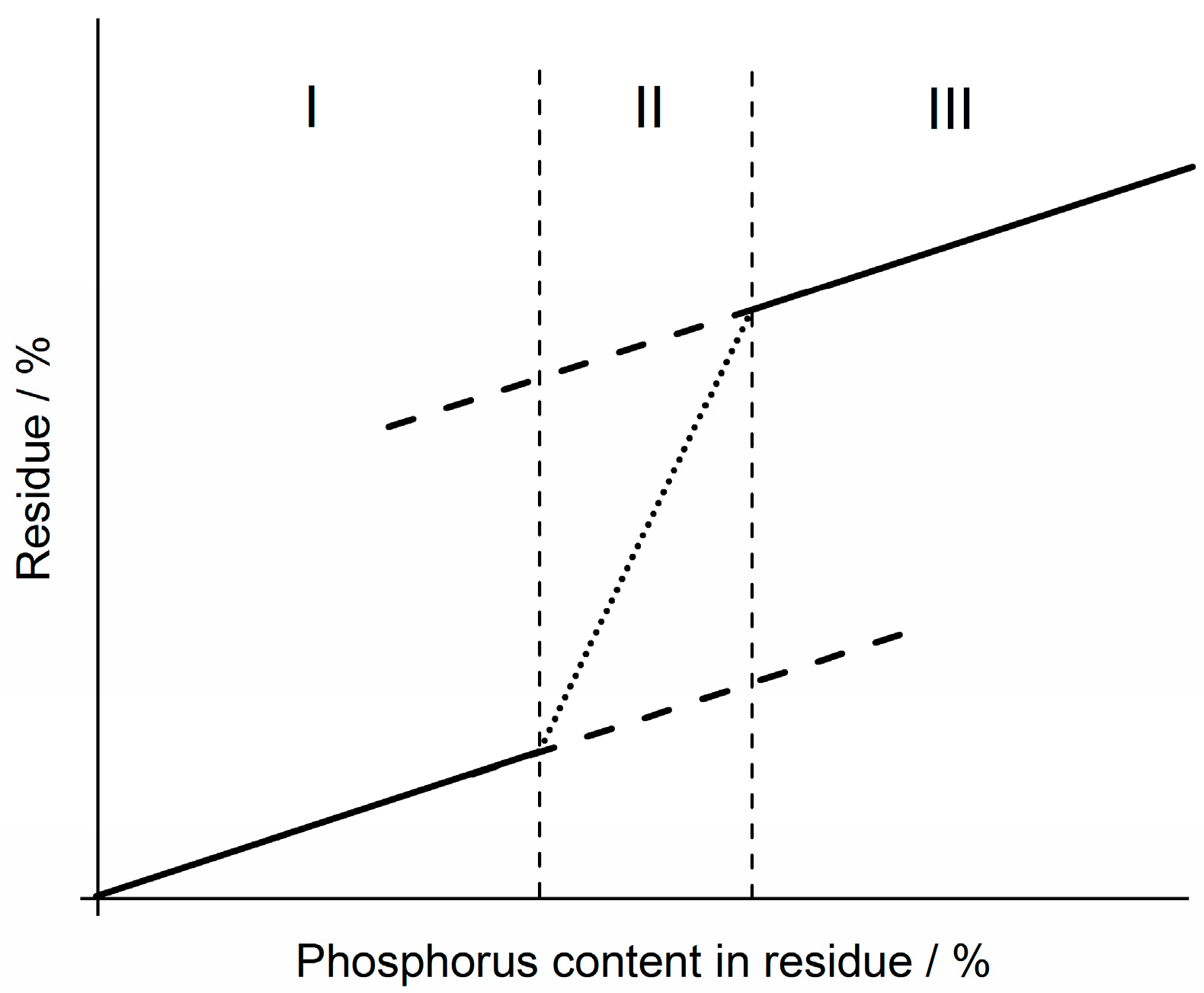
3.3.3. Dependency of Condensed Phase Activity on Phosphorus Content
3.4. Quantification of Cone Calorimeter Results
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Chigwada, G.; Gilman, J.W.; Lyon, R.E. High-throughput techniques for the evaluation of fire retardancy. J. Mater. Chem. 2006, 16, 2023–2029. [Google Scholar] [CrossRef]
- Rabe, S.; Schartel, B. The Rapid Mass Calorimeter: A route to high throughput fire testing. Fire Mater. 2016. [Google Scholar] [CrossRef]
- Rabe, S.; Schartel, B. The rapid mass calorimeter: Understanding reduced-scale fire test results. Polym. Test. 2017, 57, 165–174. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame retardants for plastics and textiles. In Practical Applications, 2nd ed.; Carl Hansen Verlag: Munich, Germany, 2015. [Google Scholar]
- Levchik, S.V. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Developments in phosphorus flame retardants. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2008; pp. 41–66. [Google Scholar]
- Brauman, S.K. Phosphorus fire retardants in polymers. 1. General mode of action. J. Fire Retard. Chem. 1977, 4, 18–37. [Google Scholar]
- Hastie, J.W. Molecular basis of flame inhibition. J. Res. Natl. Stand. Sec. A 1973, 77A, 733–754. [Google Scholar] [CrossRef]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykov, Y.; Döring, M. Phosphorus Polyester Versus Aluminium Phosphinate in Poly(butylene terephthalate) (PBT): Flame Retardancy Performance and Mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Levchick, S.V. Introduction to flame retardancy and polymer flammability. In Flame Retardant Polymer Nanocomposites; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; Chapter 1; pp. 1–29. [Google Scholar]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S. Intumescent materials. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2001; Chapter 10; pp. 318–336. [Google Scholar]
- Brauman, S.K. Phosphorus fire retardance in polymers. 2. Retardant-polymer substrate interactions. J. Fire Retard. Chem. 1977, 4, 38–58. [Google Scholar]
- Braun, U.; Schartel, B. Effect of Red Phosphorus and Melamine Polyphosphate on the Fire Behavior of HIPS. J. Fire Sci. 2005, 23, 5–30. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Cyanurate in Glass-Fibre.Reinforced Poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Langfeld, K.; Wilke, A.; Sut, A.; Greiser, A.; Ulmer, B.; Andrievici, V.; Limbach, P.; Bastian, M.; Schartel, B. Halogen-free fire retardant styrene–ethylene–butylene–styrene-based thermoplastic elastomers using synergistic aluminum diethylphosphinate-based combinations. J. Fire Sci. 2015, 33, 157–177. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Braun, U.; Russo, P.; Acierno, D. Fire Retardant Synergisms between Nanometric Fe2O3 and Aluminium Phosphinate in Poly(butylene terephthalate). Polym. Adv. Technol. 2011, 22, 2382–2391. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame Retardant Biocomposites: Synergism between Phosphinate and Nanometric Metal Oxides. Eur. Polym. J. 2011, 47, 1390–1401. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly(1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Lyons, J.W. Mechanisms of Fire Retardation with Phosphorus Compound: Some Speculations. J. Fire Flam. 1970, 1, 302–311. [Google Scholar]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Influence of the Oxidation State of Phosphorus on the Decomposition and Fire Behaviour of Flame-Retarded Epoxy Resin Composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Thompson, C.M.; Smith, J.G., Jr.; Connell, J.W.; Hinkley, J.A.; Lyon, R.E.; Moulton, R. Flame retardant aircraft epoxy resins containing phosphorus. Polymer 2005, 46, 5012–5024. [Google Scholar] [CrossRef]
- Perret, B.; Pawlowski, K.H.; Schartel, B. Fire Retardancy Mechanisms of Arylphosphates in Polycarbonate (PC) and PC/Acrylonitrile-Butadiene-Styrene: The Key Role of Decomposition Temperature. J. Therm. Anal. Calorim. 2009, 97, 949–958. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol-A bis(diphenylphosphate) in polycarbonate/acrylonitrile-butadiene-styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- Wawrzyn, E.; Schartel, B.; Ciesielski, M.; Kretzschmar, B.; Braun, U.; Döring, M. Are Novel Aryl Phosphates Competitors for Bisphenol A bis(diphenyl phosphate) in Halogen-free Flame-retarded Polycarbonate/Acrylonitrile-butadiene-styrene Blends? Eur. Polym. J. 2012, 48, 1561–1574. [Google Scholar] [CrossRef]
- Schartel, B.; Kunze, R.; Neubert, D. Red phosphorus-controlled decomposition for fire retardant PA 66. J. Appl. Polym. Sci. 2002, 83, 2060–2071. [Google Scholar] [CrossRef]
- Schartel, B.; Balabanovich, A.I.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J. Appl. Polym. Sci. 2007, 104, 2260–2269. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Brehme, S.; Köppl, T.; Schartel, B.; Altstädt, V. Competition in aluminium phosphinate-based halogen-free flame retardancy of poly(butylene terephthalate) and its glass-fibre composites. E-Polymers 2014, 14, 193–208. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, J.Y.; Sun, S.; Chen, J. A New Type of Flame Retarded Epoxy Resin Based on Metal Phosphinates. Polym. Polym. Compos. 2012, 20, 151–154. [Google Scholar]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2017, 92, 1528–1545. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. E-Polymers 2010, 10, 443–456. [Google Scholar]
- Seefeldt, H.; Duemichen, E.; Braun, U. Flame retardancy of glass fiber reinforced high temperature polyamide by use of aluminium diethylphosphinate: Thermal and thermo-oxidative effects. Polym. Int. 2013, 62, 1608–1616. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resin; Blackie Academic & Professional: London, UK, 1993. [Google Scholar]
- Pham, H.Q.; Marks, M.J. Epoxy Resins; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Täuber, K.; Marsico, F.; Wurm, F.R.; Schartel, B. Hyperbranched poly(phosphoester)s as flame retardants for technical and high performance polymers. Polym. Chem. 2014, 5, 7042–7053. [Google Scholar] [CrossRef]
- Sut, A.; Greiser, S.; Jäger, C.; Schartel, B. Interactions in multicomponent flame-retardant polymers: Solid-state NMR identifying the chemistry behind it. Polym. Degrad. Stab. 2015, 121, 116–125. [Google Scholar] [CrossRef]
- Duquesne, S.; Fontaine, G.; Cérin-Delaval, O.; Gardelle, B.; Tricot, G.; Bourbigot, S. Study of the thermal degradation of an aluminium phosphinate-aluminium trihydrate combination. Thermochim. Acta 2013, 551, 175–183. [Google Scholar] [CrossRef]
- Sonnier, R.; Viretto, A.; Dumazert, L.; Gallard, B. A method to study the two-step decomposition of binary blends in cone calorimeter. Combust. Flame 2016, 169, 1–10. [Google Scholar] [CrossRef]
- Levchik, G.F.; Levchik, S.V.; Camino, G.; Weil, E.D. Fire retardant action of red phosphorus in Nylon 6. In Fire Retardancy of Polymers the Use of Intumescence; Le Bras, M., Camino, G., Bourbigot, S., Delobel, R., Eds.; The Royal Society of Chemistry: London, UK, 1998; pp. 304–315. [Google Scholar]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Eckel, T. The most important flame retardant plastics. In Plastics Flammability Handbook; Troitzsch, J, Ed.; Hanser: Munich, Germany, 2004; pp. 158–172. [Google Scholar]
- Brehme, S.; Köppl, T.; Schartel, B.; Fischer, O.; Altstädt, V.; Pospiech, D.; Döring, M. Phosphorus Polyester—An Alternative to Low-Molecular-Weight Flame Retardants in Poly(Butylene Terephthalate)? Macromol. Chem. Phys. 2012, 213, 2386–2397. [Google Scholar] [CrossRef]
- Pappalardo, S.; Russo, P.; Acierno, D.; Rabe, S.; Schartel, B. The synergistic effect of organically modified sepiolite in intumescent flame retardant polypropylene. Eur. Polym. J. 2016, 76, 196–207. [Google Scholar] [CrossRef]



| Exolit OP935 | Exolit OP1230 | Exolit RP607 | BDP | |
|---|---|---|---|---|
| DGEBA/IPDA | EP-x-ExOP935 | EP-x-ExOP1230 | EP-x-ExRP | EP-x-BDP |
| PMMA-resin | PMMA-x-ExOP935 | PMMA-x-ExOP1230 | --- | PMMA-x-BDP |
| Polyester-resin | Pes-x-ExOP935 | Pes-x-ExOP1230 | --- | Pes-x-BDP |
| Paraffin | P-x-ExOP935 | P-x-ExOP1230 | P-x-ExRP | --- |
| Material | T5% ML | TDTGmax | Residue |
|---|---|---|---|
| °C | °C | wt % | |
| EP | 326 ± 1 | 360 ± 2 | 10.7 ± 0.1 |
| EP-10-ExOP935 | 320 ± 1 | 355 ± 1 | 12.1 ± 0.3 |
| EP-10-ExOP1230 | 320 ± 2 | 353 ± 1 | 12.1 ± 0.3 |
| EP-5-ExRP | 318 ± 1 | 349 ± 1 | 17.0 ± 0.2 |
| EP-10-BDP | 265 ± 1 | 365 ± 1 | 3.0 ± 0.1 |
| Pes | 204 ± 1 | 380 ± 2 | 2.9 ± 0.1 |
| Pes-10-ExOP935 | 211 ± 1 | 378 ± 1 | 14.0 ± 0.4 |
| Pes-10-ExOP1230 | 210 ± 1 | 382 ± 2 | 11.7 ± 0.2 |
| Pes-20-BDP | 277 ± 2 | 380 ± 2 | 2.6 ± 0.1 |
| PMMA | 192 ± 1 | 370 ± 2 | 1.7 ± 0.1 |
| PMMA-10-ExOP935 | 229 ± 2 | 368 ± 1 | 4.6 ± 0.2 |
| PMMA-10-ExOP1230 | 228 ± 1 | 370 ± 2 | 4.5 ± 0.1 |
| PMMA-10-BDP | 246 ± 2 | 364 ± 1 | 0.7 ± 0.1 |
| P | 223 ± 1 | 296 ± 1 | 0.1 ± 0.1 |
| P-10-ExOP935 | 207 ± 1 | 274 ± 1 | 0.2 ± 0.1 |
| P-10-ExOP1230 | 225 ± 2 | 454 ± 3 | 1.4 ± 0.2 |
| P-10-ExRP | 207 ± 1 | 273 ± 1 | 2.1 ± 0.2 |
| System | tig | PHRR | THE | EHC | Residue at End of Test |
|---|---|---|---|---|---|
| s | kW/m2 | MJ/m2 | MJ/kg | wt % | |
| EP | 39 ± 1 | 1858 ± 41 | 81.3 ± 0.1 | 24.7 ± 0.1 | 0.5 ± 0.0 |
| EP-5-ExOP935 | 30 ± 1 | 1632 ± 4 | 71.1 ± 2.1 | 21.6 ± 0.4 | 4.3 ± 0.3 |
| EP-10-ExOP935 | 32 ± 1 | 969 ± 57 | 59.0 ± 1.3 | 19.2 ± 0.3 | 6.3 ± 0.3 |
| EP-15-ExOP935 | 30 ± 1 | 864 ± 9 | 57.0 ± 0.3 | 18.3 ± 0.0 | 8.6 ± 0.3 |
| EP-20-ExOP935 | 30 ± 0 | 817 ± 25 | 52.3 ± 0.1 | 17.7 ± 0.1 | 11.3 ± 0.6 |
| EP-5-ExOP1230 | 31 ± 2 | 1105 ± 52 | 71.6 ± 0.3 | 19.9 ± 1.4 | 4.9 ± 0.1 |
| EP-10-ExOP1230 | 32 ± 1 | 1097 ± 50 | 61.1 ± 0.1 | 19.0 ± 0.0 | 6.3 ± 0.2 |
| EP-15-ExOP1230 | 30 ± 0 | 1007 ± 16 | 58.9 ± 0.8 | 18.2 ± 0.2 | 8.6 ± 0.2 |
| EP-20-ExOP1230 | 31 ± 2 | 861 ± 2 | 54.0 ± 0.3 | 18.0 ± 0.1 | 10.8 ± 0.0 |
| EP-5-ExRP | 29 ± 1 | 964 ± 16 | 51.1 ± 1.9 | 15.7 ± 0.3 | 6.3 ± 0.1 |
| EP-10-ExRP | 33 ± 1 | 786 ± 24 | 47.2 ± 1.0 | 15.4 ± 0.4 | 8.0 ± 0.1 |
| EP-15-ExRP | 34 ± 2 | 673 ± 125 | 42.4 ± 0.9 | 13.5 ± 0.4 | 8.7 ± 0.5 |
| EP-20-ExRP | 32 ± 2 | 873 ± 56 | 50.1 ± 0.4 | 16.6 ± 0.3 | 9.9 ± 1.5 |
| EP-10-BDP | 25 ± 3 | 1468 ± 44 | 74.0 ± 4.1 | 22.7 ± 1.4 | 5.0 ± 0.3 |
| EP-20-BDP | 26 ± 1 | 688 ± 3 | 49.0 ± 0.7 | 16.9 ± 0.1 | 14.9 ± 1.1 |
| EP-25-BDP | 22 ± 2 | 660 ± 1 | 45.0 ± 0.3 | 15.5 ± 0.1 | 15.8 ± 1.7 |
| EP-35-BDP | 28 ± 1 | 628 ± 3 | 42.5 ± 0.6 | 15.3 ± 0.3 | 17.3 ± 0.4 |
| Pes | 26 ± 1 | 1017 ± 16 | 94.3 ± 2.9 | 19.9 ± 0.9 | 0.1 ± 0.1 |
| Pes-5-ExOP935 | 33 ± 2 | 854 ± 38 | 85.5 ± 0.4 | 19.1 ± 0.1 | 4.1 ± 0.2 |
| Pes-10-ExOP935 | 32 ± 1 | 599 ± 6 | 64.5 ± 1.3 | 15.7 ± 0.1 | 7.7 ± 0.5 |
| Pes-15-ExOP935 | 36 ± 2 | 577 ± 29 | 70.9 ± 0.0 | 16.5 ± 0.2 | 8.5 ± 0.3 |
| Pes-20-ExOP935 | 34 ± 1 | 521 ± 15 | 60.9 ± 3.2 | 14.7 ± 0.6 | 8.6 ± 0.5 |
| Pes-5-ExOP1230 | 31 ± 2 | 969 ± 33 | 89.8 ± 0.1 | 19.8 ± 0.1 | 3.7 ± 0.3 |
| Pes-10-ExOP1230 | 32 ± 5 | 428 ± 27 | 69.4 ± 2.2 | 14.9 ± 2.0 | 8.5 ± 0.1 |
| Pes-15-ExOP1230 | 30 ± 2 | 603 ± 37 | 73.4 ± 4.0 | 13.9 ± 2.3 | 9.4 ± 0.4 |
| Pes-20-ExOP1230 | 33 ± 1 | 456 ± 8 | 61.2 ± 1.7 | 14.4 ± 0.4 | 10.0 ± 0.1 |
| Pes-10-BDP | 35 ± 1 | 727 ± 80 | 77.8 ± 1.4 | 16.9 ± 0.3 | 1.1 ± 0.4 |
| Pes-20-BDP | 32 ± 1 | 629 ± 63 | 66.5 ± 0.7 | 11.3 ± 3.2 | 2.1 ± 0.5 |
| Pes-25-BDP | 37 ± 6 | 570 ± 17 | 59.6 ± 0.6 | 11.7 ± 1.7 | 3.5 ± 0.5 |
| Pes-35-BDP | 34 ± 5 | 538 ± 49 | 55.9 ± 1.4 | 12.1 ± 0.4 | 4.8 ± 0.3 |
| PMMA | 32 ± 1 | 1051 ± 41 | 106.9 ± 0.6 | 25.1 ± 0.5 | 0.1 ± 0.0 |
| PMMA-5-ExOP935 | 26 ± 1 | 836 ± 1 | 93.7 ± 2.5 | 22.8 ± 0.7 | 1.2 ± 0.2 |
| PMMA-10-ExOP935 | 25 ± 1 | 792 ± 21 | 88.8 ± 0.5 | 22.8 ± 0.2 | 2.5 ± 0.1 |
| PMMA-15-ExOP935 | 24 ± 1 | 740 ± 37 | 83.8 ± 0.3 | 20.9 ± 0.1 | 3.8 ± 0.0 |
| PMMA-20-ExOP935 | 22 ± 1 | 615 ± 33 | 81.8 ± 1.0 | 21.0 ± 0.4 | 5.7 ± 0.5 |
| PMMA-5-ExOP1230 | 23 ± 1 | 852 ± 20 | 97.0 ± 1.0 | 23.2 ± 1.8 | 1.5 ± 0.1 |
| PMMA-10-ExOP1230 | 22 ± 0 | 722 ± 16 | 89.2 ± 1.1 | 22.6 ± 0.4 | 2.9 ± 0.0 |
| PMMA-15-ExOP1230 | 24 ± 2 | 678 ± 63 | 86.6 ± 3.0 | 21.6 ± 0.6 | 3.4 ± 0.1 |
| PMMA-20-ExOP1230 | 26 ± 2 | 620 ± 4 | 82.8 ± 0.6 | 21.4 ± 04 | 4.8 ± 0.5 |
| PMMA-10-BDP | 29 ± 0 | 1079 ± 25 | 108.4 ± 0.3 | 24.7 ± 2.3 | 0.5 ± 0.1 |
| PMMA-20-BDP | 31 ± 1 | 994 ± 38 | 97.4 ± 0.9 | 23.4 ± 0.4 | 0.6 ± 0.0 |
| PMMA-25-BDP | 28 ± 1 | 868 ± 48 | 91.5 ± 1.9 | 21.7 ± 0.1 | 0.7 ± 0.1 |
| PMMA-35-BDP | 28 ± 1 | 928 ± 19 | 93.0 ± 0.2 | 22.3 ± 0.4 | 0.8 ± 0.1 |
| P | 40 ± 3 | 2996 ± 24 | 210.1 ± 2.9 | 44.1 ± 0.5 | 0.1 ± 0.0 |
| P-5-ExOP935 | 32 ± 1 | 1935 ± 167 | 198.8 ± 7.1 | 42.3 ± 0.7 | 0.0 ± 0.0 |
| P-10-ExOP935 | 27 ± 2 | 1593 ± 96 | 189.9 ± 27.1 | 41.4 ± 1.0 | 0.0 ± 0.2 |
| P-15-ExOP935 | 23 ± 1 | 1371 ± 101 | 183.8 ± 1.3 | 39.4 ± 0.1 | 2.1 ± 0.3 |
| P-20-ExOP935 | 23 ± 2 | 1130 ± 69 | 187.6 ± 3.7 | 38.9 ± 0.4 | 1 ± 0.6 |
| P-5-ExOP1230 | 37 ± 2 | 2872 ± 318 | 197.2 ± 1.5 | 44.0 ± 0.2 | 0.1 ± 0.1 |
| P-10-ExOP1230 | 42 ± 6 | 2868 ± 123 | 209.9 ± 2.9 | 43.3 ± 0.3 | 1.3 ± 0.8 |
| P-15-ExOP1230 | 29 ± 1 | 2559 ± 223 | 195.8 ± 3.5 | 42.4 ± 0.7 | 2.9 ± 0.3 |
| P-20-ExOP1230 | 27 ± 4 | 2257 ± 67 | 188.6 ± 12.6 | 40.7 ± 1.6 | 4.3 ± 1.0 |
| P-5-ExRP | 33 ± 1 | 2945 ± 13 | 212.5 ± 10.9 | 43.2 ± 0.2 | 0.5 ± 0.5 |
| P-10-ExRP | 34 ± 1 | 2781 ± 150 | 201.1 ± 1.6 | 41.4 ± 0.1 | 0.5 ± 0.0 |
| P-15-ExRP | 29 ± 3 | 2559 ± 219 | 191.8 ± 1.5 | 40.4 ± 0.6 | 0.2 ± 0.1 |
| P-20-ExRP | 35 ± 4 | 2519 ± 35 | 189.9 ± 2.6 | 38.5 ± 0.7 | 0.3 ± 0.6 |
| Material | (1 − µ) | m0 | Cal. THE | THE | Cal. PHRR | PHRR | Prot. Layer | |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | |
| EP | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 |
| EP-5-ExOP935 | 96.1 | 87.7 | 103.8 | 87.5 | 87.5 | 84.3 | 87.9 | −4.3 |
| EP-10-ExOP935 | 94.2 | 77.9 | 98.9 | 72.5 | 72.6 | 73.3 | 52.2 | 28.9 |
| EP-15-ExOP935 | 91.9 | 74.3 | 102.7 | 70.1 | 70.1 | 68.3 | 46.5 | 31.9 |
| EP-20-ExOP935 | 89.2 | 71.7 | 100.7 | 64.4 | 64.4 | 63.9 | 44.0 | 31.2 |
| EP-5-ExOP1230 | 95.6 | 80.6 | 105.5 | 81.3 | 88.2 | 77.1 | 59.5 | 22.8 |
| EP-10-ExOP1230 | 94.1 | 77.1 | 103.6 | 75.2 | 75.2 | 72.5 | 59.0 | 18.6 |
| EP-15-ExOP1230 | 91.8 | 73.8 | 107.0 | 72.5 | 72.5 | 67.8 | 54.2 | 20.0 |
| EP-20-ExOP1230 | 89.7 | 72.9 | 101.7 | 66.5 | 66.5 | 65.4 | 46.3 | 29.2 |
| EP-5-ExRP | 94.2 | 63.7 | 104.8 | 62.9 | 62.9 | 60.0 | 51.9 | 13.5 |
| EP-10-ExRP | 92.4 | 62.3 | 101.0 | 58.1 | 58.1 | 57.5 | 42.3 | 26.5 |
| EP-15-ExRP | 91.8 | 54.8 | 103.7 | 52.2 | 52.1 | 50.3 | 36.2 | 27.9 |
| EP-20-ExRP | 90.5 | 67.2 | 101.4 | 61.7 | 61.6 | 60.8 | 47.0 | 22.7 |
| EP-10-BDP | 95.4 | 92.0 | 103.7 | 91.1 | 91.0 | 87.8 | 79.0 | 10.0 |
| EP-20-BDP | 85.5 | 68.6 | 102.8 | 60.3 | 60.3 | 58.7 | 37.0 | 36.9 |
| EP-25-BDP | 84.6 | 63.0 | 104.0 | 55.4 | 55.4 | 53.3 | 35.5 | 33.3 |
| EP-35-BDP | 83.1 | 62.0 | 101.4 | 52.3 | 52.3 | 51.5 | 33.8 | 34.4 |
| Pes | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 |
| Pes-5-ExOP935 | 96.0 | 95.9 | 101.3 | 93.3 | 90.7 | 92.1 | 84.0 | 8.8 |
| Pes-10-ExOP935 | 92.4 | 78.7 | 96.6 | 70.2 | 68.4 | 72.7 | 58.9 | 18.9 |
| Pes-15-ExOP935 | 91.6 | 82.9 | 102.3 | 77.6 | 75.2 | 75.9 | 56.8 | 25.2 |
| Pes-20-ExOP935 | 91.5 | 73.6 | 98.9 | 66.6 | 64.6 | 67.4 | 51.2 | 24.0 |
| Pes-5-ExOP1230 | 96.4 | 99.5 | 102.4 | 98.2 | 95.2 | 95.9 | 95.3 | 0.5 |
| Pes-10-ExOP1230 | 91.6 | 74.8 | 102.4 | 70.2 | 73.7 | 68.6 | 42.1 | 38.6 |
| Pes-15-ExOP1230 | 90.8 | 69.7 | 101.1 | 63.9 | 77.8 | 63.2 | 59.3 | 6.2 |
| Pes-20-ExOP1230 | 90.1 | 72.4 | 101.3 | 66.1 | 64.9 | 65.3 | 44.8 | 31.3 |
| Pes-10-BDP | 99.0 | 84.6 | 100.3 | 84.0 | 82.5 | 83.8 | 71.5 | 14.7 |
| Pes-20-BDP | 98.0 | 56.5 | 100.3 | 55.5 | 70.5 | 55.4 | 61.8 | -11.7 |
| Pes-25-BDP | 96.6 | 58.9 | 99.3 | 56.5 | 63.2 | 56.9 | 56.0 | 1.4 |
| Pes-35-BDP | 95.4 | 60.8 | 98.9 | 57.3 | 59.3 | 58.0 | 52.9 | 8.8 |
| PMMA | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 |
| PMMA-5-ExOP935 | 98.9 | 90.9 | 97.5 | 87.6 | 87.6 | 89.9 | 79.6 | 11.4 |
| PMMA-10-ExOP935 | 97.6 | 91.2 | 93.4 | 83.1 | 83.1 | 89.0 | 75.4 | 15.2 |
| PMMA-15-ExOP935 | 96.3 | 83.6 | 97.4 | 78.4 | 78.4 | 80.5 | 70.4 | 12.6 |
| PMMA-20-ExOP935 | 94.4 | 83.9 | 96.5 | 76.4 | 76.5 | 79.2 | 58.6 | 26.0 |
| PMMA-5-ExOP1230 | 98.6 | 92.6 | 99.4 | 90.8 | 90.7 | 91.3 | 81.1 | 11.1 |
| PMMA-10-ExOP1230 | 97.2 | 90.3 | 95.0 | 83.4 | 83.4 | 87.8 | 68.7 | 21.7 |
| PMMA-15-ExOP1230 | 96.7 | 86.3 | 97.1 | 80.9 | 81.0 | 83.4 | 64.5 | 22.6 |
| PMMA-20-ExOP1230 | 95.3 | 85.3 | 95.2 | 77.4 | 77.4 | 81.3 | 59.0 | 27.4 |
| PMMA-10-BDP | 99.6 | 104.6 | 97.6 | 101.7 | 101.4 | 104.2 | 102.6 | 1.5 |
| PMMA-20-BDP | 99.5 | 93.5 | 97.9 | 91.1 | 91.1 | 93.0 | 94.6 | -1.7 |
| PMMA-25-BDP | 99.4 | 86.8 | 99.2 | 85.6 | 85.6 | 86.3 | 82.6 | 4.3 |
| PMMA-35-BDP | 99.3 | 89.1 | 98.3 | 87.0 | 87.0 | 88.5 | 88.3 | 0.2 |
| P | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 |
| P-5-ExOP935 | 100.1 | 95.9 | 98.2 | 94.2 | 94.7 | 96.0 | 64.6 | 32.7 |
| P-10-ExOP935 | 100.1 | 93.2 | 96.3 | 90.4 | 90.4 | 93.9 | 53.2 | 43.4 |
| P-15-ExOP935 | 98.0 | 89.4 | 99.1 | 86.8 | 87.5 | 87.5 | 45.8 | 47.7 |
| P-20-ExOP935 | 98.1 | 88.3 | 103.3 | 89.5 | 89.3 | 86.6 | 37.7 | 56.5 |
| P-5-ExOP1230 | 99.9 | 99.7 | 94.5 | 94.1 | 93.9 | 99.7 | 95.9 | 3.8 |
| P-10-ExOP1230 | 98.7 | 98.2 | 102.9 | 99.8 | 99.9 | 97.0 | 95.7 | 1.3 |
| P-15-ExOP1230 | 97.1 | 96.3 | 98.0 | 91.6 | 93.2 | 93.5 | 85.4 | 8.6 |
| P-20-ExOP1230 | 95.8 | 92.3 | 102.2 | 90.4 | 89.8 | 88.5 | 75.3 | 14.9 |
| P-5-ExRP | 99.6 | 97.9 | 103.1 | 100.5 | 101.1 | 97.5 | 98.3 | -0.8 |
| P-10-ExRP | 99.5 | 93.9 | 102.5 | 95.8 | 95.7 | 93.5 | 92.8 | 0.7 |
| P-15-ExRP | 99.8 | 100.7 | 100.1 | 91.5 | 91.3 | 91.4 | 85.4 | 6.5 |
| P-20-ExRP | 99.8 | 87.3 | 104.2 | 90.8 | 90.4 | 87.1 | 84.1 | 3.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. https://doi.org/10.3390/ma10050455
Rabe S, Chuenban Y, Schartel B. Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems. Materials. 2017; 10(5):455. https://doi.org/10.3390/ma10050455
Chicago/Turabian StyleRabe, Sebastian, Yuttapong Chuenban, and Bernhard Schartel. 2017. "Exploring the Modes of Action of Phosphorus-Based Flame Retardants in Polymeric Systems" Materials 10, no. 5: 455. https://doi.org/10.3390/ma10050455






