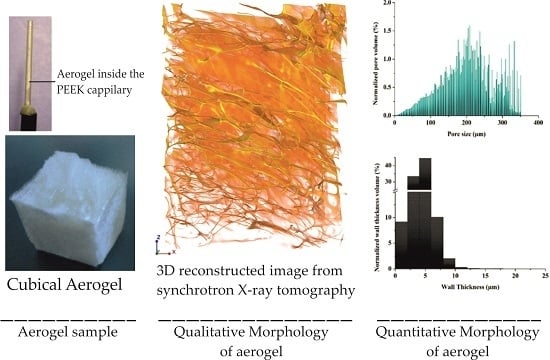
Synchrotron Microtomography Reveals the Fine Three-Dimensional Porosity of Composite Polysaccharide Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. 3D Structural Visualization
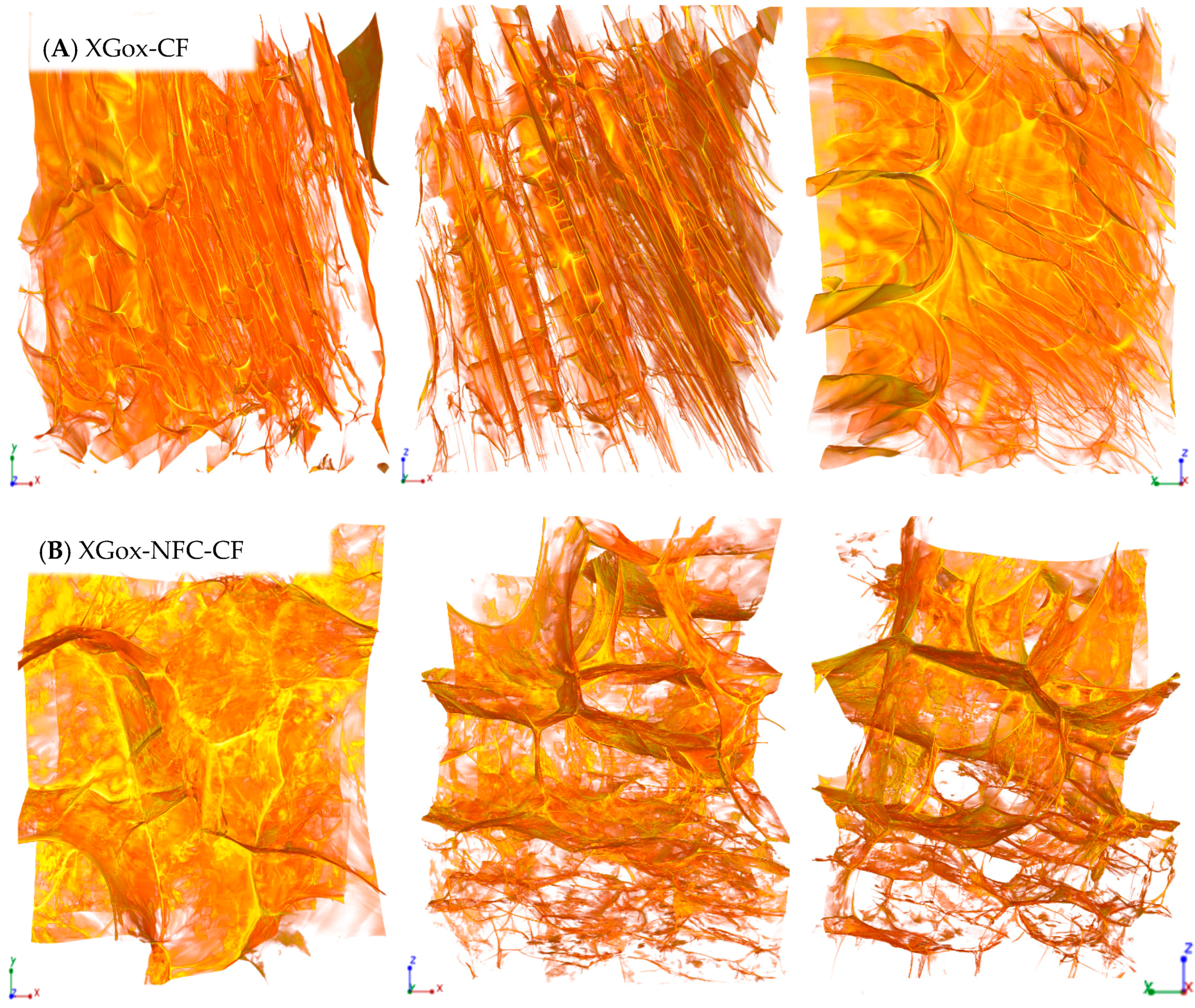
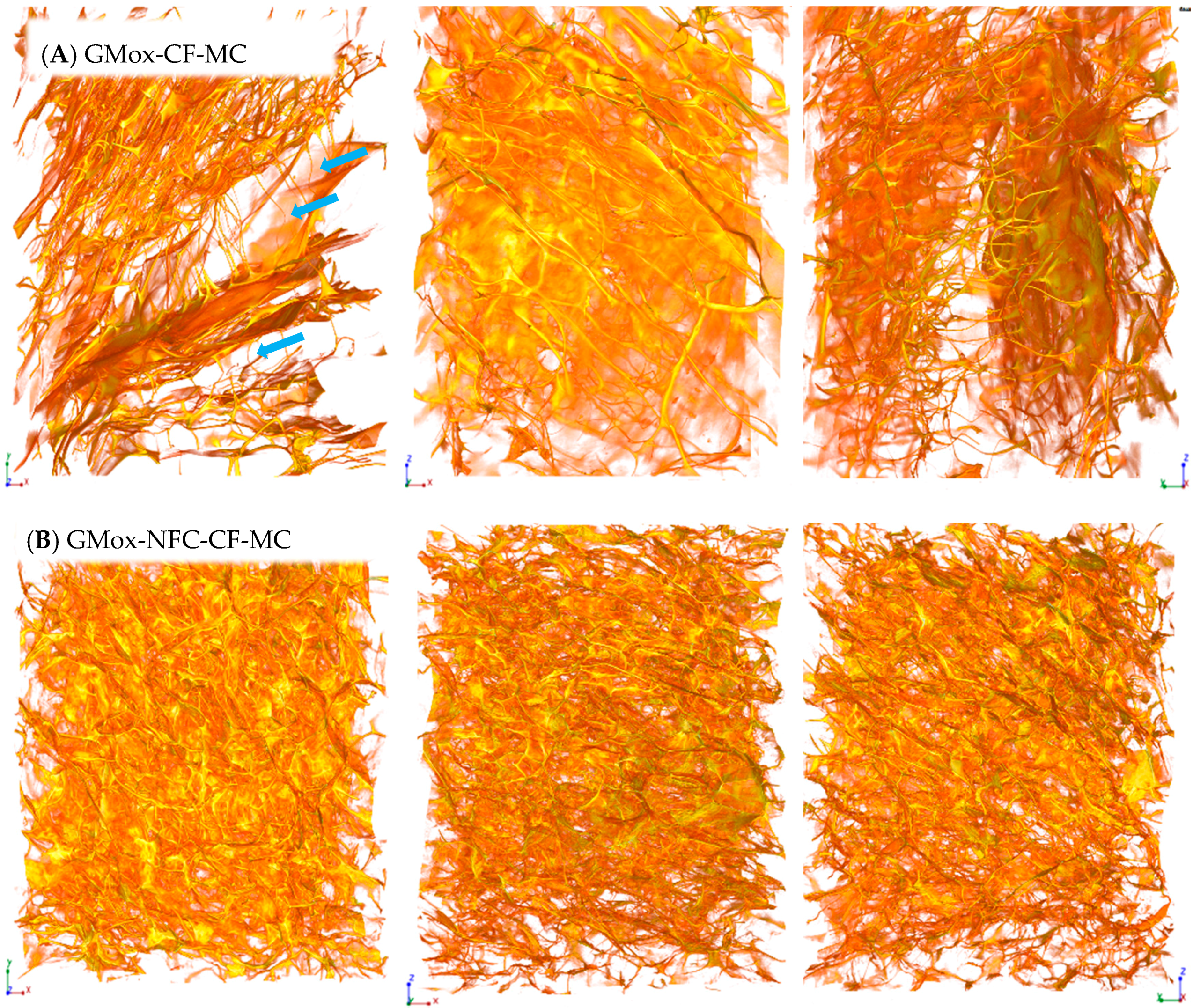
2.1.1. Effect of NFC Reinforcement on the Aerogels’ Morphology
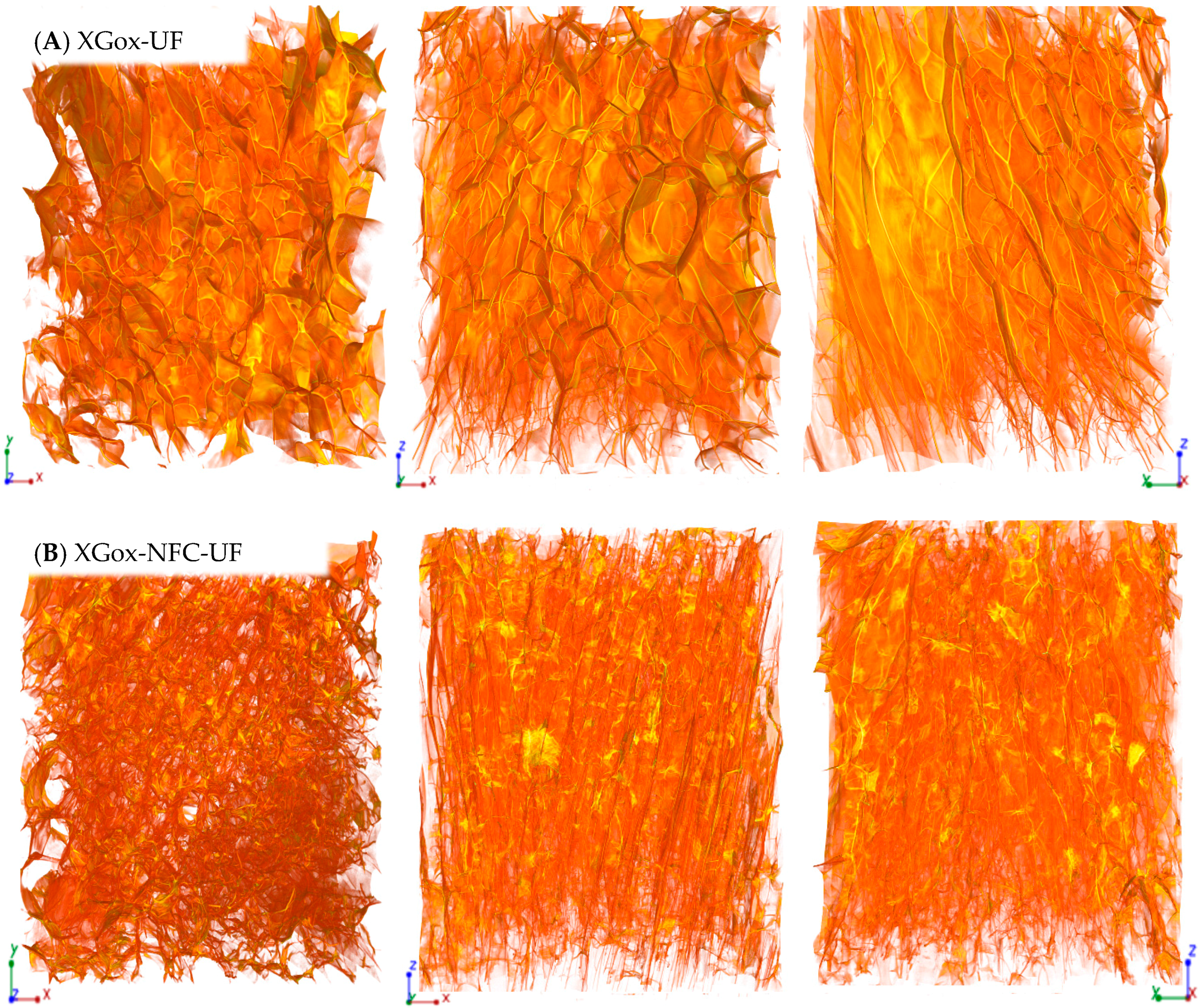
2.1.2. Effect of Freezing Method on the Aerogels’ Morphology
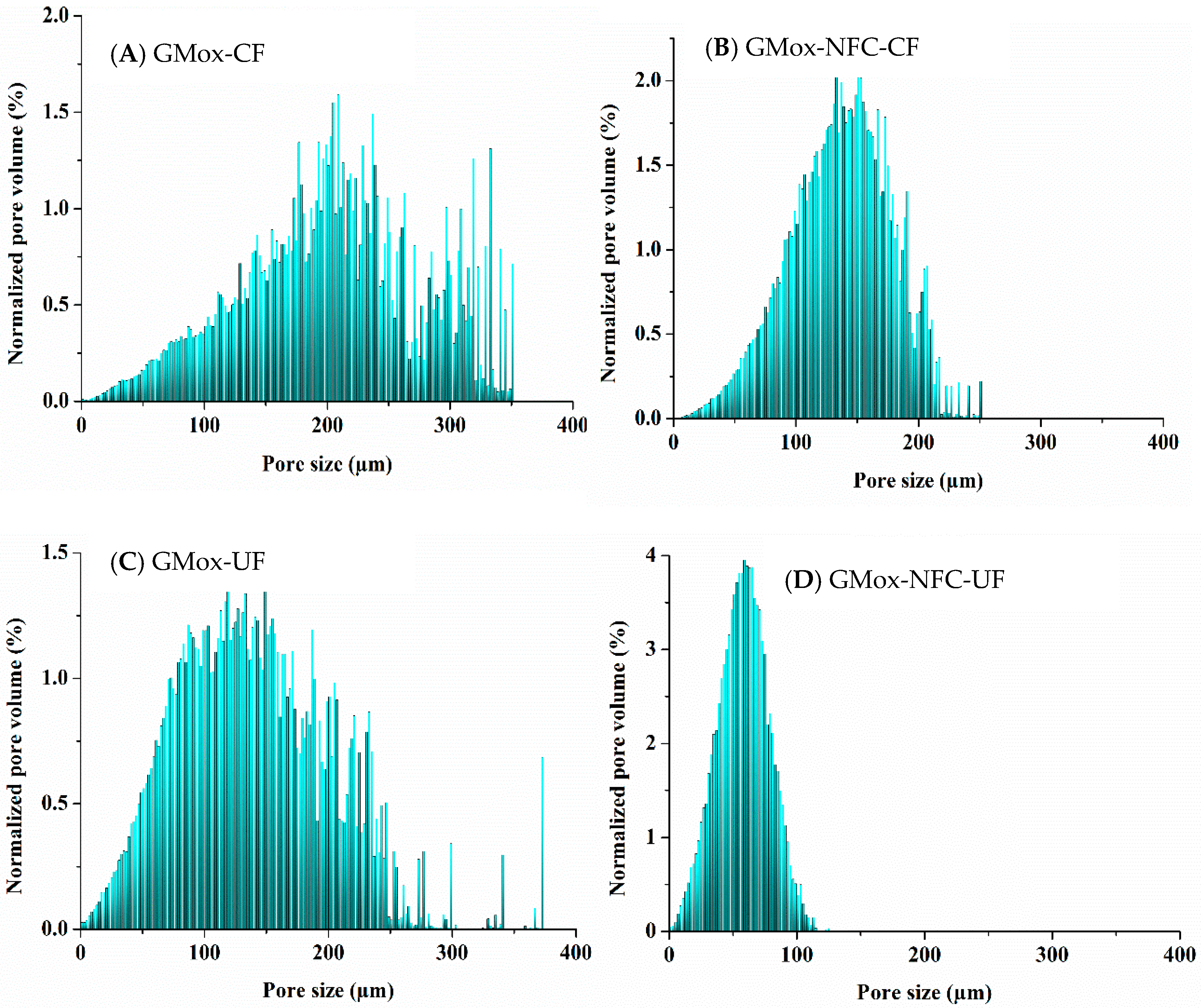
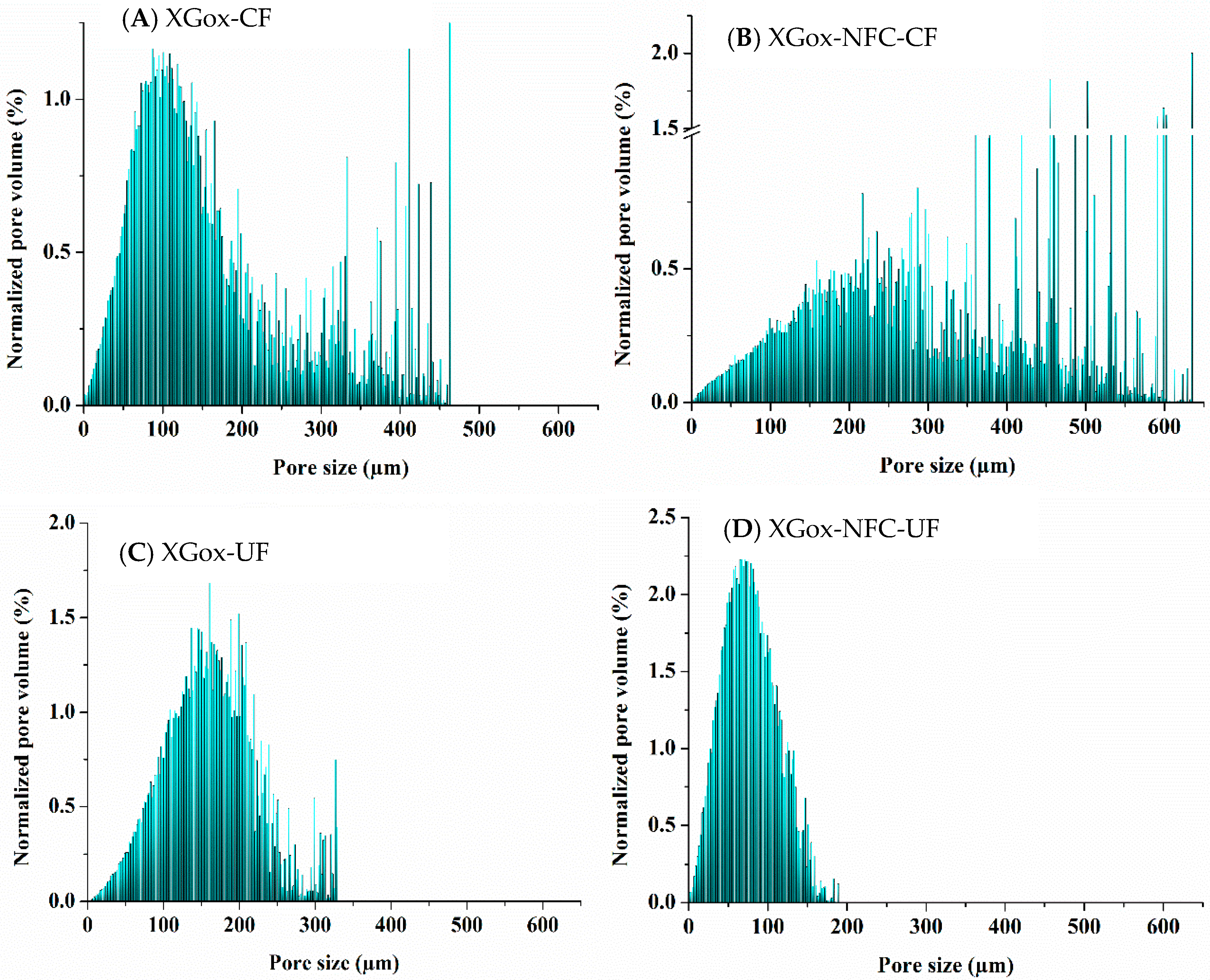
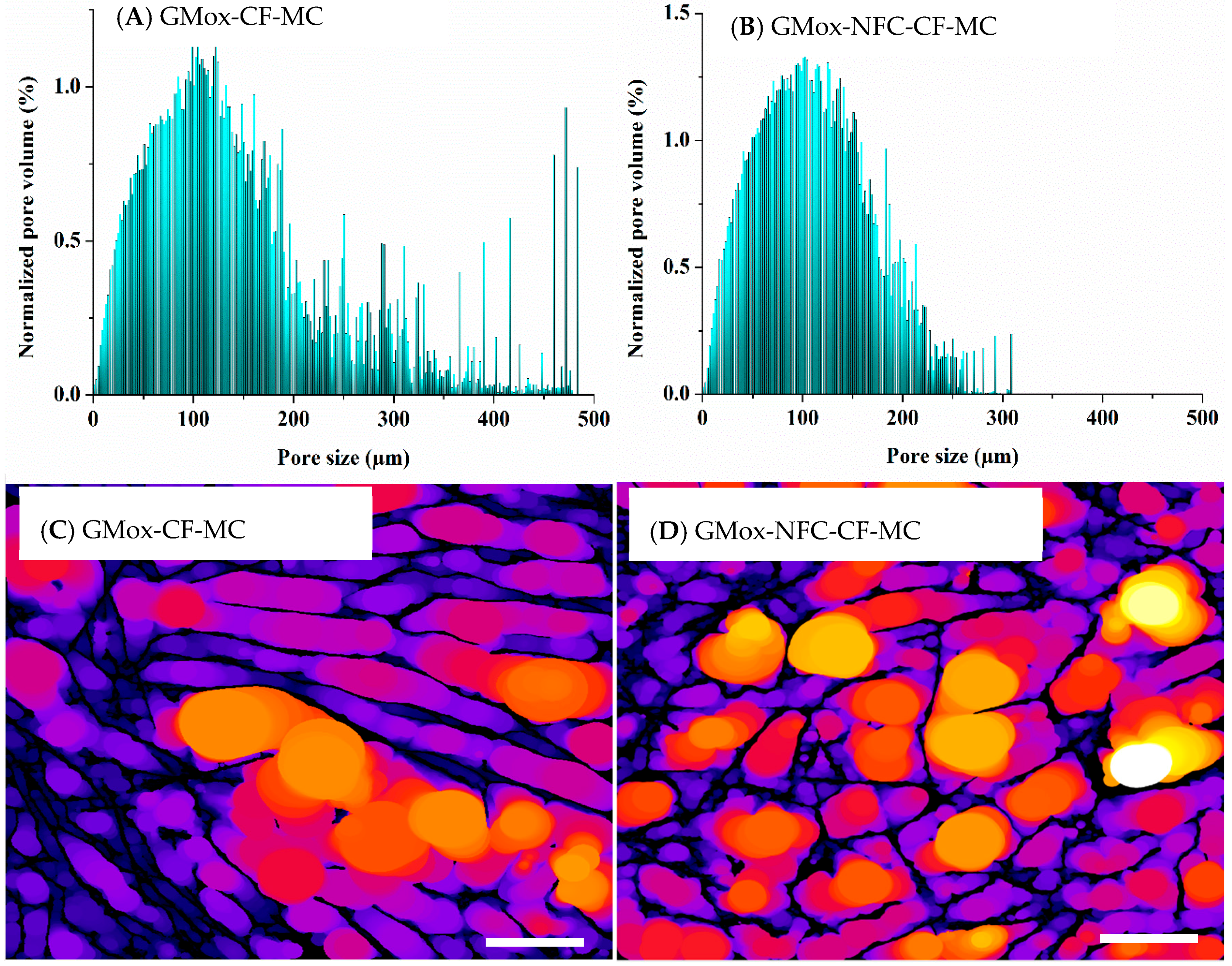
2.2. Quantitative Analyses
2.2.1. Volume-Weighted Pore Size Distribution
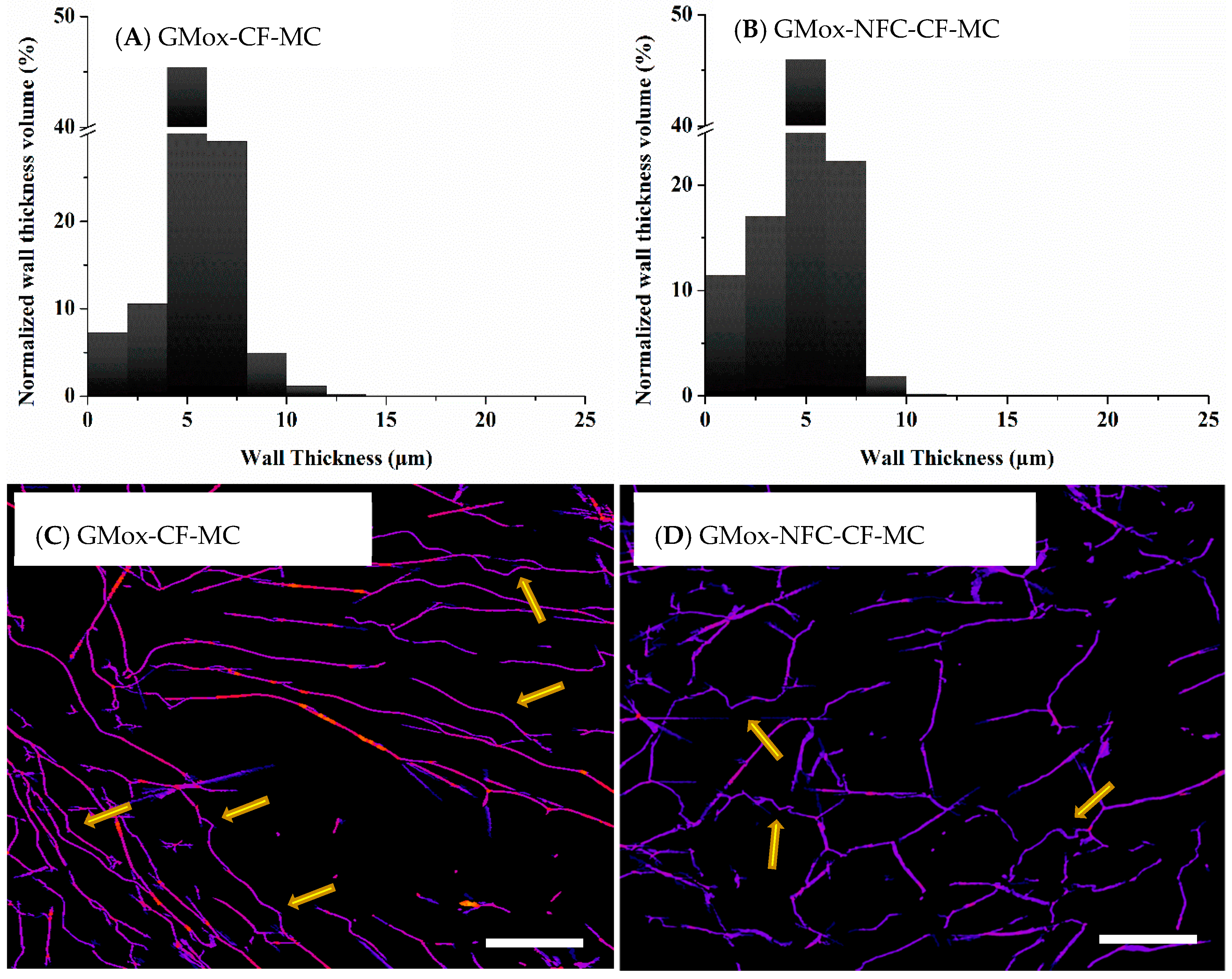
2.2.2. Volume-Weighted Pores Wall Thickness Distribution
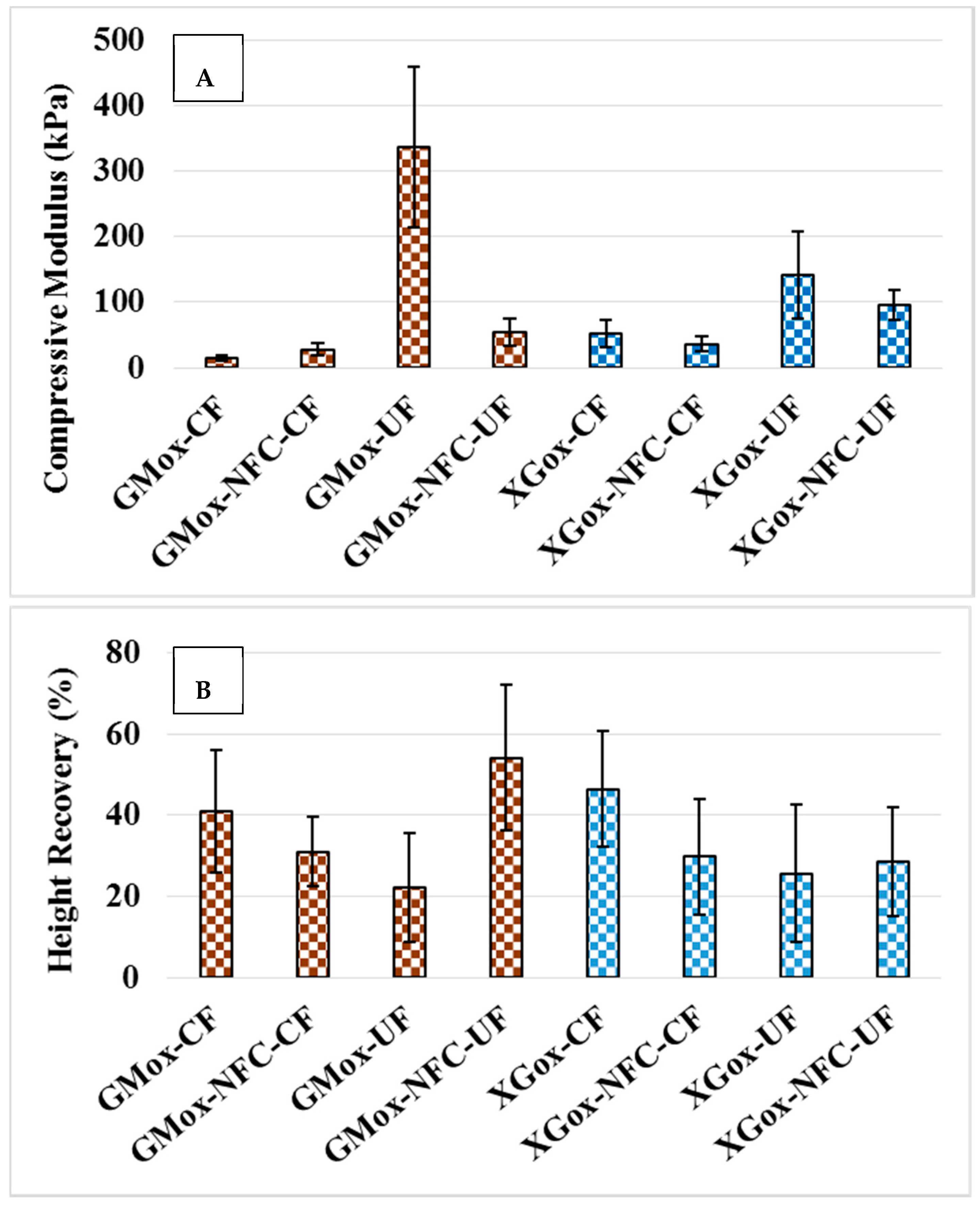
2.3. Structural Response to Uniaxial Mechanical Compression
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Crosslinking and Aerogel Formation
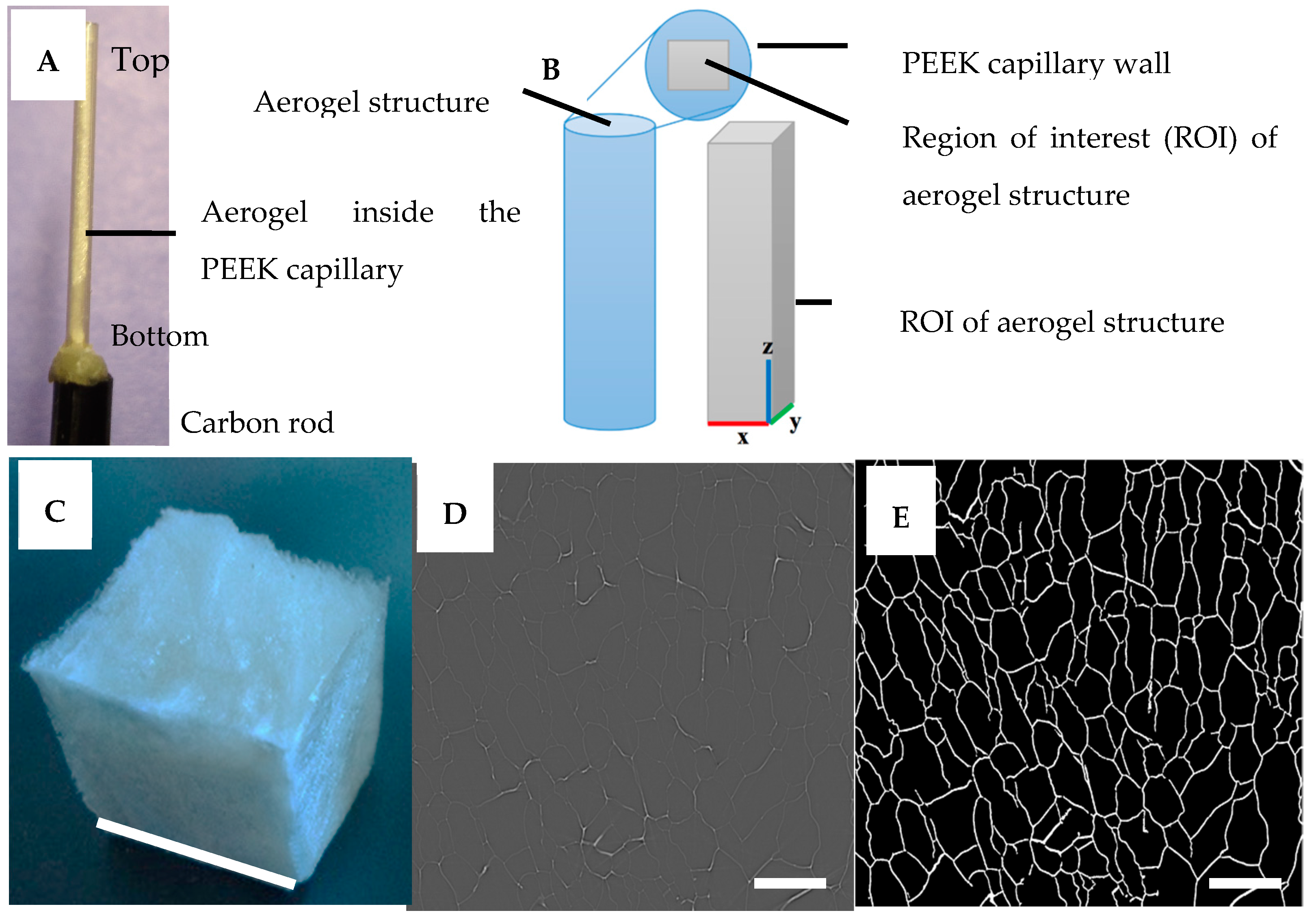
3.3. Synchrotron X-rays Tomography (XMT) Scanning and Image Reconstruction
3.4. Mechanical Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 1–11. [Google Scholar] [CrossRef]
- Liebner, F.; Potthast, A.; Rosenau, T.; Haimer, E.; Wendland, M. Cellulose aerogels: Highly porous, ultra-lightweight materials. Holzforschung 2008, 62, 129–135. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded-aerogels. J. Phys. Chem. 1931, 36, 52–64. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Borisova, A.; De Bruyn, M.; Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Segatto, M.L.; Clark, J.H. A sustainable freeze-drying route to porous polysaccharides with tailored hierarchical meso- and macroporosity. Macromol. Rapid Commun. 2015, 36, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Valentin, R.; Molvinger, K.; Viton, C.; Domard, A.; Quignard, F. From hydrocolloids to high specific surface area porous supports for catalysis. Biomacromolecules 2005, 6, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Parikka, K.; Leppänen, A.; Pitkänen, L.; Reunanen, M.; Willför, S.; Tenkanen, M. Oxidation of polysaccharides by galactose oxidase. J. Agric. Food Chem. 2010, 58, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Parikka, K.; Suuronen, J.; Ghafar, A.; Serimaa, R.; Tenkanen, M. Enzymatic oxidation as a potential new route to produce polysaccharide aerogels. RSC Adv. 2014, 4, 11884–11892. [Google Scholar] [CrossRef]
- Ghafar, A.; Gurikov, P.; Subrahmanyam, R.; Parikka, K.; Tenkanen, M.; Smirnova, I.; Mikkonen, K.S. Mesoporous guar galactomannan based biocomposite aerogels through enzymatic crosslinking. Compos. Part A Appl. Sci. Manuf. 2017, 94, 93–103. [Google Scholar] [CrossRef]
- Ghafar, A.; Parikka, K.; Sontag-Strohm, T.; Österberg, M.; Tenkanen, M.; Mikkonen, K.S. Strengthening effect of nanofibrillated cellulose is dependent on enzymatically oxidized polysaccharide gel matrices. Eur. Polym. J. 2015, 71, 171–184. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Zhao, H.; Zhou, K.; Feng, X. Functional map of biological and biomimetic materials with hierarchical surface structures. RSC Adv. 2015, 5, 66901–66926. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Mukai, S.R.; Nishihara, H.; Tamon, H. Formation of monolithic silica gel microhoneycombs (SMHs) using pseudosteady state growth of microstructural ice crystals. Chem. Commun. 2004, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Mukai, S.R.; Yamashita, D.; Tamon, H. Ordered macroporous silica by ice templating. Chem. Mater. 2005, 17, 683–689. [Google Scholar] [CrossRef]
- Deville, S.; Meille, S.; Seuba, J. A meta-analysis of the mechanical properties of ice-templated ceramics and metals. Sci. Technol. Adv. Mater. 2015, 16, 043501. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. A design protocol for tailoring ice-templated scaffold structure. J. R. Soc. Interface 2014, 11, 20130958. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Deng, Y. The morphology and mechanical properties of layer structured cellulose microfibril foams from ice-templating methods. Soft Matter 2011, 7, 6034–6040. [Google Scholar] [CrossRef]
- Martoïa, F.; Cochereau, T.; Dumont, P.J.J.; Orgéas, L.; Terrien, M.; Belgacem, M.N. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Des. 2016, 104, 376–391. [Google Scholar] [CrossRef]
- Pourhaghgouy, M.; Zamanian, A. Physical and mechanical properties of the fully interconnected chitosan ice-templated scaffolds. J. Appl. Polym. Sci. 2015, 132, 41476. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Deville, S. Freeze-casting of porous biomaterials: Structure, properties and opportunities. Materials 2010, 3, 1913–1927. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Atanasio, R.; Williams, R.A.; Jia, X. Combining X-ray microtomography with computer simulation for analysis of granular and porous materials. Particuology 2010, 8, 81–99. [Google Scholar] [CrossRef]
- Sedighi Gilani, M.; Boone, M.N.; Fife, J.L.; Zhao, S.; Koebel, M.M.; Zimmermann, T.; Tingaut, P. Structure of cellulose -silica hybrid aerogel at sub-micron scale, studied by synchrotron X-ray tomographic microscopy. Compos. Sci. Technol. 2016, 124, 71–80. [Google Scholar] [CrossRef]
- Kohnke, T.; Lin, A.; Elder, T.; Theliander, H.; Ragauskas, A.J. Nanoreinforced xylan-cellulose composite foams by freeze-casting. Green Chem. 2012, 14, 1864–1869. [Google Scholar] [CrossRef]
- Lee, K.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Schecter, M.; Donius, A.E.; Hunger, P.M. Biomaterials by freeze casting. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 2099–2121. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Sun, D. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Arboleda, J.C.; Hughes, M.; Lucia, L.A.; Laine, J.; Ekman, K.; Rojas, O.J. Soy protein–nanocellulose composite aerogels. Cellulose 2013, 20, 2417–2426. [Google Scholar] [CrossRef]
- Sauter, T.; Kratz, K.; Lendlein, A. Pore-size distribution controls shape-memory properties on the macro- and microscale of polymeric foams. Macromol. Chem. Phys. 2013, 214, 1184–1188. [Google Scholar] [CrossRef]
- Alakalhunmaa, S.; Parikka, K.; Penttilä, P.A.; Cuberes, M.T.; Willför, S.; Salmén, L.; Mikkonen, K.S. Softwood-based sponge gels. Cellulose 2016, 23, 3221–3238. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hintermüller, C.; Marone, F.; Isenegger, A.; Stampanoni, M. Image processing pipeline for synchrotron-radiation-based tomographic microscopy. J. Synchrotron Radiat. 2010, 17, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Marone, F.; Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Radiat. 2012, 19, 1020–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, R.; Kunzelmann, K. Computing local thickness of 3D structures with ImageJ. Microsc. Microanal. 2007, 13, 1678–1679. [Google Scholar] [CrossRef]


| Samples Codes * | Amount of NFC (wt %) | Ice-Templateing Method * |
|---|---|---|
| GMox-CF | 0 | CF |
| GMox-NFC-CF | 25 | CF |
| GMox-UF | 0 | UF |
| GMox-NFC-UF | 25 | UF |
| XGox-CF | 0 | CF |
| XGox-NFC-CF | 25 | CF |
| XGox-UF | 0 | UF |
| XGox-NFC-UF | 25 | UF |
| GMox-CF-MC | 0 | CF |
| GMox-NFC-CF-MC | 25 | CF |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafar, A.; Parikka, K.; Haberthür, D.; Tenkanen, M.; Mikkonen, K.S.; Suuronen, J.-P. Synchrotron Microtomography Reveals the Fine Three-Dimensional Porosity of Composite Polysaccharide Aerogels. Materials 2017, 10, 871. https://doi.org/10.3390/ma10080871
Ghafar A, Parikka K, Haberthür D, Tenkanen M, Mikkonen KS, Suuronen J-P. Synchrotron Microtomography Reveals the Fine Three-Dimensional Porosity of Composite Polysaccharide Aerogels. Materials. 2017; 10(8):871. https://doi.org/10.3390/ma10080871
Chicago/Turabian StyleGhafar, Abdul, Kirsti Parikka, David Haberthür, Maija Tenkanen, Kirsi S. Mikkonen, and Jussi-Petteri Suuronen. 2017. "Synchrotron Microtomography Reveals the Fine Three-Dimensional Porosity of Composite Polysaccharide Aerogels" Materials 10, no. 8: 871. https://doi.org/10.3390/ma10080871