From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals
Abstract
:1. Introduction
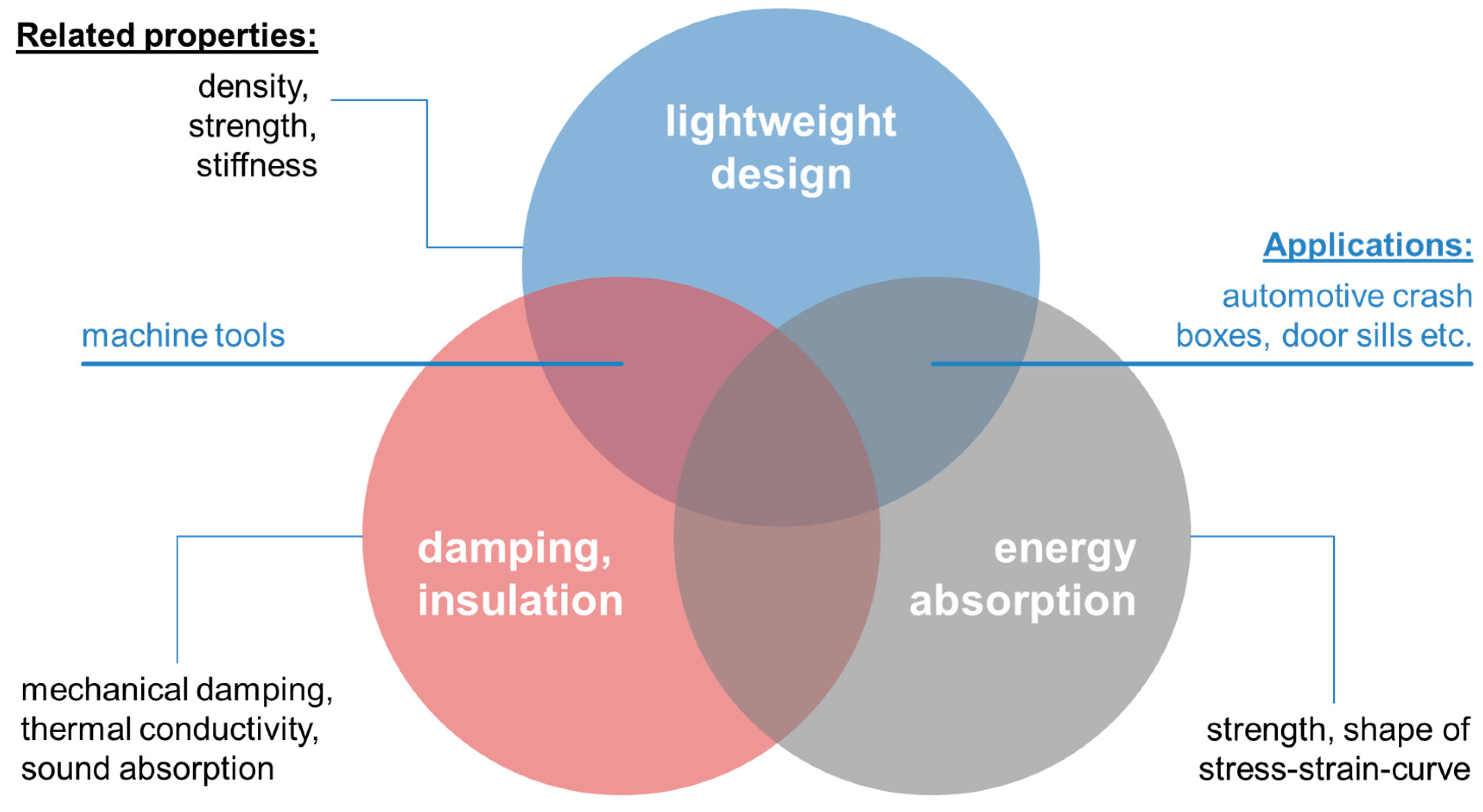
- Mechanical Properties
- ○
- Material stiffness (e.g., Young’s modulus): structural applications
- ○
- Material strength
- ■
- X% offset yield stress: structural applications
- ■
- Plateau stress (magnitude and stability): energy absorption
- ○
- Mechanical damping: structural and functional (e.g., damping elements) applications
- ○
- Designed mechanical characteristics like anisotropy, auxetic behavior: diverse applications.
- Functional Properties
- ○
- Thermal conductivity: thermal energy storage and conduction
- ○
- Flow resistance: heat exchangers
- ○
- Electrical conductivity: electrodes
- ○
- Acoustic damping: sound absorption
- ○
- Internal surface area: thermal applications, catalysts.
- Raising property levels via the matrix material, e.g., through:
- Raising property levels via structural features, e.g., through:
- ○
- material and/or process adaptations improving structural control
- ○
- alternative processes with inherently better control of/less freedom in structure development, such as,
- Lowering the scatter of properties to allow for reduction of safety factors, thus increasing the practically accessible material properties, as done in
- Use of metal foams as one of several components in multi-material, hybrid, and similar structures
- ○
- integral foams
- ○
- sandwich structures
- ○
- foam-filled hollow components
- ■
- ■
- co-extrusion of conventional and foamable material followed by in situ foaming [8]
- ■
- ■
- ■
- etc.
- ○
- hybrid foams
- process automation to minimize cost-intensive manual labor.
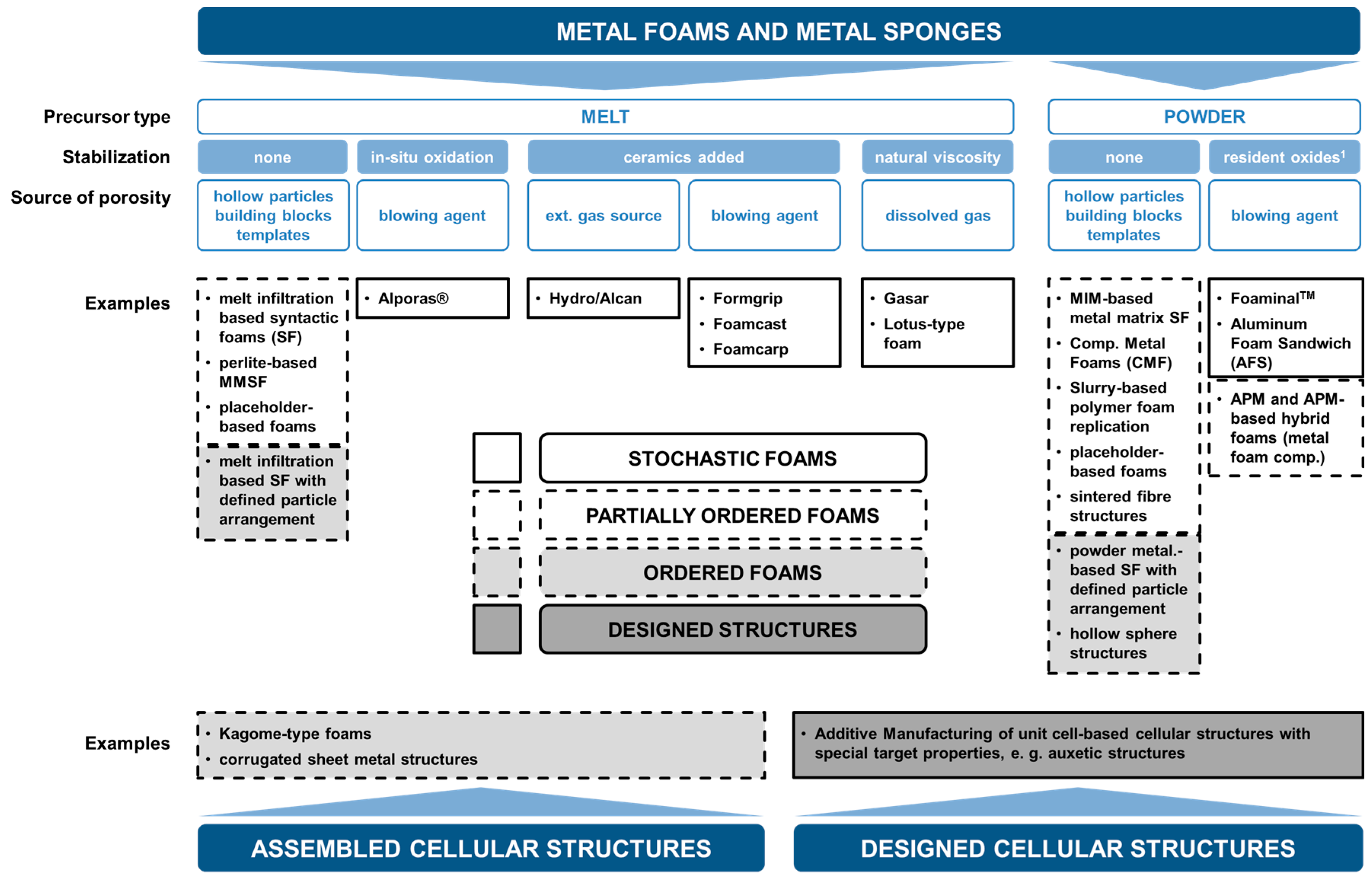
2. Categorization of Foams and Associated Manufacturing Processes
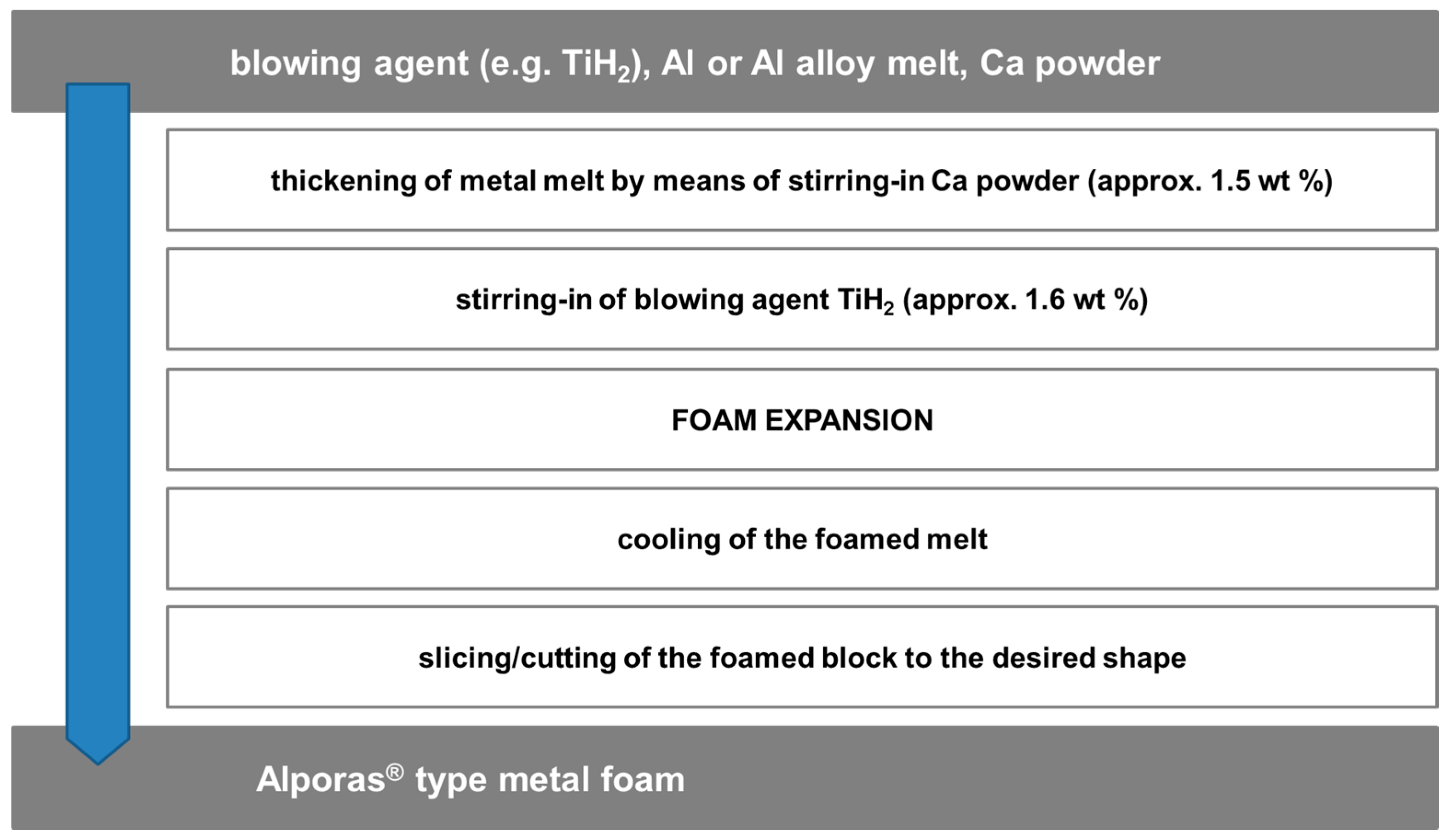
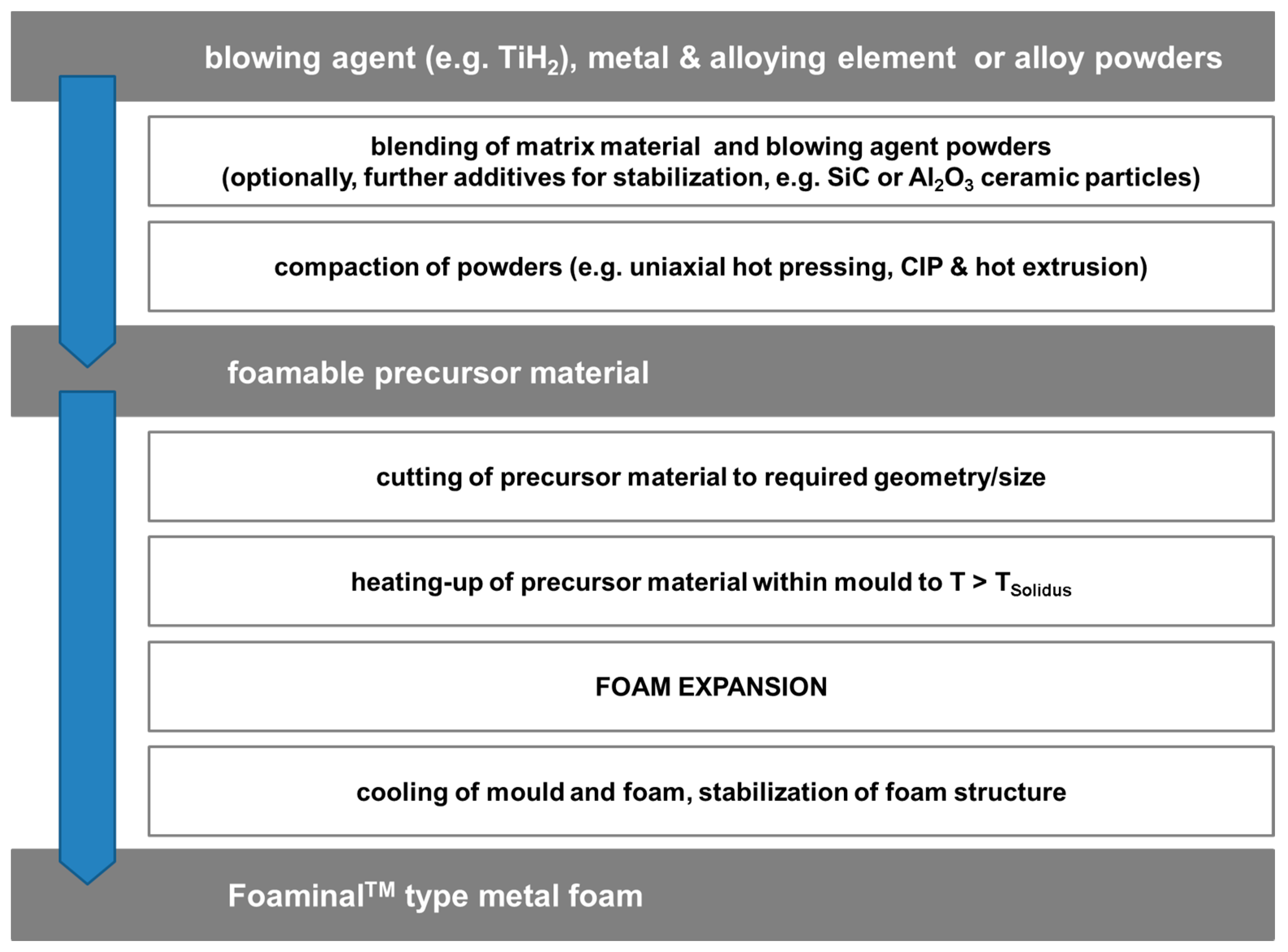
- Stochastic Foams
- (a)
- Melt foaming using blowing agents, gas introduction (uncontrolled)
- (b)
- Powder metallurgy using blowing agents
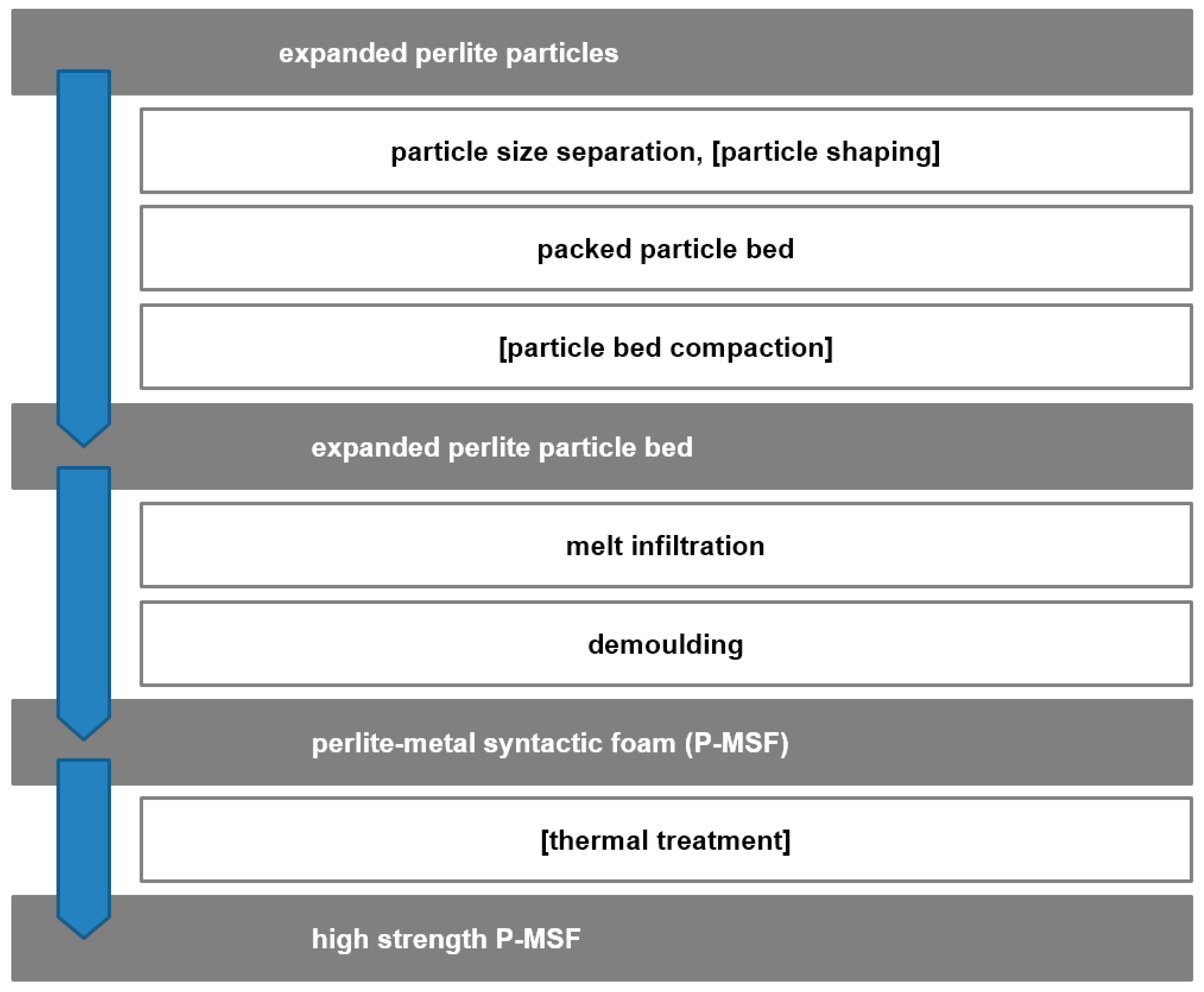
- Partially Ordered Foams: Enhanced Structural Control on Pore Level
- (a)
- Melt foaming using controlled gas introduction
- (b)
- Melt foaming using solidification control
- (c)
- Placeholder methods
- (d)
- Precursor/replication methods
- (e)
- APM foams
- (f)
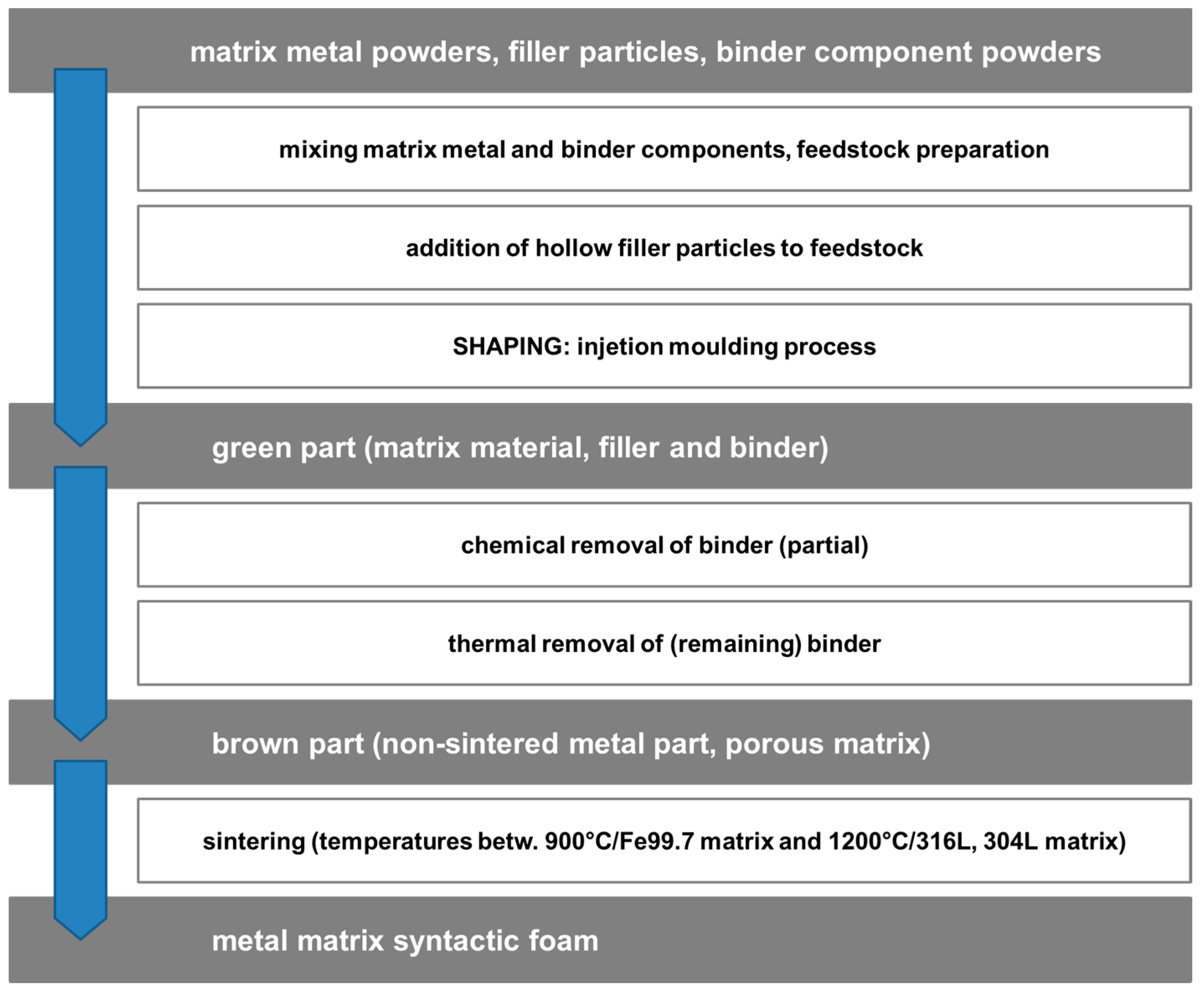
- Syntactic foams
- (g)
- Placeholder methods with ordered assembly of placeholders
- (h)
- Syntactic foams through ordered assembly of hollow particles
- (i)
- Metal fiber structures
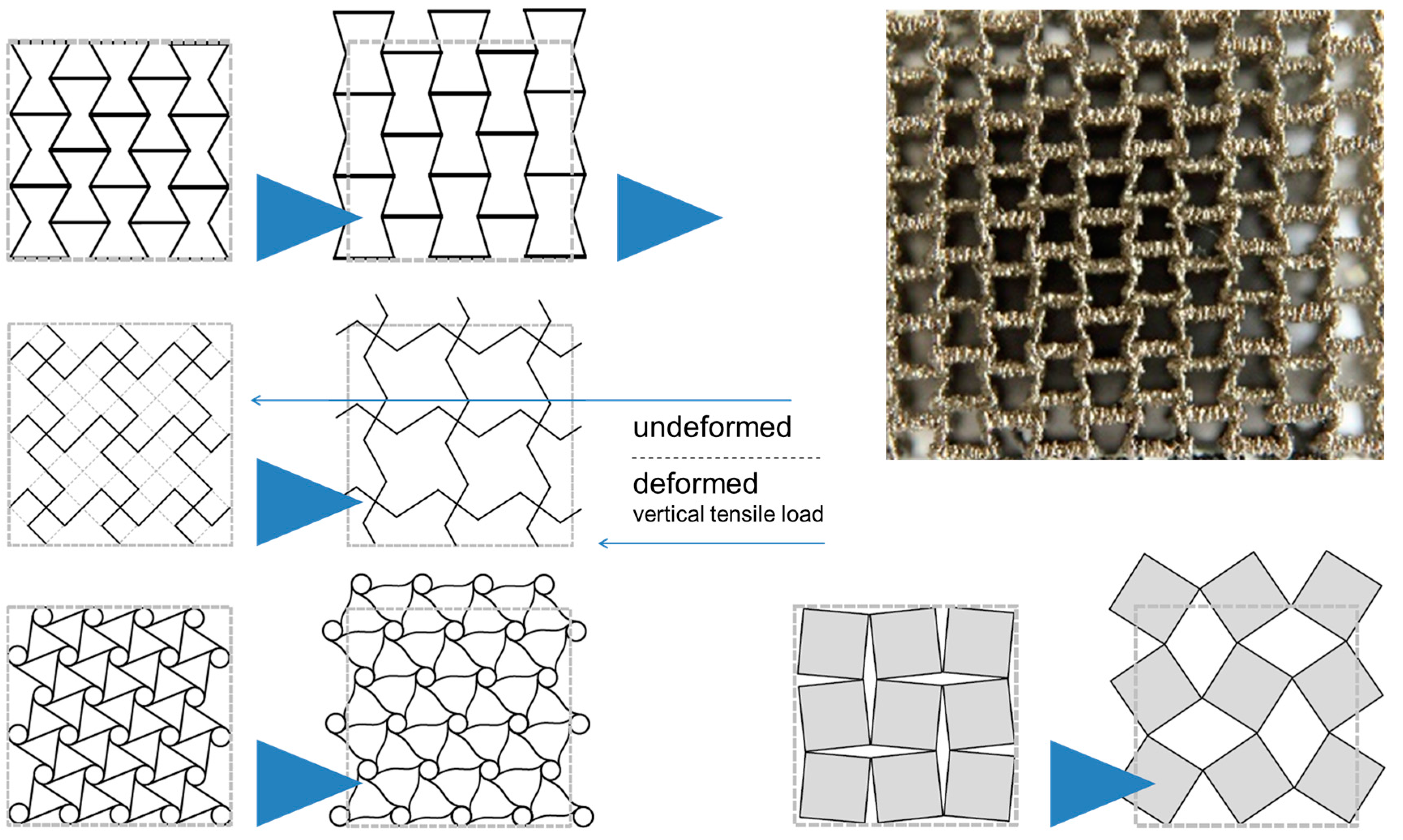
- Ordered Foams: Ordered Assembly of Identical Structural Elements
- (a)
- Kagome-type foams
- (b)
- Corrugated sheet metal structures
- (c)
- UniPore structures
- Designed-to-Purpose Cellular Structures
- (a)
- Additive manufacturing approaches.
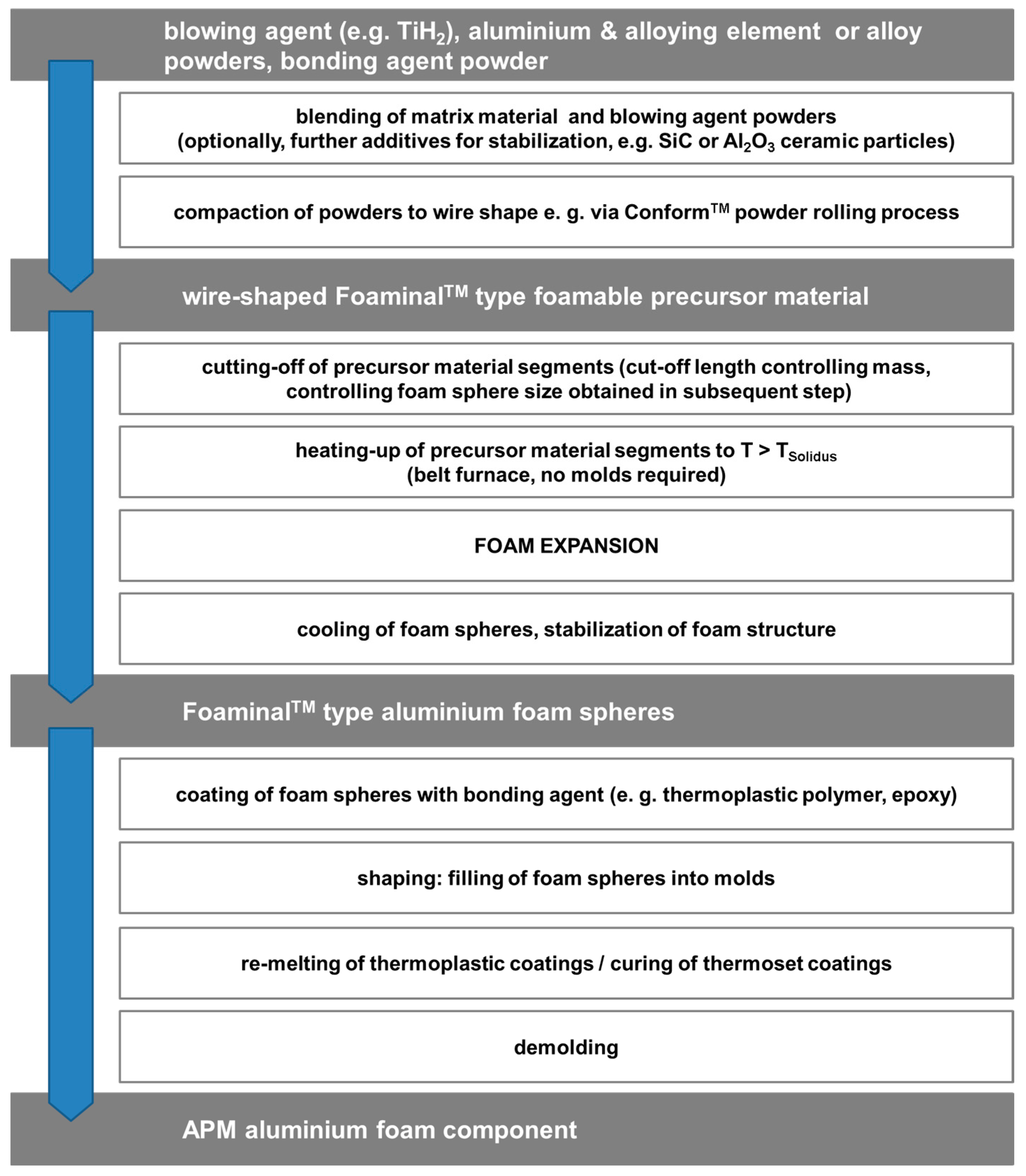
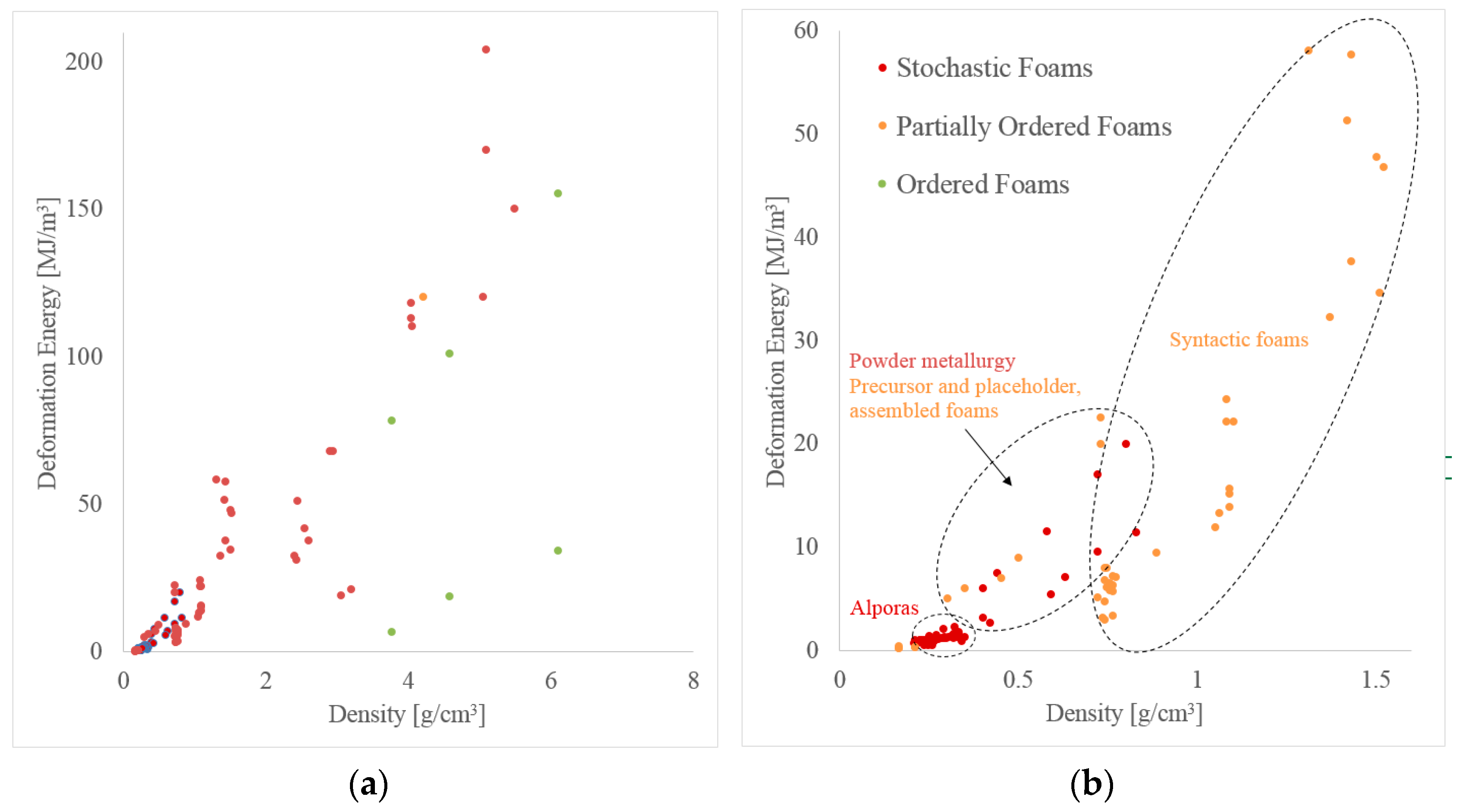
2.1. Category I: Stochastic Foam—Alporas®, Foaminal™, and Others
2.2. Category II: Partially Ordered Foams—Limited Structural Control on Pore and Spatial Distribution of Pore Levels
2.3. Category III: Ordered Foams—Ordered Assemblies of Identical Structural Elements
2.4. Category IV: Designed-to-Purpose Cellular Structures
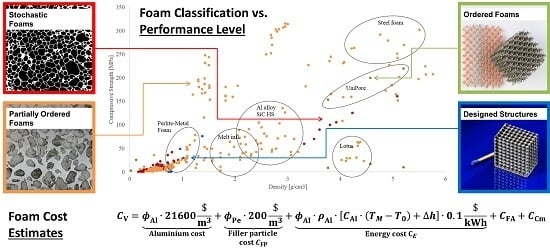
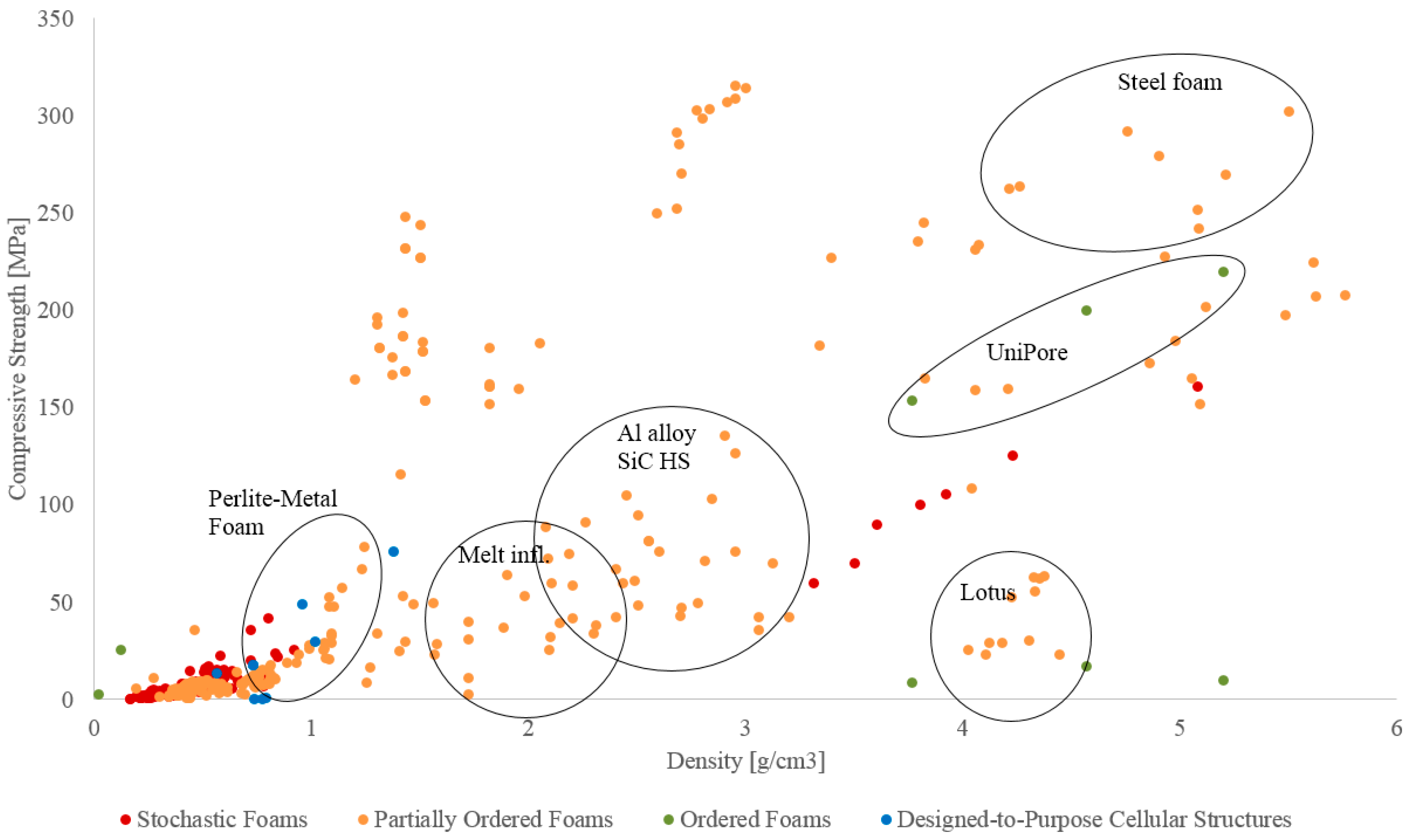
3. Mechanical Performance
3.1. Compressive Strength
3.2. Energy Absorption
4. Cost of Performance
4.1. General Approach
4.2. Strength and Cost
4.3. Energy Absorption and Cost
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A
| Density | Metal (Matrix) | Comp. Strength (C), Yield Stress (Y) or Plateau Stress (P) | Energy Density | Specific Cost Estimate | Volumetric Cost Estimate | Reference |
|---|---|---|---|---|---|---|
| (g/cm3) | (-) | (MPa) | (MJ/m3) | (EUR/kg) | (EUR/m3) | |
| 0.17–2.1 | Al | 0.5–0.9 (P) | 0.22–0.34 | 10.7 | 1390 | [117,118] |
| 0.19–0.84 | Al | 1.5–22.1 (C) | [24] | |||
| 0.2–0.8 | Al | 2–7 (Y) | [24] | |||
| 0.21–0.33 | Al | 0.71–1.33 | 12.1 | 2600 | [119] | |
| 0.25–0.34 | Al | 1.8–3 (P) | 0.93–1.55 | 10.3 | 5770 | [54] |
| 0.26–0.27 | Al | 1.05–1.09 | [120] | |||
| 0.26–0.59 | Al | 3.4–15.6 (C) | [24] | |||
| 0.29–0.4 | Al | 2.7–8.6 (P) | 2.12–6.07 | [54,60] | ||
| 0.35–0.66 | Al | 3.8–10.6 (C) | [121] | |||
| 0.42–0.83 | Al | 5.8–24 (P) | 2.66–11.45 | [60,65] | ||
| 0.44–0.8 | Al | 15–42 (P) | 7.5–20 | [122] | ||
| 0.6 | Al | 11.7–14.6 (C) | [17] | |||
| 0.66–0.8 | Al | 10.2–11.7 (P) | [123] | |||
| 3.31–5.08 | Steel | 60.1–161.3 (C) | [124] |
| Density | Metal (Matrix) | Comp. Strength (C), Yield Stress (Y), or Plateau Stress (P) | Energy Density | Specific Cost Estimate | Volumetric Cost Estimate | Reference |
|---|---|---|---|---|---|---|
| (g/cm3) | (-) | (MPa) | (MJ/m3) | (EUR/kg) | (EUR/m3) | |
| 0.19–0.46 | Al | 0.3–12 (Y) | [104] | |||
| 0.3–0.5 | Al | 6.2 (P) | 5–9 | [43] | ||
| 0.3–0.6 | Steel | 1.7–4.6 (P) | [125] | |||
| 0.34–0.74 | Al | 2.5–12.7 (C) | [44,121] | |||
| 0.41–1.08 | Al | 5.6–26.2 (C) | [126] | |||
| 0.43–0.8 | Al | 1.2–11.4 (P) | [105] | |||
| 0.67–0.77 | Al | 3.5–12.4 (Y) | 7.2–8 | 8.5 | 6060 | [127] |
| 0.74–0.76 | Al | 7.5–8.8 (P) | 4.8–5.7 | 10.8 | 7030 | [102] |
| 0.74–1.08 | Al | 7.1–52.6 (P) | 3.2–24.3 | 8.1 | 8790 | [92] |
| 1.05–1.09 | Al | 25.4–34.4 (P) | 11.9–15.6 | [38] | ||
| 1.31–1.52 | Al | 154–248 (C) | 32.3–58.1 | [128] | ||
| 1.56–4.76 | Steel | 50–292 (Y) | [129] | |||
| 1.72–2.5 | Al | 2.8–48.6 (C) | [130] | |||
| 1.82 | Al | 152–181 (C) | [131] | |||
| 2.4 | Al | 67 (P) | 32.3 | [132] | ||
| 2.43–2.45 | Steel | 60–105 (P) | 31–35 | [133] | ||
| 2.55–3.06 | Fe | 42.3–81.4 (C) | [98] | |||
| 2.55–3.06 | Steel | 36.2–127 (P) | 18.9–67.8 | [98] | ||
| 2.6–3.2 | Steel | 42.3–136 (P) | 21–68 | [132] | ||
| 2.95 | Steel | 76 (C) | [98] | |||
| 3.34–5.21 | Fe | 182–270 (Y) | [93] | |||
| 3.39–4.93 | Fe | 227–231.3 (C) | [94] | |||
| 3.79–5.01 | Fe | 235.4–262.8 (C) | [94] | |||
| 4.02–4.44 | Steel | 25.5–63.3 (C) | [134] | |||
| 4.06–5.05 | Fe | 159–165 (Y) | 110–120 | [95] | ||
| 4.21–5.49 | Invar | 160–198 (Y) | 113–204 | [95] | ||
| 4.86–5.76 | Steel | 173.2–208.2 (C) | [36] | |||
| 4.9–6 | Steel | 279.5–607.5 (Y) | [94] |
| Density | Metal (Matrix) | Comp. Strength (C), Yield Stress (Y), or Plateau Stress (P) | Energy Density | Specific Cost Estimate | Volumetric Cost Estimate | Reference |
|---|---|---|---|---|---|---|
| (g/cm3) | (-) | (MPa) | (MJ/m3) | (EUR/kg) | (EUR/m3) | |
| 0.018 | Ti | 2.8 (Y) | [106] | |||
| 0.12 | Ti | 26 (Y) | [106] | |||
| 3.76–4.57 | Cu | 153.6–200.1 (P) | 78.2–100.9 | [50,52] |
| Density | Metal (Matrix) | Comp. Strength (C), Yield Stress (Y), or Plateau Stress (P) | Energy Density | Specific Cost Estimate | Volumetric Cost Estimate | Reference |
|---|---|---|---|---|---|---|
| (g/cm3) | (-) | (MPa) | (MJ/m3) | (EUR/kg) | (EUR/m3) | |
| 0.56–1.34 | Ti | 13.5–76.3 (Y) | [135] | |||
| 0.73 | Al | 8 (C) | [136] | |||
| 0.74–0.79 | Cu | 0.3–0.9 (C) | [137] |
References
- De Meller, M.A. Produit métallique pour l’obtention d’objets laminés, moulés ou autres, et procédés pour sa fabrication. Fr. Pat. 1926, 147, 615. [Google Scholar]
- Sosnick, B. Foamlike Metal. U.S. Patent US2553016 A, 15 May 1951. [Google Scholar]
- Sosnick, B. Process for Making Foamlike Mass of Metal. U.S. Patent US2434775 A, 20 January 1948. [Google Scholar]
- Elliott, J.C. Metal Foaming Process. U.S. Patent US3005700 A, 24 October 1961. [Google Scholar]
- Elliott, J.C. Metal Foam and Method for Making. U.S. Patent US2983597 A, 9 May 1961. [Google Scholar]
- Elliott, J.C. Method of Producing Metal Foam. U.S. Patent US2751289 A, 19 June 1956. [Google Scholar]
- Baumeister, J. Verfahren zur Herstellung Poröser Metallkörper. German Patent 40 18 360, 14 March 1991. [Google Scholar]
- Baumeister, J.; Schrader, H. Verfahren zur Herstellung Aufschäumbarer Metallkörper und Verwendung Derselben. German Patent 41 01 630, 29 January 1992. [Google Scholar]
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.W. Recent trends in aluminum foam sandwich technology. Adv. Eng. Mater. 2012, 14, 1082–1087. [Google Scholar] [CrossRef]
- Lefebvre, L.P.; Banhart, J.; Dunand, D.C. Porous Metals and Metallic Foams: Current Status and Recent Developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Duarte, I.; Ferreira, J.M.F. Composite and nanocomposite metal foams. Materials 2016, 9, 79. [Google Scholar] [CrossRef]
- Lehmhus, D.; von Hehl, A.; Kayvantash, K.; Gradinger, R.; Becker, T.; Schimanski, K.; Avalle, M. Taking a downward turn on the weight spiral—Lightweight materials in transport applications. Mater. Des. 2015, 66, 385–389. [Google Scholar] [CrossRef]
- Weise, J.; Fraunhofer IFAM, Bremen, Germany. Private Communication, 2016.
- Lufthansa Fuel Efficiency im Lufthansa Konzern—Kosten Sparen und die Umwelt Schonen. Available online: https://www.lufthansagroup.com/fileadmin/downloads/de/LH-Fuel-Efficiency-0612.pdf (accessed on 1 July 2017).
- Garcia-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef]
- Lehmhus, D.; Banhart, J.; Rodriguez-Perez, M.A. Adaptation of aluminium foam properties by means of precipitation hardening. Mater. Sci. Technol. 2002, 18, 474–479. [Google Scholar] [CrossRef]
- Campana, F.; Pilone, D. Effect of wall microstructure and morphometric parameters on the crush behaviour of Al alloy foams. Mater. Sci. Eng. A 2008, 479, 58–64. [Google Scholar] [CrossRef]
- Lehmhus, D.; Banhart, J. Properties of heat-treated aluminium foams. Mater. Sci. Eng. A 2003, 349, 98–110. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. On the mechanical properties of heat-treated expanded perlite-aluminium syntactic foam. Mater. Des. 2014, 63, 375–383. [Google Scholar] [CrossRef]
- Esmaeelzadeh, S.; Simchi, A.; Lehmhus, D. Effect of ceramic particle addition on the foaming behavior, cell structure and mechanical properties of P/M AlSi7 foam. Mater. Sci. Eng. A 2006, 424, 290–299. [Google Scholar] [CrossRef]
- Kennedy, A.R. The effect of TiH2 heat treatment on gas release and foaming in Al-TiH2 preforms. Scr. Mater. 2002, 47, 763–767. [Google Scholar] [CrossRef]
- Matijasevic-Lux, B.; Banhart, J.; Fiechter, S.; Görke, O.; Wanderka, N. Modification of titanium hydride for improved aluminium foam manufacture. Acta Mater. 2006, 54, 1887–1900. [Google Scholar] [CrossRef]
- Lehmhus, D.; Busse, M. Mechanical performance of structurally optimized AlSi7 aluminum foams—An experimental study. Mater. Werkst. 2014, 45, 1061–1071. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, S.; Li, W.; Liu, J.; Wei, M. Compressive behavior of SiCp/AlSi9Mg composite foams. J. Alloys Compd. 2008, 460, 294–298. [Google Scholar] [CrossRef]
- Daoud, A. Compressive response and energy absorption of foamed A359-Al2O3 particle composites. J. Alloys Compd. 2009, 486, 597–605. [Google Scholar] [CrossRef]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS Aluminum Foam: Production Process, Properties, and Applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Miyoshi, T.; Hara, S.; Mukai, T.; Higashi, K. Development of a Closed Cell Aluminum Alloy Foam with Enhancement of the Compressive Strength. Mater. Trans. 2001, 42, 2118–2123. [Google Scholar] [CrossRef]
- Shapovalov, V.I. Method for Manufacturing Porous Articles. U.S. Patent US5181549 A, 26 January 1993. [Google Scholar]
- Shapovalov, V.; Boyko, L. Gasar—A new class of porous materials. Adv. Eng. Mater. 2004, 6, 407–410. [Google Scholar] [CrossRef]
- Nakajima, H.; Ikeda, T.; Hyun, S.K. Fabrication of Lotus-type Porous Metals and their Physical Properties. Adv. Eng. Mater. 2004, 6, 377–384. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, properties and application of porous metals with directional pores. Prog. Mater. Sci. 2007, 52, 1091–1173. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication of Lotus-type Porous Metals through Hydride Decomposition. Adv. Eng. Mater. 2008, 10, 816–819. [Google Scholar] [CrossRef]
- Vesenjak, M.; Kovačič, A.; Tane, M.; Borovinšek, M.; Nakajima, H.; Ren, Z. Compressive properties of lotus-type porous iron. Comput. Mater. Sci. 2012, 65, 37–43. [Google Scholar] [CrossRef]
- Peroni, L.; Scapin, M.; Avalle, M.; Weise, J.J.; Lehmhus, D.; Baumeister, J.; Busse, M. Syntactic iron foams—On deformation mechanisms and strain-rate dependence of compressive properties. Adv. Eng. Mater. 2012, 14, 909–918. [Google Scholar] [CrossRef]
- Weise, J.; Lehmhus, D.; Baumeister, J.; Kun, R.; Bayoumi, M.; Busse, M. Production and properties of 316l stainless steel cellular materials and syntactic foams. Steel Res. Int. 2014, 85, 486–497. [Google Scholar] [CrossRef]
- Luong, D.; Lehmhus, D.; Gupta, N.; Weise, J.; Bayoumi, M. Structure and compressive properties of invar-cenosphere syntactic foams. Materials 2016, 9, 115. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Low-density expanded perlite–aluminium syntactic foam. Mater. Sci. Eng. A 2014, 604, 127–134. [Google Scholar] [CrossRef]
- Borovinšek, M.; Taherishargh, M.; Vesenjak, M.; Ren, Z.; Fiedler, T. Geometrical characterization of perlite-metal syntactic foam. Mater. Charact. 2016, 119, 209–215. [Google Scholar] [CrossRef]
- Vesenjak, M.; Sulong, M.A.; Krstulović-Opara, L.; Borovinšek, M.; Mathier, V.; Fiedler, T. Dynamic compression of aluminium foam derived from infiltration casting of salt dough. Mech. Mater. 2016, 93, 96–108. [Google Scholar] [CrossRef]
- Kang, K.J. Wire-woven cellular metals: The present and future. Prog. Mater. Sci. 2015, 69, 213–307. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-printing of lightweight cellular composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef] [PubMed]
- Stöbener, K.; Lehmhus, D.; Avalle, M.; Peroni, L.; Busse, M. Aluminum foam-polymer hybrid structures (APM aluminum foam) in compression testing. Int. J. Solids Struct. 2008, 45, 5627–5641. [Google Scholar] [CrossRef]
- Lehmhus, D.; Baumeister, J.; Stutz, L.; Schneider, E.; Stöbener, K.; Avalle, M.; Peroni, L.; Peroni, M. Mechanical characterization of particulate aluminum foams-strain-rate, density and matrix alloy versus adhesive effects. Adv. Eng. Mater. 2010, 12, 596–603. [Google Scholar] [CrossRef]
- Vesenjak, M.; Borovinšek, M.; Fiedler, T.; Higa, Y.; Ren, Z. Structural characterisation of advanced pore morphology (APM) foam elements. Mater. Lett. 2013, 110, 201–203. [Google Scholar] [CrossRef]
- Sulong, M.A.; Vesenjak, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Compressive properties of Advanced Pore Morphology (APM) foam elements. Mater. Sci. Eng. A 2014, 607, 498–504. [Google Scholar] [CrossRef]
- Andersen, O.; Waag, U.; Schneider, L.; Stephani, G.; Kieback, B. Novel Metallic Hollow Sphere Structures. Adv. Eng. Mater. 2000, 2, 192–195. [Google Scholar] [CrossRef]
- Borovinšek, M.; Vesenjak, M.; Ren, Z. Estimating the base material properties of sintered metallic hollow spheres by inverse engineering procedure. Mech. Mater. 2016, 100, 22–30. [Google Scholar] [CrossRef]
- Gupta, N.R.P. Metal Matrix Syntactic Foams: Processing, Microstructure, Properties and Applications; DEStech Publications Inc.: Lancaster, PA, USA, 2014. [Google Scholar]
- Hokamoto, K.; Vesenjak, M.; Ren, Z. Fabrication of cylindrical uni-directional porous metal with explosive compaction. Mater. Lett. 2014. [Google Scholar] [CrossRef]
- Vesenjak, M.; Hokamoto, K.; Matsumoto, S.; Marumo, Y.; Ren, Z. Uni-directional porous metal fabricated by rolling of copper sheet and explosive compaction. Mater. Lett. 2016, 170, 39–43. [Google Scholar] [CrossRef]
- Vesenjak, M.; Hokamoto, K.; Sakamoto, M.; Nishi, T.; Krstulović-Opara, L.; Ren, Z. Mechanical and microstructural analysis of unidirectional porous (UniPore) copper. Mater. Des. 2016, 90, 867–880. [Google Scholar] [CrossRef]
- Güner, A.; Arıkan, M.; Nebioglu, M. New Approaches to Aluminum Integral Foam Production with Casting Methods. Metals 2015, 5, 1553–1565. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L. Variation of quasi-static and dynamic compressive properties in a single aluminium foam block. Mater. Sci. Eng. A 2014, 616, 171–182. [Google Scholar] [CrossRef]
- Wiehler, H.; Körner, C.; Singer, R.F. High pressure integral foam moulding of aluminium—Process technology. Adv. Eng. Mater. 2008, 10, 171–178. [Google Scholar] [CrossRef]
- Weise, J.; Baumeister, J.; Petzoldt, F. MIM components with hollow-sphere additions—Production, properties and market potential. Powder Inject. Mould. Int. 2013, 7, 73–77. [Google Scholar]
- Banhart, J.; Seeliger, H.W. Aluminium foam sandwich panels: Manufacture, metallurgy and applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Neugebauer, R.; Hipke, T. Machine Tools With Metal Foams. Adv. Eng. Mater. 2006, 8, 858–863. [Google Scholar] [CrossRef]
- Hohe, J.; Hardenacke, V.; Fascio, V.; Girard, Y.; Baumeister, J.; Stöbener, K.; Weise, J.; Lehmhus, D.; Pattofatto, S.; Zeng, H.; et al. Numerical and experimental design of graded cellular sandwich cores for multi-functional aerospace applications. Mater. Des. 2012, 39, 20–32. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L.; Ren, Z. Static and dynamic axial crush performance of in-situ foam-filled tubes. Compos. Struct. 2015, 124, 128–139. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L.; Anžel, I.; Ferreira, J.M.F. Manufacturing and bending behaviour of in situ foam-filled aluminium alloy tubes. Mater. Des. 2015, 66, 532–544. [Google Scholar] [CrossRef]
- Hangai, Y.; Nakano, Y.; Koyama, S.; Kuwazuru, O.; Kitahara, S.; Yoshikawa, N. Fabrication of aluminum tubes filled with aluminum alloy foam by frictionwelding. Materials 2015, 8, 7180–7190. [Google Scholar] [CrossRef]
- Strano, M.; Marra, A.; Mussi, V.; Goletti, M.; Bocher, P. Endurance of damping properties of foam-filled tubes. Materials 2015, 8, 4061–4079. [Google Scholar] [CrossRef] [Green Version]
- Santosa, S.P.; Wierzbicki, T.; Hanssen, A.G.; Langseth, M. Experimental and numerical studies of foam-filled sections. Int. J. Impact Eng. 2000, 24, 509–534. [Google Scholar] [CrossRef]
- Duarte, I.; Krstulović-Opara, L.; Vesenjak, M. Characterisation of aluminium alloy tubes filled with aluminium alloy integral-skin foam under axial compressive loads. Compos. Struct. 2015, 121, 154–162. [Google Scholar] [CrossRef]
- Chirwa, C.; Lehmhus, D.; Mao, M.; Chen, T.; Lanzi, L. Mechanics of lightweight aluminium foam wrapped in carbon fibre reinforced composites. In Impact Loading of Lightweight Structures; Alves, M., Ed.; WIT Press: Florianopolis, Brazil, 2005; pp. 157–176. [Google Scholar]
- Sun, G.; Li, S.; Liu, Q.; Li, G.; Li, Q. Experimental study on crashworthiness of empty/aluminum foam/honeycomb-filled CFRP tubes. Compos. Struct. 2016, 152, 969–993. [Google Scholar] [CrossRef]
- Weise, J.; Baumeister, J.; Hohe, J.; Boehme, W.; Beckmann, C. Aluminum Hybrid Foam—An Innovative Sandwich Core Material with Improved Energy Absorption Characteristics. In 10th International Conference on Sandwich Structures ICSS-10; Casari, P., Ed.; Université de Nantes: Nantes, France, 2012. [Google Scholar]
- Weise, J.; Queiroz Barbosa, A.F.; Yezerska, O.; Lehmhus, D.; Baumeister, J. Mechanical Behavior of Particulate Aluminium-Epoxy Hybrid Foams Based on Cold-Setting Polymers. Adv. Eng. Mater. 2017, 1700090. [Google Scholar] [CrossRef]
- Gergely, V.; Clyne, B. The FORMGRIP process: Foaming of reinforced metals by gas release in precursors. Adv. Eng. Mater. 2000, 2, 175–178. [Google Scholar] [CrossRef]
- Gergely, V.; Curran, D.C.; Clyne, T.W. The FOAMCARP process: Foaming of aluminium MMCs by the chalk-aluminium reaction in precursors. Compos. Sci. Technol. 2003, 63, 2301–2310. [Google Scholar] [CrossRef]
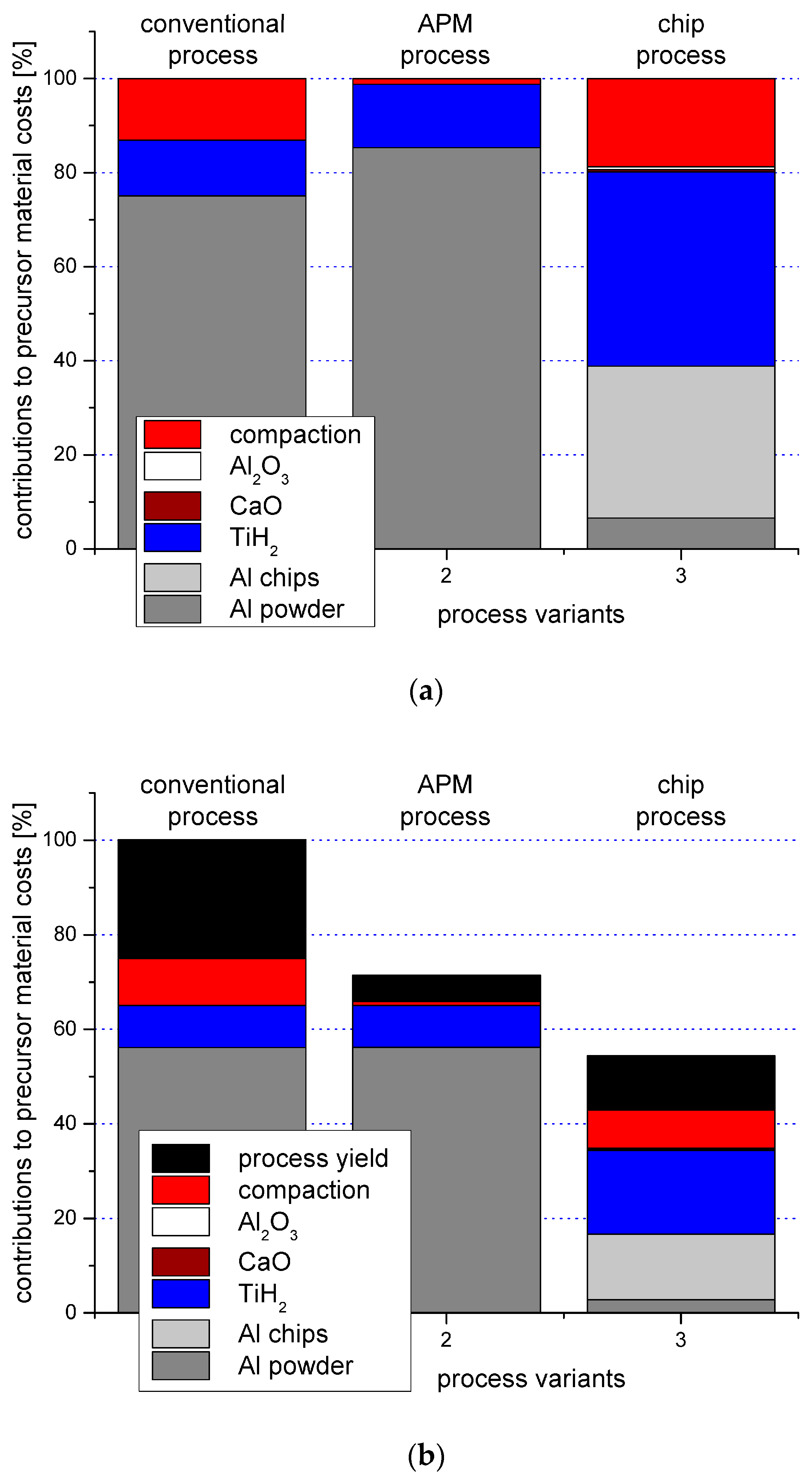
- Lehmhus, D.; Baumeister, J.; Haesche, M.; Stöbener, K.; Weise, J.; Lange, D.; Meyer, N. Paths towards cost reduction in precursor material based aluminium foam production processes. In Aluminium Alloys—Their Physical and Mechanical Properties; Hirsch, J., Skrotzki, B., Gottstein, G., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2008; pp. 2180–2186. [Google Scholar]
- Haesche, M.; Lehmhus, D.; Weise, J.; Wichmann, M.; Mocellin, I.C.M. Carbonates as foaming agent in chip-based aluminium foam precursor. J. Mater. Sci. Technol. 2010, 26, 845–850. [Google Scholar] [CrossRef]
- Wright, A.; Kennedy, A. The Processing and Properties of Syntactic Al Foams Containing Low Cost Expanded Glass Particles. Adv. Eng. Mater. 2016. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Kim, J.K.; Gupta, N.; Alaraj, S.; Daoud, A. Compressive characteristics of A356/fly ash cenosphere composites synthesized by pressure infiltration technique. Compos. Part A Appl. Sci. Manuf. 2006, 37, 430–437. [Google Scholar] [CrossRef]
- Banhart, J. Manufacturing routes for metallic foams. JOM 2000, 52, 22–27. [Google Scholar] [CrossRef]
- Fiedler, T.; Sulong, M.A.; Vesenjak, M.; Higa, Y.; Belova, I.V.; Oechsner, A.; Murch, G.E. Determination of the thermal conductivity of periodic APM foam models. Int. J. Heat Mass Transf. 2014, 73, 826–833. [Google Scholar] [CrossRef]
- Ulbin, M.; Borovinšek, M.; Higa, Y.; Shimojima, K.; Vesenjak, M.; Ren, Z. Internal structure characterization of AlSi7 and AlSi10 advanced pore morphology (APM) foam elements. Mater. Lett. 2014, 136, 416–419. [Google Scholar] [CrossRef]
- Strucwire|KIESELSTEIN International GmbH—Hersteller Moderner Drahtziehanlagen und Drahtziehschälmaschinen Sowie Drahtverarbeiter. Available online: http://www.kieselstein.com/de/Drahtprodukte/strucwire_1106.html (accessed on 1 July 2017).
- Baumgärtner, F.; Duarte, I.; Banhart, J. Industrialization of Powder Compact Foaming Process. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Lehmhus, D.; Rausch, G. Tailoring titanium hydride decomposition kinetics by annealing in various atmospheres. Adv. Eng. Mater. 2004, 6, 313–330. [Google Scholar] [CrossRef]
- Lehmhus, D.; Busse, M. Potential new matrix alloys for production of PM aluminium foams. Adv. Eng. Mater. 2004, 6, 391–396. [Google Scholar] [CrossRef]
- Grenestedt, J.L. Influence of wavy imperfections in cell walls on elastic stiffness of cellular solids. J. Mech. Phys. Solids 1998, 46, 29–50. [Google Scholar] [CrossRef]
- Irretier, A.; Banhart, J. Lead and lead alloy foams. Acta Mater. 2005, 53, 4903–4917. [Google Scholar] [CrossRef]
- Chethan, A.; Garcia-Moreno, F.; Wanderka, N.; Murty, B.S.; Banhart, J. Influence of oxides on the stability of zinc foam. J. Mater. Sci. 2011, 46, 7806–7814. [Google Scholar] [CrossRef]
- Rausch, G.; Weber, M.; Knüwer, M. Neue Entwicklungen zur Herstellung von Stahlschäumen. Mater. Werkst. 2000, 31, 424–427. [Google Scholar] [CrossRef]
- Wang, C.-F.; Zhou, F.; Gu, H.-Z.; Zhu, H.-X.; Li, Y.-W. Preparation and Microstructure of Al-intercalated Vermiculite. In International Conference on Chemical, Material and Metallurgical Engineering, ICCMME 2011; Trans Tech Publications: Beihai, China, 2011; pp. 683–687. [Google Scholar]
- Bazzaz Bonabi, S.; Kahani Khabushan, J.; Kahani, R.; Honarbakhsh Raouf, A. Fabrication of metallic composite foam using ceramic porous spheres “Light Expanded Clay Aggregate” via casting process. Mater. Des. 2014, 64, 310–315. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Pumice/aluminium syntactic foam. Mater. Sci. Eng. A 2015, 635, 102–108. [Google Scholar] [CrossRef]
- Taherishargh, M.; Sulong, M.A.; Belova, I.V.; Murch, G.E.; Fiedler, T. On the particle size effect in expanded perlite aluminium syntactic foam. Mater. Des. 2015, 66, 294–303. [Google Scholar] [CrossRef]
- Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. The effect of particle shape on mechanical properties of perlite/metal syntactic foam. J. Alloys Compd. 2017, 693, 55–60. [Google Scholar] [CrossRef]
- Broxtermann, S.; Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. On the compressive behaviour of high porosity expanded Perlite-Metal Syntactic Foam (P-MSF). J. Alloys Compd. 2017, 691, 690–697. [Google Scholar] [CrossRef]
- Peroni, L.; Scapin, M.; Avalle, M.; Weise, J.; Lehmhus, D. Dynamic mechanical behavior of syntactic iron foams with glass microspheres. Mater. Sci. Eng. A 2012, 552, 364–375. [Google Scholar] [CrossRef]
- Peroni, L.; Scapin, M.; Fichera, C.; Lehmhus, D.; Weise, J.; Baumeister, J.; Avalle, M. Investigation of the mechanical behaviour of AISI 316L stainless steel syntactic foams at different strain-rates. Compos. Part B Eng. 2014, 66, 430–442. [Google Scholar] [CrossRef]
- Luong, D.D.; Shunmugasamy, V.C.; Gupta, N.; Lehmhus, D.; Weise, J.; Baumeister, J. Quasi-static and high strain rates compressive response of iron and Invar matrix syntactic foams. Mater. Des. 2015, 66, 516–531. [Google Scholar] [CrossRef]
- Weise, J.; Salk, N.; Jehring, U.; Baumeister, J.; Lehmhus, D.; Bayoumi, M.A. Influence of powder size on production parameters and properties of syntactic invar foams produced by means of metal powder injection moulding. Adv. Eng. Mater. 2013, 15, 118–122. [Google Scholar] [CrossRef]
- Rabiei, A.; O’Neill, A.T. A study on processing of a composite metal foam via casting. Mater. Sci. Eng. A 2005, 404, 159–164. [Google Scholar] [CrossRef]
- Neville, B.P.; Rabiei, A. Composite metal foams processed through powder metallurgy. Mater. Des. 2008, 29, 388–396. [Google Scholar] [CrossRef]
- Marx, J.; Rabiei, A. Overview of Composite Metal Foams and Their Properties and Performance. Adv. Eng. Mater. 2017, 1600776. [Google Scholar] [CrossRef]
- Fiedler, T.; Ochsner, A.; Gracio, J. Numerical Investigations on the Mechanical Properties of Adhesively Bonded Hollow Sphere Structures. J. Compos. Mater. 2010, 44, 1165–1178. [Google Scholar] [CrossRef]
- Shishkin, A.; Drozdova, M.; Kozlov, V.; Hussainova, I.; Lehmhus, D. Vibration-Assisted Sputter Coating of Cenospheres: A New Approach for Realizing Cu-Based Metal Matrix Syntactic Foams. Metals 2017, 7, 16. [Google Scholar] [CrossRef]
- Vesenjak, M.; Gačnik, F.; Krstulović-Opara, L.; Ren, Z. Mechanical Properties of Advanced Pore Morphology Foam Elements. Mech. Adv. Mater. Struct. 2015, 22, 359–366. [Google Scholar] [CrossRef]
- Veyhl, C.; Fiedler, T.; Andersen, O.; Meinert, J.; Bernthaler, T.; Belova, I.V.; Murch, G.E. On the thermal conductivity of sintered metallic fibre structures. Int. J. Heat Mass Transf. 2012, 55, 2440–2448. [Google Scholar] [CrossRef]
- Veyhl, C.; Fiedler, T.; Jehring, U.; Andersen, O.; Bernthaler, T.; Belova, I.V.V.; Murch, G.E.E. On the mechanical properties of sintered metallic fibre structures. Mater. Sci. Eng. A 2013, 562, 83–88. [Google Scholar] [CrossRef]
- Andersen, O.; Vesenjak, M.; Fiedler, T.; Jehring, U.; Krstulović-Opara, L. Experimental and Numerical Evaluation of the Mechanical Behavior of Strongly Anisotropic Light-Weight Metallic Fiber Structures under Static and Dynamic Compressive Loading. Materials 2016, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Elambasseril, J.; Brandt, M.; Feih, S. Performance of bio-inspired Kagome truss core structures under compression and shear loading. Compos. Struct. 2014, 118, 294–302. [Google Scholar] [CrossRef]
- Lee, M.-G.; Ko, G.-D.; Song, J.; Kang, K.-J. Compressive characteristics of a wire-woven cellular metal. Mater. Sci. Eng. A 2012, 539, 185–193. [Google Scholar] [CrossRef]
- Lee, M.-G.; Kang, K.-J. Mechanical Behaviors of Wire-woven Metals Composed of Two Different Thickness of Wires. Procedia Mater. Sci. 2014, 4, 21–25. [Google Scholar] [CrossRef]
- Li, M.Z.; Stephani, G.; Kang, K.J. New cellular metals with enhanced energy absorption: Wire-woven bulk kagome (WBK)-metal hollow sphere (MHS) hybrids. Adv. Eng. Mater. 2011, 13, 33–37. [Google Scholar] [CrossRef]
- Warmuth, F.; Körner, C. Phononic band gaps in 2D quadratic and 3D cubic cellular structures. Materials 2015, 8, 8327–8337. [Google Scholar] [CrossRef]
- Novak, N.; Vesenjak, M.; Ren, Z. Auxetic Cellular Materials—A Review. Strojniški Vestn. J. Mech. Eng. 2016, 62, 485–493. [Google Scholar] [CrossRef]
- Rehme, O. Selective Laser Melting of Honeycombs with Negative Poisson’s Ratio. J. Laser Micro/Nanoeng. 2009, 4, 128–134. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Heinl, P.; Singer, R.F.; Körner, C. Auxetic cellular structures through selective electron-beam melting. Phys. Status Solidi Basic Res. 2010, 247, 269–272. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Schury, F.; Stingl, M.; Wein, F.; Singer, R.F.; Körner, C. Mechanical characterisation of a periodic auxetic structure produced by SEBM. Phys. Status Solidi Basic Res. 2012, 249, 1347–1352. [Google Scholar] [CrossRef]
- Lakes, R. Cellular solids with tunable positive or negative thermal expansion of unbounded magnitude. Appl. Phys. Lett. 2007, 90, 221905. [Google Scholar] [CrossRef]
- Liebold-Ribeiro, Y.; Körner, C. Phononic band gaps in periodic cellular materials. Adv. Eng. Mater. 2014, 16, 328–334. [Google Scholar] [CrossRef]
- Vesenjak, M.; Krstulović-Opara, L.; Ren, Z. Characterization of irregular open-cell cellular structure with silicone pore filler. Polym. Test. 2013, 32, 1538–1544. [Google Scholar] [CrossRef]
- Vesenjak, M.; Veyhl, C.; Fiedler, T. Analysis of anisotropy and strain rate sensitivity of open-cell metal foam. Mater. Sci. Eng. A 2012, 541, 105–109. [Google Scholar] [CrossRef]
- Jang, W.-Y.; Hsieh, W.-Y.; Miao, C.-C.; Yen, Y.-C. Microstructure and mechanical properties of ALPORAS closed-cell aluminium foam. Mater. Charact. 2015, 107, 228–238. [Google Scholar] [CrossRef]
- Ramamurty, U.; Paul, A. Variability in mechanical properties of a metal foam. Acta Mater. 2004, 52, 869–876. [Google Scholar] [CrossRef]
- Avalle, M.; Lehmhus, D.; Peroni, L.; Pleteit, H.; Schmiechen, P.; Belingardi, G.; Busse, M. AlSi7 metallic foams—Aspects of material modelling for crash analysis. Int. J. Crashworth. 2009, 14, 269–285. [Google Scholar] [CrossRef]
- Duarte, I.; Krstulović-Opara, L.; Vesenjak, M. Analysis of performance of in-situ carbon steel bar reinforced Al-alloy foams. Compos. Struct. 2016, 152, 432–443. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Krstulović-Opara, L. Compressive behaviour of unconstrained and constrained integral-skin closed-cell aluminium foam. Compos. Struct. 2016, 154, 231–238. [Google Scholar] [CrossRef]
- Park, C.; Nutt, S.R. Strain rate sensitivity and defects in steel foam. Mater. Sci. Eng. A 2002, 323, 358–366. [Google Scholar] [CrossRef]
- Friedl, O.; Motz, C.; Peterlik, H.; Puchegger, S.; Reger, N.; Pippan, R. Experimental Investigation of Mechanical Properties of Metallic Hollow Sphere Structures. Metall. Mater. Trans. B 2008, 39, 135–146. [Google Scholar] [CrossRef]
- Vesenjak, M.; Fiedler, T.; Ren, Z.; Öchsner, A. Behaviour of Syntactic and Partial Hollow Sphere Structures under Dynamic Loading. Adv. Eng. Mater. 2008, 10, 185–191. [Google Scholar] [CrossRef]
- Fiedler, T.; Sulong, M.A.; Mathier, V.; Belova, I.V.; Younger, C.; Murch, G.E. Mechanical properties of aluminium foam derived from infiltration casting of salt dough. Comput. Mater. Sci. 2014, 81, 246–248. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Ginsztler, J. Compressive characteristics of metal matrix syntactic foams. Compos. Part A Appl. Sci. Manuf. 2012, 43, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, I.; Oktay, E. Production and aging of highly porous 17-4 PH stainless steel. J. Porous Mater. 2012, 19, 433–440. [Google Scholar] [CrossRef]
- Casolco, S.R.; Dominguez, G.; Sandoval, D.; Garay, J.E. Processing and mechanical behavior of Zn–Al–Cu porous alloys. Mater. Sci. Eng. A 2007, 471, 28–33. [Google Scholar] [CrossRef]
- Luong, D.D.; Strbik, O.M.; Hammond, V.H.; Gupta, N.; Cho, K. Development of high performance lightweight aluminum alloy/SiC hollow sphere syntactic foams and compressive characterization at quasi-static and high strain rates. J. Alloys Compd. 2013, 550, 412–422. [Google Scholar] [CrossRef]
- Rabiei, A.; Vendra, L.; Reese, N.; Young, N.; Neville, B.P. Processing and Characterization of a New Composite Metal Foam. Mater. Trans. 2006, 47, 2148–2153. [Google Scholar] [CrossRef]
- Rabiei, A.; Vendra, L.J. A comparison of composite metal foam’s properties and other comparable metal foams. Mater. Lett. 2009, 63, 533–536. [Google Scholar] [CrossRef]
- Tane, M.; Kawashima, T.; Yamada, H.; Horikawa, K.; Kobayashi, H.; Nakajima, H. Strain rate dependence of anisotropic compression behavior in porous iron with unidirectional pores. J. Mater. Res. 2010, 25, 1179–1190. [Google Scholar] [CrossRef]
- Yang, L.; Harrysson, O.; West, H.; Cormier, D. Compressive properties of Ti–6Al–4V auxetic mesh structures made by electron beam melting. Acta Mater. 2012, 60, 3370–3379. [Google Scholar] [CrossRef]
- Vesenjak, M.; Krstulović-Opara, L.; Ren, Z.; Öchsner, A.; Domazet, Ž. Experimental Study of Open-Cell Cellular Structures with Elastic Filler Material. Exp. Mech. 2008, 49, 501–509. [Google Scholar] [CrossRef]
- Lakes, R.S.; Elms, K. Indentability of conventional and negative Poisson’s ratio foams. J. Compos. Mater. 1993, 27, 1193–1202. [Google Scholar] [CrossRef]

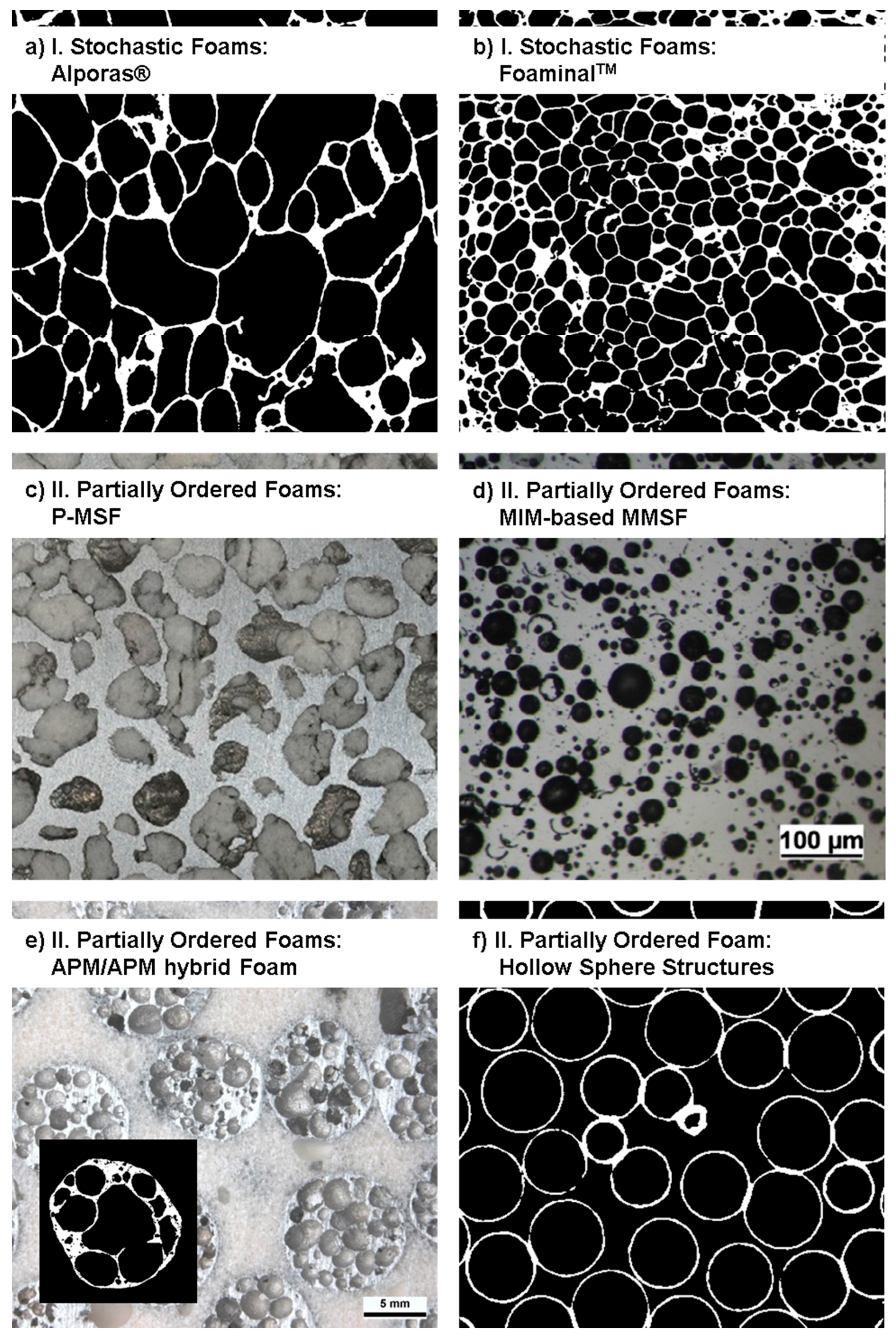


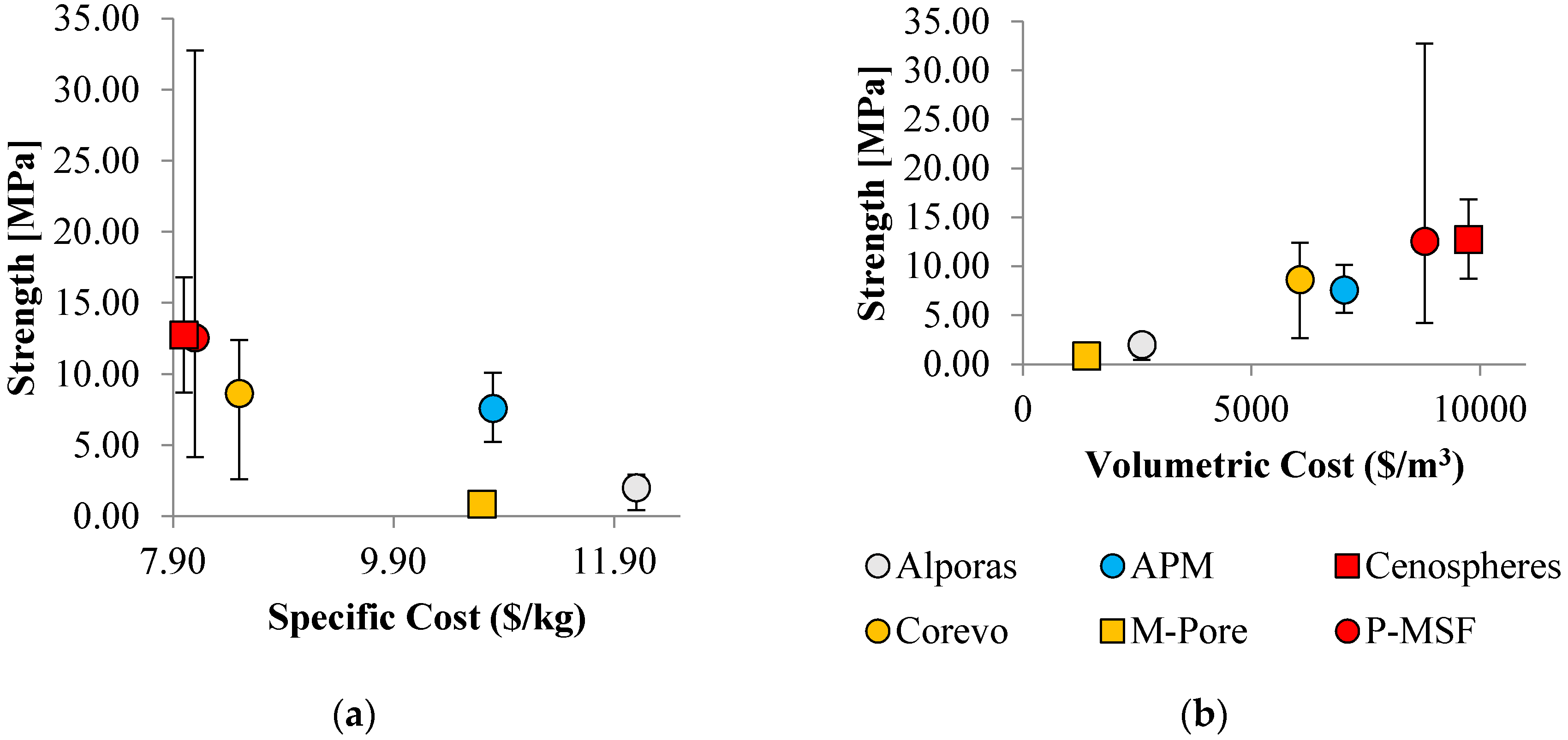
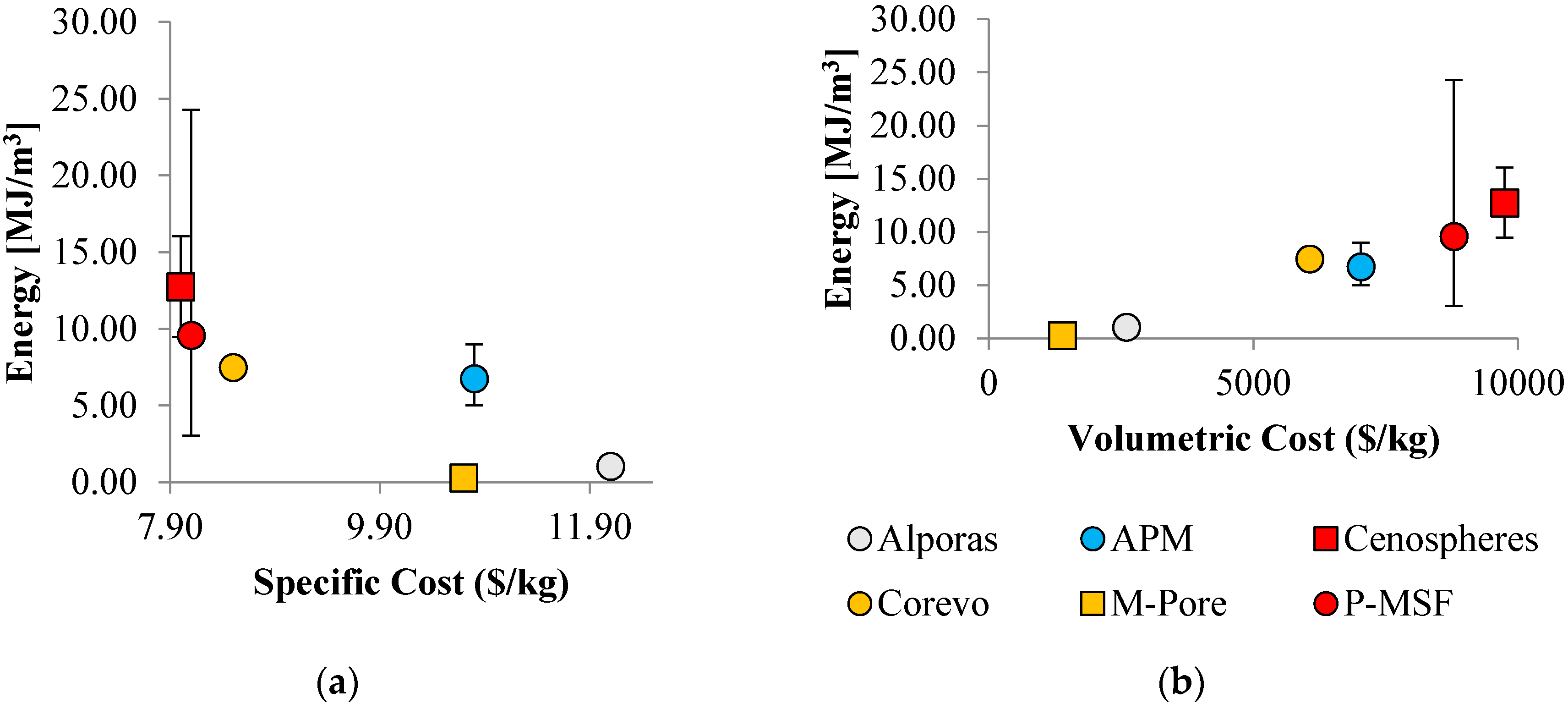
| Property | Alporas | APM | Cenospheres | Corevo | M.Pore | P-MSF |
|---|---|---|---|---|---|---|
| (%) | 8 | 25 | 45 | 25 | 5 | 40 |
| ($/m3) | 871 | 819 | - | - | ||
| ($/m) | - | - | free | 120 | ||
| ($/m) | - | - | - | 320 | 100 | - |
| ($/m) | 6.0 | 18 | 33.6 | 19.8 | 3.7 | 29.9 |
| ($/m) | 2600 | 7030 | 9750 | 6060 | 1390 | 8790 |
| (kg/m) | 216 | 650 | 1215 | 715 | 130 | 1080 |
| ($/k) | 12.1 | 10.8 | 8.0 | 8.5 | 10.7 | 8.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmhus, D.; Vesenjak, M.; Schampheleire, S.D.; Fiedler, T. From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals. Materials 2017, 10, 922. https://doi.org/10.3390/ma10080922
Lehmhus D, Vesenjak M, Schampheleire SD, Fiedler T. From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals. Materials. 2017; 10(8):922. https://doi.org/10.3390/ma10080922
Chicago/Turabian StyleLehmhus, Dirk, Matej Vesenjak, Sven De Schampheleire, and Thomas Fiedler. 2017. "From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals" Materials 10, no. 8: 922. https://doi.org/10.3390/ma10080922