Electrochemical Impedance Analysis of a PEDOT:PSS-Based Textile Energy Storage Device
Abstract
:1. Introduction
2. Results and Discussion
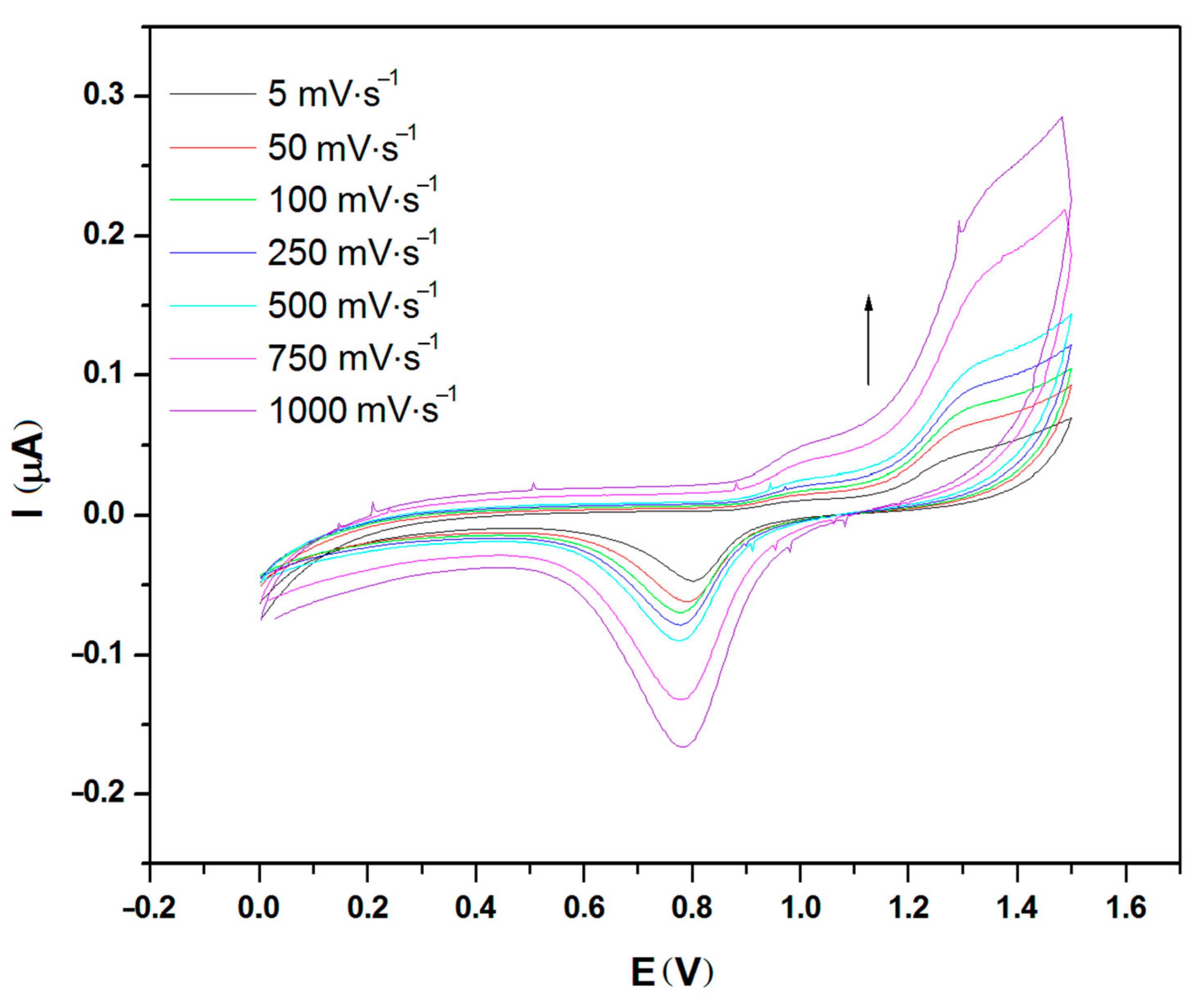
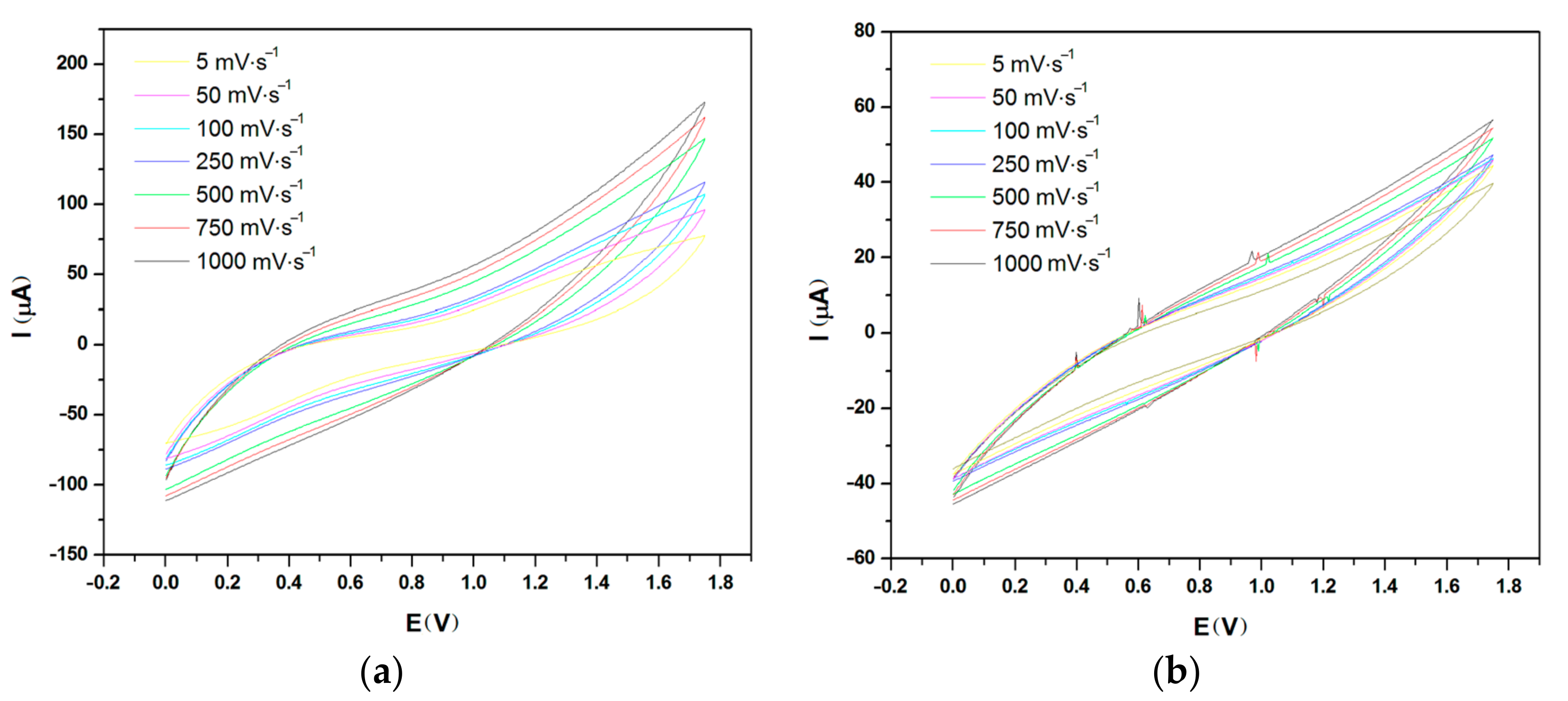
2.1. Cyclic Voltammetry
2.2. Nyquist and Bode Plots
2.3. Simulation of Circuit Model
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhattacharya, R.; de Kok, M.M.; Zhou, J. Rechargable electronic textile battery. Appl. Phys. Lett. 2009, 95, 223305. [Google Scholar] [CrossRef]
- Odhiambo, S.A.; De Mey, G.; Hertleer, C.; Schwarz, A.; Van Langenhove, L. Discharge characteristics of poly(3,4-ethylene dioxythiophene): poly(styrenesulfonate) (PEDOT:PSS) textile batteries; comparison of silver coated yarn electrode devices and pure stainless steel filament yarn electrode devices. Text. Res. J. 2014, 84, 347–354. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technologycal Application; Springer: New York, NY, USA, 1999; p. 163. ISBN 9781475730586. [Google Scholar]
- Su, Z.; Yang, C.; Xu, C.; Wu, H.; Zhang, Z.; Liu, T.; Zhang, C.; Yang, Q.; Li, B.; Kang, F. Co-electro-deposition of the MnO2-PEDOT:PSS nanostructured composite for high-areal mass, flexible asymmetric supercapacitor devices. J. Mater. Chem. A 2013, 1, 12432–12440. [Google Scholar] [CrossRef]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Nuramdhani, I.; Odhiambo, S.A.; Hertleer, C.; De Mey, G.; Van Langenhove, L. Electric Field Effect on Charge-Discharge Characteristics of Textile-Based Energy Storage Devices: In Search of the Underlying Mechanism. Tekstilec 2016, 59, 162–167. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Marathe, D.M.; Ghosh, S.S.; Ganesan, V.; Sali, J.V. Changes in in-plane electrical conductivity of PEDOT:PSS thin films due to electric field induced dipolar reorientation. RSC Adv. 2015, 5, 86393–86401. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High Conductivity Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) Film and Its Application in Polymer Optoelectronic Devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Timpanaro, S.; Kemerink, M.; Touwslager, F.J.; De Kok, M.M.; Schrader, S. Morphology and conductivity of PEDOT/PSS films studied by scanning-tunneling microscopy. Chem. Phys. Lett. 2004, 394, 339–343. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; Van Breemen, A.J.J.M.; de Kok, M.M. Microscopic Understanding of the Anisotropic Conductivity of PEDOT:PSS Thin Films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Ouyang, L.; Musumeci, C.; Jafari, M.J.; Ederth, T.; Inganäs, O. Imaging the Phase Separation between PEDOT and Polyelectrolytes during Processing of Highly Conductive PEDOT:PSS Films. ACS Appl. Mater. Interfaces 2015, 7, 19764–19773. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N.; Alam, F.; Swami, S.K.; Dutta, V. Effect of electric field on the spray deposited poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) layer and its use in organic solar cell. J. Appl. Phys. 2013, 114, 184501. [Google Scholar] [CrossRef]
- Maddala, J.; Sambath, K.; Kumar, V. Identification of reaction mechanism for anodic dissolution of metals using Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2010, 638, 183–188. [Google Scholar] [CrossRef]
- Yu, A.; Cahbot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781138077119. [Google Scholar]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776–10786. [Google Scholar] [CrossRef]
- Su, F.; Miao, M. Flexible, high performance two-ply yarn supercapacitors based on irradiated carbon nanotube yarn and PEDOT/PSS. Electrochim. Acta 2014, 127, 433–438. [Google Scholar] [CrossRef]
- Yang, N.; Zoski, C.G. Polymer Films on Electrodes: Investigation of Ion Transport at Poly(3,4-ethylenedioxythiophene) Films by Scanning Electrochemical Microscopy. Langmuir 2006, 22, 10338–10347. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, X. Towards Textile Energy Storage from Cotton T-Shirts. Adv. Mater. 2012, 24, 3246–3252. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Song, M.K.; Park, Y.J.; Kim, J.M.; Liu, M.; Wang, Z.L. Fiber Supercapacitors Made of Nanowire-Fiber Hybrid Structures for Wearable/Flexible Energy Storage. Angew. Chem. Int. Ed. 2011, 50, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.; Kao, P.; Best, A. Conducting polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, H.; Ye, S.; Cai, X.; Yu, X.; Hou, S.; Kafafy, H.; Zou, D. Integrated power fiber for energy conversion and storage. Energy Environ. Sci. 2013, 6, 805–812. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Application of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420069129. [Google Scholar]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy. In Impedance Spectroscopy: Theory, Experiment and Applications, 2nd ed.; Barsoukow, E., Macdonald, J.R., Eds.; Wiley Interscience: New York, NY, USA, 2005; pp. 1–26. [Google Scholar] [CrossRef]
- Stocker, T.; Kohler, A.; Moos, R. Why Does the Electrical Conductivity in PEDOT:PSS Decrease with PSS Content? A Study Combinig Thermoelectric Measurements with Impedance Spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 976–983. [Google Scholar] [CrossRef]

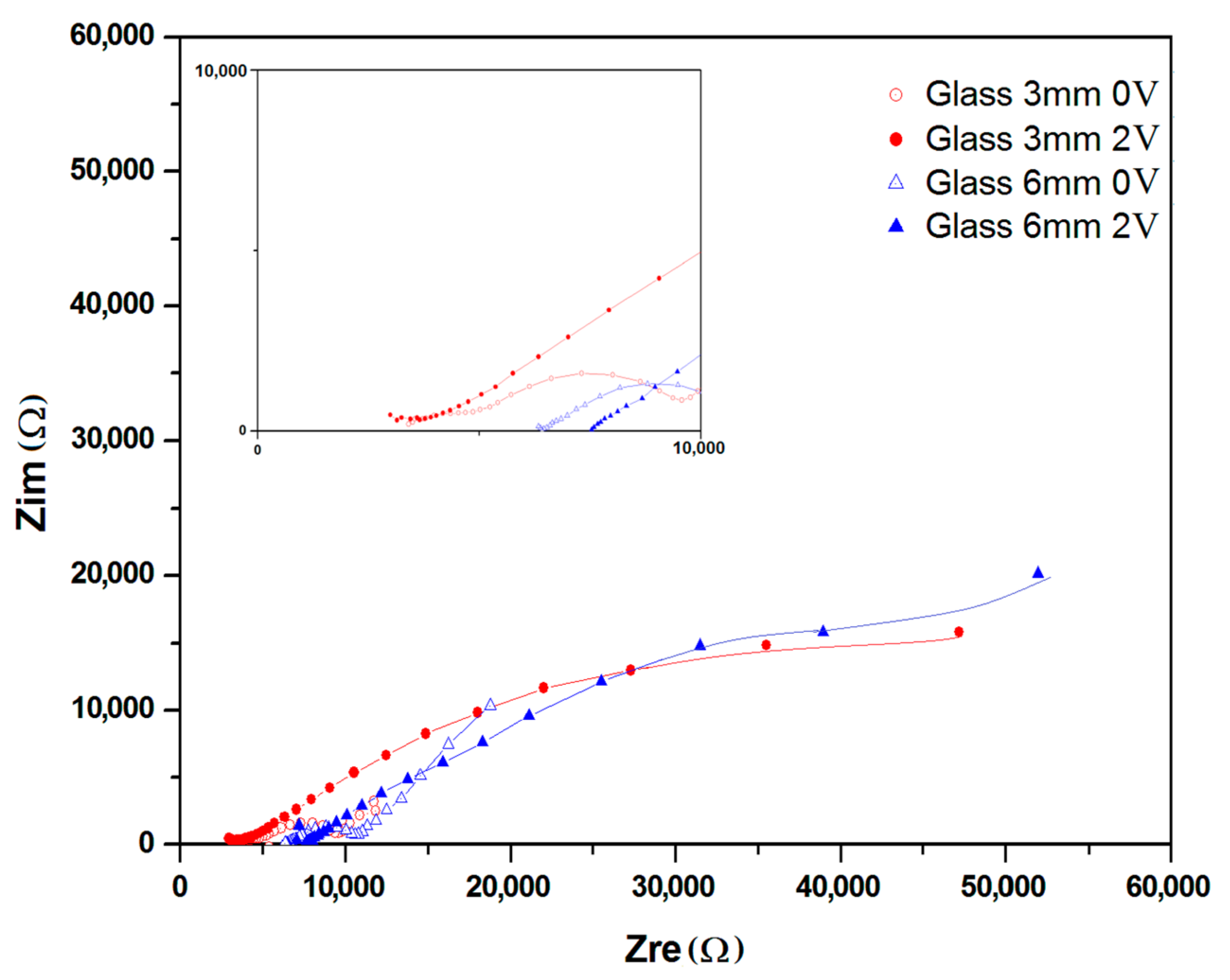
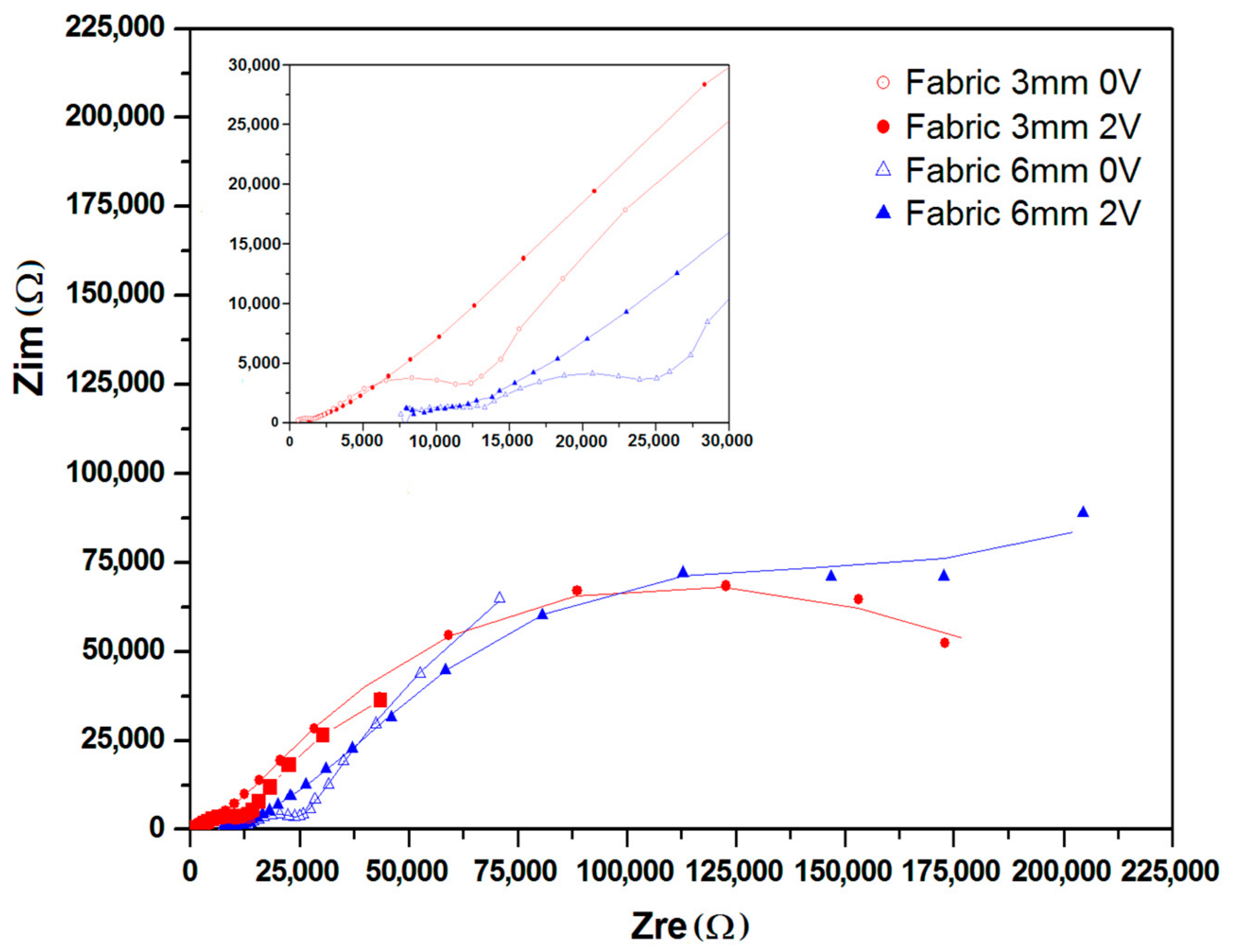

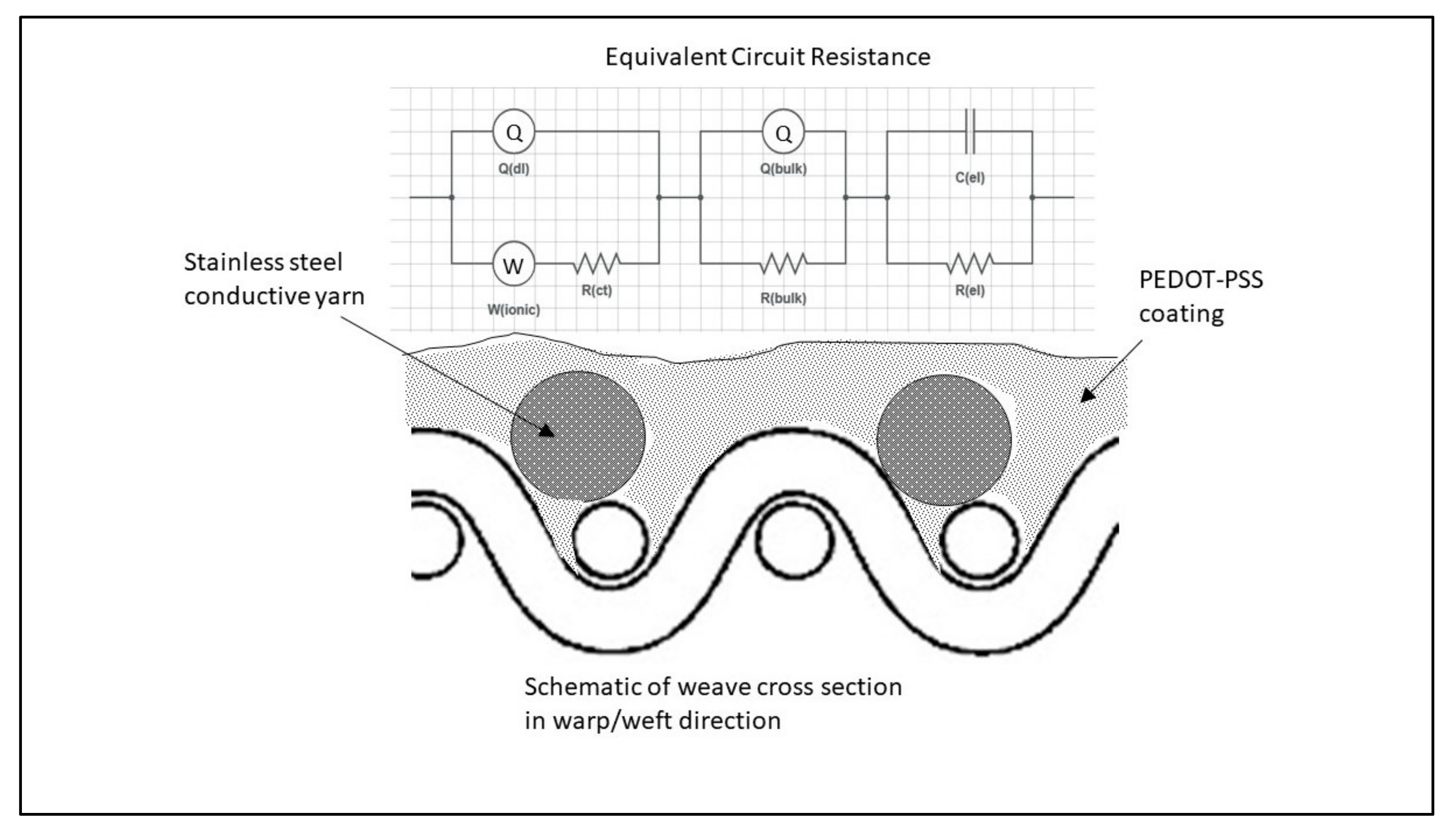
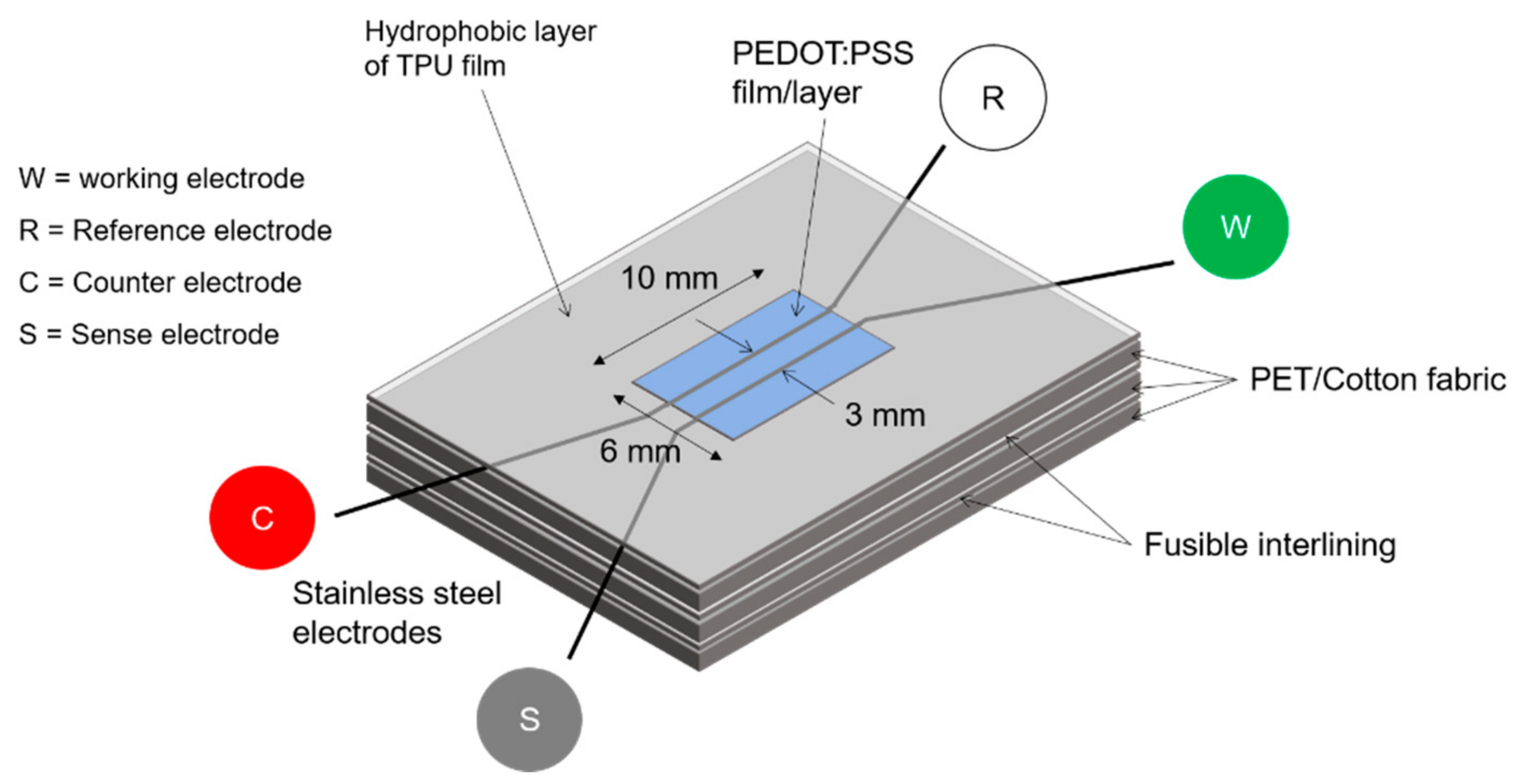
| Sample | Qel * (μS·s−n) | n | Rel (kΩ) | Qbulk (μF) | n | Rbulk (kΩ) | Qdl (μS·s−n) | n | Rct (kΩ) | Wionic (μS·s−1/2) | X |
|---|---|---|---|---|---|---|---|---|---|---|---|
| On glass | |||||||||||
| 3 mm | 1.11 | 0.6479 | 1.3 | 5.10 | 0.7856 | 2.46 | 64.6 | 0.4736 | 375.48 | - | 1.25 × 10−4 |
| 3 mm_ch*) | 124.7 | 0.2144 | 4.9 | 4.00 | 0.9999 | 1.7 | 40.23 | 0.6564 | 55.43 | 92.9 | 1.11 × 10−3 |
| 6 mm | 38.68 | 0.9052 | 2.19 | 0.23 | 0.4888 | 17.3 | 49.43 | 0.3281 | 185.1 | - | 9.90 × 10−5 |
| 6 mm_ch*) | 87.6 | 0.717 | 95.7 | 6.98 | 0.6834 | 2.2 | 22.53 | 0.002 | 23.73 | 9.26 | 8.02 × 10−5 |
| On fabric | |||||||||||
| 3 mm | 8.06 | 0.3754 | 2.35 | 902.9 | 0.7812 | 128 | 34.62 | 0.6781 | 10.93 | - | 6.71 × 10−4 |
| 3 mm_ch*) | 293.75 | 0.0869 | 1.93 | 86.5 | 0.8384 | 124 | 56.02 | 0.3951 | 171.05 | 316.6 | 1.19 × 10−4 |
| 6 mm | 18.0 | 0.7861 | 5.41 | 456.67 | 0.7329 | 39.69 | 38.41 | 0.7398 | 18.2 | - | 4.01 × 10−4 |
| 6 mm_ch*) | 192.8 | 0.09875 | 59.9 | 17.35 | 0.1392 | 21.64 | 32.94 | 0.4822 | 215.3 | 73.7 | 6.28 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuramdhani, I.; Gokceoren, A.T.; Odhiambo, S.A.; De Mey, G.; Hertleer, C.; Van Langenhove, L. Electrochemical Impedance Analysis of a PEDOT:PSS-Based Textile Energy Storage Device. Materials 2018, 11, 48. https://doi.org/10.3390/ma11010048
Nuramdhani I, Gokceoren AT, Odhiambo SA, De Mey G, Hertleer C, Van Langenhove L. Electrochemical Impedance Analysis of a PEDOT:PSS-Based Textile Energy Storage Device. Materials. 2018; 11(1):48. https://doi.org/10.3390/ma11010048
Chicago/Turabian StyleNuramdhani, Ida, Argun Talat Gokceoren, Sheilla Atieno Odhiambo, Gilbert De Mey, Carla Hertleer, and Lieva Van Langenhove. 2018. "Electrochemical Impedance Analysis of a PEDOT:PSS-Based Textile Energy Storage Device" Materials 11, no. 1: 48. https://doi.org/10.3390/ma11010048





